Abstract
Asthma is characterized by airway inflammation, mucus overproduction, and airway hyperreactivity. Cytokines, especially T helper 2-derived cytokines interleukin (IL)-4, IL-5, and IL-13, are involved in the pathogenesis of asthma. IL-21 has a variety of effects on the immune system. However, the contribution of IL-21 to the development of allergic diseases is currently controversial. The aim of this study was to investigate the effect of IL-21 on asthma airway inflammation in vivo. A murine ovalbumin (OVA)-induced allergic asthma model was used. The concentration of IL-21 in the bronchoalveolar lavage fluid (BALF) of mice was evaluated by enzyme-linked immunosorbent assay. BALF cellularity, lung histopathology, and sera IgE levels were compared between the normal control group, OVA sensitization/challenge group, and OVA sensitization/challenge plus IL-21-administered group. An OVA-induced allergic rhinitis model with IL-21 was used as a positive control and the infiltration of eosinophils in the nasal mucosa was evaluated. The concentration of IL-21 in the BALF was lower in the asthmatic group compared with the normal control group. However, no significant differences in airway eosinophilia, lung histopathology, and sera IgE levels were observed between the OVA sensitization/challenge group and OVA sensitization/challenge plus IL-21-administered group. Decreased eosinophilic infiltration of nasal mucosa was observed in the positive control allergic rhinitis model administered IL-21 during the challenge period. Exogenous administration of IL-21 alone may not alleviate allergic lung inflammation. The role of IL-21 in allergic lung inflammation needs further research.
Keywords: Allergic lung inflammation, asthma, challenge, cytokine, interleukin-21
Introduction
Asthma is a common respiratory disorder characterized by airway inflammation, bronchoconstriction, and airway hyperresponsiveness. These features clinically manifest themselves as exacerbations of wheezing and breathlessness, which have significant impact on the quality of life. Airway inflammation that occurs during atopic asthma is associated with exposure to specific allergens such as house dust mite allergens or nonspecific triggers, such as air pollution [1]. Cytokines are critical in allergic intercellular communication networks, and they contribute to disease pathology through the recruitment and activation of proinflammatory leukocytes and in the chronic phase by mediating pro-fibrotic/remodeling events. A number of studies have clearly established the importance of T helper (Th) 2-derived cytokines interleukin (IL)-4, IL-5, and IL-13 in mediating the airway inflammatory response using a murine model of allergic lung inflammation [2-5]. Th2 cytokines are produced predominantly by activated Th2 cells, which induce inflammatory cell infiltration into the airways. Recently, Th17 cells, a subset of CD4+ T cells with distinct properties from Th1, Th2, and regulatory T cells [6], are found in the lungs of patients with severe asthma [7]. In addition, the Th17 cytokine IL-17A is present in the bronchoalveolar lavage fluid (BALF) and bronchial biopsies of patients with moderate to severe asthma [8]. IL-21 is a member of the type I cytokine family with significant sequence homology to IL-2, IL-4, and IL-15 [9]. IL-21 is produced by activated CD4+ T cells, and recently it has been reported that IL-21 is produced by Th17 cells and NKT cells [10]. IL-21 biological functions are mediated by a heterodimeric receptor, formed by the common γ-chain subunit, which is shared with IL-2, IL-4, IL-7, IL-9, and IL-15 receptors, and its own unique receptor (designated IL-21R). Binding of IL-21 to IL-21R induces the activation of Janus kinase (JAK)-1 and JAK3 and hence activation of STAT1, STAT3, and, to a lesser degree, STAT4 and STAT5. IL-21 regulates the activation and proliferation of CD4+ T cells and B cells. In vitro experiments have demonstrated that IL-21 can have both positive and negative effects on B lineage cells depending on the presence or absence of other signals [11,12]. IL-21 stimulates the differentiation of follicular helper T [13] and Th17 cells [14,15], and it has antitumor actions in vitro and in vivo [16-18]. Little is known about the impact of IL-21 signaling pathway on the initiation and progression of IgE-dependent allergic diseases [19]. In an ovalbumin (OVA)-induced mouse model of allergic rhinitis, administering IL-21 during the initial antigen challenge significantly reduced allergic symptoms, with diminished antigen-specific serum IgE and Th2 cytokines (IL-4, IL-5, and IL-13) in the nasal tissue and decreased levels of IL-4-induced eotaxin-1 and eotaxin-2 in nasal fibroblasts, leading to suppressed eosinophilic migration into the nasal tissue [20]. A previous study showed that IL-21 gene polymorphisms were associated with susceptibility to atopic asthma [21]. Therefore, this study determined whether IL-21 influenced eosinophilic inflammation in a murine asthma model. Unexpectedly, the administration of IL-21 did not improve allergic lung inflammation in an OVA-induced murine asthma model.
Materials and methods
Mice
Female BALB/c mice (6-8 weeks old) were purchased from the Laboratory Animal Center of Hubei Province China and housed under specific pathogen-free conditions in the Laboratory Animal Center of Huazhong University of Science and Technology. The experimental procedure was approved by the committee of Huazhong University of Science and Technology for animal research.
Animal model and recombinant mouse (rm) IL-21 administration
The allergic asthma model was induced as previously described [22]. Briefly, mice were sensitized on day 0 by intraperitoneal (i.p.) injection of 20 μg OVA (Grade V; Sigma, MO, USA) absorbed in 2 mg of alum (Pierce, Rockford, IL, USA) (200 μL/mouse). On day 14, mice were sensitized a second time with 100 μg OVA. On days 24, 26, and 28, mice were anesthetized by i.p. injection of 0.1 mL of a mixture of 10 mg/mL ketamine and 1 mg/mL xylazine diluted in sterile phosphate-buffered saline (PBS) and challenged with 200 μg OVA in 40 μL of sterile PBS by intratracheal instillation. The control group received sterile PBS with alum i.p. on days 0 and 14 and 40 μL of sterile PBS on days 24, 26, and 28 by intratracheal instillation. In the IL-21 treatment group, mice were administered 20 ng of rmIL-21 (PeproTech, Rocky Hill, NJ) intratracheally 30 min before challenge.
A nasal allergic model was used as a positive control, and mice were treated as previously described [20]. Briefly, mice were administered 100 μg OVA and 4 mg alum in saline i.p. at a dosage of 0.2 mL/mouse. The sensitization was repeated three times at weekly intervals (days 0, 7, and 14) followed by daily injections of OVA solution (40 mg/mL in saline) into the nostrils (0.02 mL/mouse) on days 21-28 (challenge). In the IL-21 treatment group, mice were administered 20 ng of rmIL-21 intranasally 30 min before challenge (Figure 1).
Figure 1.
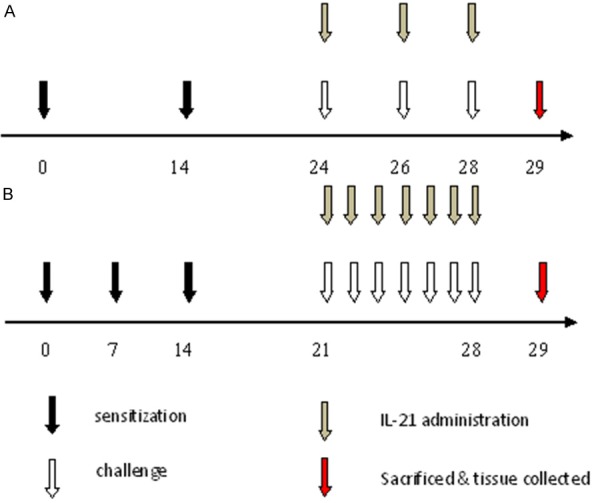
Protocols for sensitization, challenge, and rmIL-21 administration. A: Murine asthma model: mice were sensitized by i.p. immunization with OVA plus alum on days 0 and 14 followed by challenge with OVA solution by intratracheal instillation on days 24, 26, and 28. Some groups of mice were treated with rmIL-21 before challenge. B: Allergic rhinitis model: mice were sensitized by i.p. administration of OVA plus alum on days 0, 7, and 14 followed by daily injections of OVA solution into the nostrils on days 21-28 (challenge). Some groups of mice were treated with rmIL-21 administration into the nasal cavity before challenge. On day 29, mice were sacrificed and samples were collected.
Bronchoalveolar lavage and lung histopathologic examination
Bronchoalveolar lavage was performed with 0.8 mL PBS three times. The lavage fluid was centrifuged, and the supernatants were kept at -80°C until used for cytokine measurements. The cell pellets were resuspended in 0.5 mL of PBS and used for total and differential cell counts. The total number of cells in BALF was counted by hemacytometer. Eosinophils, neutrophils, and macrophages were counted in BALF using cytospins (centrifuged preparations) stained with Diff-Quik staining. A total of 200 cells in each sample were counted. Lungs were then inflated with 0.8 mL 10% formalin, fixed in 10% formalin for at least 24 h, dehydrated through a gradient ethanol, embedded in paraffin, and the sagittal sections were cut at a thickness of 5 μm. Sections were stained with hematoxylin and eosin.
Enzyme-linked immunosorbent assay
Sera were collected from mice within 24 h after the last challenge. Concentrations of IgE were evaluated using an enzyme-linked immunosorbent assay (ELISA) kit (BioLegend, San Diego, CA). Concentrations of IL-21 in the BALF were measured by ELISA kits (R & D Systems, Minneapolis, MN).
Statistical analysis
Data were expressed as mean ± SD. Statistical significance was assessed by one-way analysis of variance followed by the Tukey’s multiple comparison test or the unpaired Student’s t-test. Statistical significance was set at p < 0.05.
Results
BALF concentration of IL-21 was decreased in asthmatic mice
The concentration of IL-21 in the BALF was determined by ELISA. IL-21 levels were decreased in asthmatic mice (40.17 ± 5.919 pg/mL) compared with those in normal mice (102.7 ± 14.44 pg/mL) (Figure 2).
Figure 2.
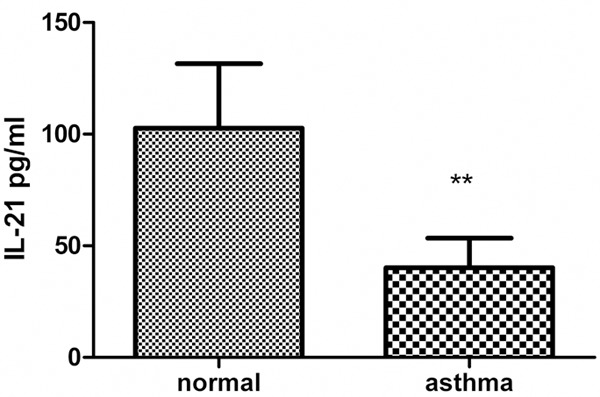
The concentration of IL-21 in the supernatants of BALF. Asthmatic mice were sacrificed within 24 h after the last challenge. BALF was collected and the concentration of IL-21 was determined by ELISA. Values represent mean ± SD (n = 6 in each group). **p < 0.005.
Intranasal IL-21 administration alleviates infiltration of eosinophils in nasal mucosa in murine allergic rhinitis
To validate the effect of IL-21 as a positive control, we performed an experiment using an allergic rhinitis model treated with IL-21 before challenge as previously described [20]. Intranasal IL-21 administration decreased eosinophilic infiltration of the nasal mucosa in an OVA-induced murine allergic rhinitis model (Figure 3).
Figure 3.
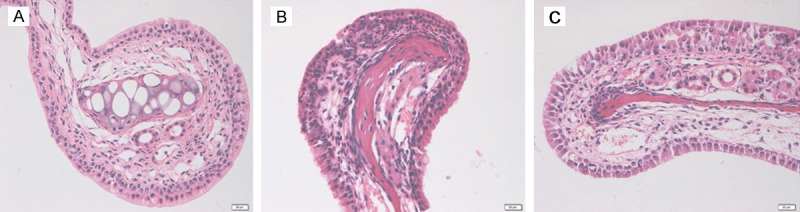
Histological examination of nasal mucosa. A: Nasal mucosa from the normal control group. B: Nasal mucosa from the OVA-induced mice allergic rhinitis model. C: Nasal mucosa from the rmIL-21-treated group. Mice were sensitized and challenged with the allergen with or without rmIL-21 administration before challenge. In rmIL-21-treated mice, the infiltration of eosinophils in the nasal mucosa was decreased significantly compared with the OVA sensitized/challenge group.
Administration of IL-21 did not ameliorate allergic airway inflammation
Because the concentration of IL-21 in the BALF was lower in asthmatic mice and the administration of IL-21 decreased eosinophilic infiltration of nasal mucosa in allergic rhinitis, we determined whether allergic airway inflammation could be improved by administration of IL-21 before challenge. rmIL-21 was administered intratracheally before each challenge as in Figure 1A. Inflammation around the bronchus and vessels was not affected by the administration of IL-21 (Figure 4). After the last challenge, mice were sacrificed and the lungs were lavaged, cells were counted and a differential cell count was performed. There was no significant change in the number of total cells, macrophages, neutrophils, eosinophils, or lymphocytes between the asthmatic group and the IL-21 administration group (Figure 5). Similarly, there were no significant changes in sera IgE levels between the groups with and without treatment of rmIL-21 before challenge in asthmatic mice (Figure 6).
Figure 4.
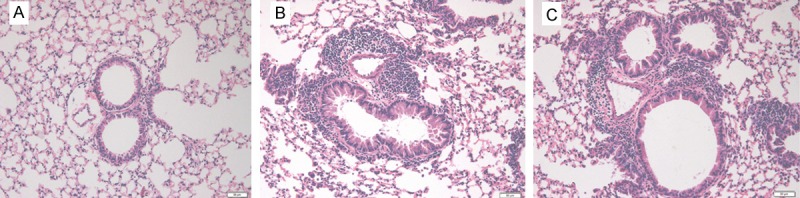
Administration of IL-21 before challenge does not ameliorate OVA-induced peribronchial inflammation. Lung sections were stained with hematoxylin and eosin for the measurement of inflammatory cells around the airways. The extent of cellular infiltration of the peri-airway region at 24 h after the last OVA challenge was comparable between the OVA sensitized/challenge group (B) and the IL-21-treated group (C). (A) Histologic section of lung from the normal control group.
Figure 5.
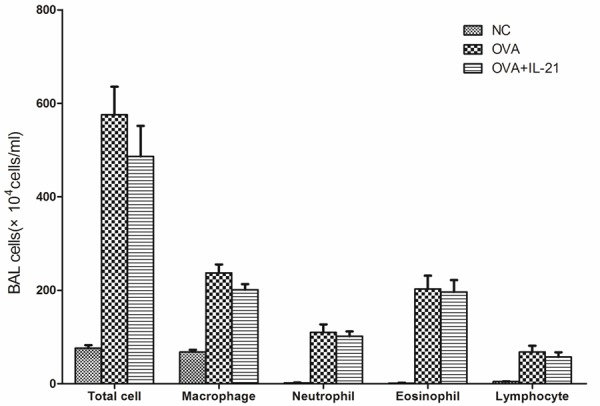
Administration of IL-21 does not decrease cell infiltration into the BALF in OVA sensitized/challenge mice. Mice were treated with sensitization and challenge protocols and lungs were lavaged after the last challenge. Total cells were counted and a differential cell count was performed based on staining with Diff-Quik. Compared with the OVA sensitized/challenge group, significant differences in total cells and cell differential were not observed in the IL-21-treated group. NC, negative control. OVA, OVA sensitization and challenge group. OVA+IL-21, OVA sensitized/challenge and IL-21 treated before challenge (n = 6 in each group).
Figure 6.
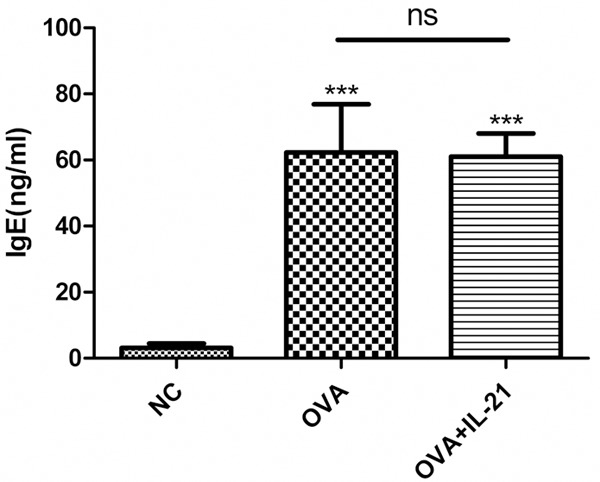
IL-21 administration does not decrease sera IgE levels in OVA sensitized/challenge mice. Mice were treated with sensitization and challenge protocols, and IL-21 was administered before each challenge. Mice were sacrificed 24 h after the last challenge, and the concentration of IgE in the sera was evaluated by ELISA. Each value represents the mean ± SD of six mice in each group. ***p < 0.005 vs control group; ns, not significant.
Discussion
In the present study, we examined the effect of IL-21 treatment on allergic asthmatic mice. IL-21 had no effect on allergic airway inflammation in mice. During the challenge period, intratracheally administration of IL-21 may not alleviate airway inflammation and sera IgE levels were not affected.
Conflicting data on the effects of IL-21 on allergic inflammation in animal models of various diseases have been reported. IL-21 receptor deficiency inhibited allergic cutaneous inflammation by suppressing the trafficking of cutaneous dendritic cells to draining lymph nodes in a murine model of epicutaneous sensitization using tape stripping [23]. And studies by Fröhlich A et al. [24] and Lajoie S et al. [25] found that IL-21 may play roles in enhancing allergic asthma in IL-21 receptor deficient mice using OVA-induced allergic model and HDM allergen-induced allergic mouse model respectively. In contrast, IL-21 suppressed experimental allergic rhinitis by down-regulating Th2 cytokines and preventing nasal fibroblasts from producing eotaxins [20]. To validate the effect of IL-21 as a positive control in our study, we also used an allergic rhinitis model treated with IL-21 before challenge. We found decreased eosinophilic infiltration of the nasal mucosa. These results illustrate that IL-21 levels were lower in the asthma group compared with the normal group, but the extent of inflammation was not reduced by administration of IL-21, suggesting that the function of IL-21 in the pathogenesis of various diseases may be different.
The function of IL-21 on the immune system is complex. IL-21 regulates the activation, proliferation, and survival of both CD4+ T cells and B cells, the functional activity of CD8+ T cells and NK cells, limits the differentiation of inducible regulatory T cells, and counteracts their suppressive properties on effector T cells [26,27]. IL-21 can also negatively regulate the maturation and function of dendritic cells [28,29]. As IgE may play a role in allergic disorders, many studies have focused on the influence of IL-21 on IgE synthesis. However, these reports also remain inconsistent. Studies by Suto et al. indicated that IL-21 down-regulated IL-4-dependent IgE production from B cells but did not affect Th2 cell differentiation [30]. Caven et al. found that IL-21 inhibited IgE production at high cell concentrations, but only a modest enhancement when using low cell input in vitro culture systems [31]. IL-21 has been reported to have pleiotropic effects on B cells in terms of cell survival, proliferation, and differentiation in a context-dependent fashion [19]. Studies also revealed that IL-21 induced B-cell apoptosis when combined with prostaglandin E2, while IL-21 alone supported the viability of cultured mouse B cells [32]. In this study, we found that IL-21 had no effect on IgE production in vivo. This may because of the differential effects of IL-21 on B cells under different inflammatory conditions.
In conclusion, this study showed that although IL-21 levels were decreased in asthmatic mice, exogenous administration of IL-21 may not alleviate allergic lung inflammation. Further research may explore the function of IL-21 on various cell types in asthmatic inflammation and its association with other cytokines.
Acknowledgements
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript. This work was supported by the National Natural Science Foundation of China (No. 81170021 and No. 30900647).
Disclosure of conflict of interest
None.
References
- 1.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med. 1998;187:939–948. doi: 10.1084/jem.187.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohn L, Homer RJ, MacLeod H, Mohrs M, Brombacher F, Bottomly K. Th2-Induced Airway Mucus Production Is Dependent on IL-4Rα, But Not on Eosinophils. J Immunol. 1999;162:6178–6183. [PubMed] [Google Scholar]
- 5.Mojtabavi N, Dekan G, Stingl G, Epstein MM. Long-Lived Th2 Memory in experimental allergic asthma. J Immunol. 2002;169:4788–4796. doi: 10.4049/jimmunol.169.9.4788. [DOI] [PubMed] [Google Scholar]
- 6.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Voo KS, Liu B, Chen C, Uygungil B, Spoede W, Bernstein JA, Huston DP, Liu Y. A novel subset of CD4+ TH2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207:2479–2491. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, Boulet L, Hamid Q. Airway remodeling-associated mediators in moderate to severe asthma: Effect of steroids on TGF-β, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immun. 2003;111:1293–1298. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 9.Parrish-Novak J, Foster DC, Holly RD, Clegg CH. Interleukin-21 and the IL-21 receptor: novel effectors of NK and T cell responses. J Leukocyte Biol. 2002;72:856–863. [PubMed] [Google Scholar]
- 10.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 11.Jin H, Carrio R, Yu A, Malek TR. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or bim-dependent apoptosis. J Immunol. 2004;173:657–665. doi: 10.4049/jimmunol.173.1.657. [DOI] [PubMed] [Google Scholar]
- 12.Ozaki K, Spolski R, Ettinger R, Kim H, Wang G, Qi C, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC, Morse HC, Lipsky PE, Leonard WJ. Regulation of B Cell Differentiation and Plasma Cell Generation by IL-21, a Novel Inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 13.Liu R, Wu Q, Su D, Che N, Chen H, Geng L, Chen J, Chen W, Li X, Sun L. A regulatory effect of IL-21 on T follicular helper-like cell and B cell in rheumatoid arthritis. Arthritis Res Ther. 2012;14:R255. doi: 10.1186/ar4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raveney BJE, Oki S, Yamamura T. Nuclear receptor NR4A2 orchestrates Th17 cell-mediated autoimmune inflammation via IL-21 signalling. PLoS One. 2013;8:e56595. doi: 10.1371/journal.pone.0056595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terrier B, Geri G, Chaara W, Allenbach Y, Rosenzwajg M, Costedoat-Chalumeau N, Fouret P, Musset L, Benveniste O, Six A, Klatzmann D, Saadoun D, Cacoub P. Interleukin-21 modulates Th1 and Th17 responses in giant cell arteritis. Arthritis Rheum. 2012;64:2001–2011. doi: 10.1002/art.34327. [DOI] [PubMed] [Google Scholar]
- 16.Petrella TM, Tozer R, Belanger K, Savage KJ, Wong R, Smylie M, Kamel-Reid S, Tron V, Chen BE, Hunder NN, Hagerman L, Walsh W, Eisenhauer EA. Interleukin-21 has activity in patients with metastatic melanoma: a phase II study. J. Clin. Oncol. 2012;30:3396–3401. doi: 10.1200/JCO.2011.40.0655. [DOI] [PubMed] [Google Scholar]
- 17.Souza AP, Bonorino C, Muraro SP, Rodrigues LC Jr. Interleukin-21 expanded NKDC in vitro reduces the B16F10 tumor growth in vivo. Cytokine. 2013;61:154–160. doi: 10.1016/j.cyto.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Santegoets S, Turksma A, Suhoski M, Stam A, Albelda S, Hooijberg E, Scheper R, van den Eertwegh A, Gerritsen W, Powell D, June C, de Gruijl T. IL-21 promotes the expansion of CD27+CD28+ tumor infiltrating lymphocytes with high cytotoxic potential and low collateral expansion of regulatory T cells. J Transl Med. 2013;11:37. doi: 10.1186/1479-5876-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konforte D, Simard N, Paige CJ. IL-21: an executor of B cell fate. J Immunol. 2009;182:1781–1787. doi: 10.4049/jimmunol.0803009. [DOI] [PubMed] [Google Scholar]
- 20.Hiromura Y, Kishida T, Nakano H, Hama T, Imanishi J, Hisa Y, Mazda O. IL-21 administration into the nostril alleviates murine allergic rhinitis. J Immunol. 2007;179:7157–7165. doi: 10.4049/jimmunol.179.10.7157. [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee R, Batra J, Ghosh B. A common exonic variant of interleukin21 confers susceptibility to atopic asthma. Int Arch Allergy Immunol. 2009;148:137–146. doi: 10.1159/000155744. [DOI] [PubMed] [Google Scholar]
- 22.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin H, Oyoshi MK, Le Y, Bianchi T, Koduru S, Mathias CB, Kumar L, Le Bras S, Young D, Collins M, Grusby MJ, Wenzel J, Bieber T, Boes M, Silberstein LE, Oettgen HC, Geha RS. IL-21R is essential for epicutaneous sensitization and allergic skin inflammation in humans and mice. J Clin Invest. 2009;119:47–60. doi: 10.1172/JCI32310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frohlich A, Marsland BJ, Sonderegger I, Kurrer M, Hodge MR, Harris NL, Kopf M. IL-21 receptor signaling is integral to the development of Th2 effector responses in vivo. Blood. 2007;109:2023–2031. doi: 10.1182/blood-2006-05-021600. [DOI] [PubMed] [Google Scholar]
- 25.Lajoie S, Lewkowich I, Herman NS, Sproles A, Pesce JT, Wynn TA, Grusby MJ, Hamid Q, Wills-Karp M. IL-21 receptor signalling partially mediates Th2-mediated allergic airway responses. Clin Exp Allergy. 2014;44:976–985. doi: 10.1111/cea.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 27.Vogelzang A, McGuire HM, Liu SM, Gloss B, Mercado K, Earls P, Dinger ME, Batten M, Sprent J, King C. IL-21 contributes to fatal inflammatory disease in the absence of Foxp3+ T regulatory cells. J Immunol. 2014;192:1404–1414. doi: 10.4049/jimmunol.1302285. [DOI] [PubMed] [Google Scholar]
- 28.Monteleone G, Pallone F, MacDonald TT. Interleukin-21: a critical regulator of the balance between effector and regulatory T-cell responses. Trends Immunol. 2008;29:290–294. doi: 10.1016/j.it.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Spolski R, Leonard WJ. The Yin and Yang of interleukin-21 in allergy, autoimmunity and cancer. Curr Opin Immunol. 2008;20:295–301. doi: 10.1016/j.coi.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suto A, Nakajima H, Hirose K, Suzuki K, Kagami S, Seto Y, Hoshimoto A, Saito Y, Foster DC, Iwamoto I. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line Cepsilon transcription of IL-4-stimulated B cells. Blood. 2002;100:4565–4573. doi: 10.1182/blood-2002-04-1115. [DOI] [PubMed] [Google Scholar]
- 31.Caven TH, Shelburne A, Sato J, Chan-Li Y, Becker S, Conrad DH. IL-21 dependent IgE production in human and mouse in vitro culture systems is cell density and cell division dependent and is augmented by IL-10. Cell Immunol. 2005;238:123–134. doi: 10.1016/j.cellimm.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Magari M, Nishikawa Y, Fujii Y, Nishio Y, Watanabe K, Fujiwara M, Kanayama N, Ohmori H. IL-21-dependent B Cell Death Driven by Prostaglandin E2, a Product Secreted from Follicular Dendritic Cells. J Immunol. 2011;187:4210–4218. doi: 10.4049/jimmunol.1100934. [DOI] [PubMed] [Google Scholar]


