Abstract
This systematic review and meta-analysis was performed to determine accuracy and usefulness of adenosine deaminase (ADA) in diagnosis of tuberculosis pleurisy. Medline, Google scholar and Web of Science databases were searched to identify related studies until 2014. Two reviewers independently assessed quality of studies included according to standard Quality Assessment of Diagnosis Accuracy Studies (QUADAS) criteria. The sensitivity, specificity, diagnostic odds ratio and other parameters of ADA in diagnosis of tuberculosis pleurisy were analyzed with Meta-DiSC1.4 software, and pooled using the random effects model. Twelve studies including 865 tuberculosis pleurisy patients and 1379 non-tuberculosis pleurisy subjects were identified from 110 studies for this meta-analysis. The sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR) and diagnosis odds ratio (DOR) of ADA in the diagnosis of tuberculosis pleurisy were 45.25 (95% CI 27.63-74.08), 0.86 (95% CI 0.84-0.88), 0.88 (95% CI 0.86-0.90), 6.32 (95% CI 4.83-8.26) and 0.15 (95% 0.11-0.22), respectively. The area under the summary receiver operating characteristic curve (SROC) was 0.9340. Our results demonstrate that the sensitivity and specificity of ADA are high in the diagnosis of tuberculosis pleurisy especially when ADA≥50 (U/L). Thus, ADA is a relatively sensitive and specific marker for tuberculosis pleurisy diagnosis. However, it is cautious to apply these results due to the heterogeneity in study design of these studies. Further studies are required to confirm the optimal cut-off value of ADA.
Keywords: Tuberculosis pleural effusion, ADA, diagnostic test, meta analysis
Introduction
Tuberculosis is one of the most common infectious bacterial diseases and has threatened the human health worldwide [1]. Tuberculosis has high morbidity and mortality around of the world, and caused estimated 1.4 million deaths in 2011 [2]. Tuberculosis can be classified as intrapulmonary, extra-pulmonary and disseminated tuberculosis. Tuberculosis pleurisy (TP) is a common manifestation of extra-pulmonary tuberculosis (EPTB) [3]. The pleural tissue biopsy and pleural fluid examination are two major methods for the diagnosis of tuberculosis pleurisy effusion (TPE). However, mycobacterium culture of the pleural fluid has a relative lower success rate (36%) [4], and thus its role in the diagnosis of TP is still controversial. Due to the non-specific clinical manifestations and negative laboratory findings, it is difficult to distinguish TPE from malignant pleural effusion (MPE), both of which are the most common types of pleural effusion [5]. Moreover, there are still conflicting findings on the sensitivity and specificity of biopsy and mycobacterium culture of pleural fluid in the diagnosis of TP. Consequently, it is imperative to develop a reliable molecular marker that can be used to rapidly and accurately diagnose TPE and MPE [6].
Adenosine deaminase (ADA), interferon-γ, C-reactive protein (CRP), carcinoembryonic antigen, interleukin-6 (IL-6), lactate dehydrogenase (LDH), tumor necrosis factor-α (TNF-α) and vascular endothelial growth factor (VEGF) are markers used in the diagnosis of TPE [7]. The ADA activity in TPE is one of the most common biomarkers used for the diagnosis and treatment decision of tuberculosis, having a high sensitivity [8]. The role of ADA in the diagnosis of TPE has been evaluated, and results showed it is helpful to distinguish TPE from MPE [9]. Numerous studies have been conducted to improve our understanding of the diagnostic value of ADA in TPE. Two meta-analyses have displayed that ADA has a favorable diagnostic value in [10,11].
The diagnostic accuracy of ADA in TPE has been extensively studied. However, the optimal cut-off value of ADA is still to be elucidated. In the present study, we systematically assessed and analyzed the overall efficiency and accuracy of ADA in the diagnosis of TPE through meta-analysis, and distinguished factors related to the heterogeneity of results among studies. This study aimed to perform as systemic review to evaluate the diagnostic value of ADA as compared to the gold standard. The sensitivity, specificity, positive likelihood ratio (+LR), negative likelihood ratio (-LR) and the area of SROC were used for the evaluation.
Materials and methods
Search strategy and study selection
We systematically hand-searched three database: Medline, Google scholar and Web of science using the following key words (“tuberculosis pleurisy” or “tuberculosis pleural effusion”) and (“adenosine deaminase” or “ADA”) from 1990-2014.
Although there was no language restriction in the initial searching of studies, only English articles were obtained for reviewing and final analysis due to limitations in the resources. Conferences, letters to editor, case reports and reviews were not included because of incomplete original data. Studies having involvement of the accuracy of ADA in diagnosing TP were included. These studies had complete information, such as sensitivity, specificity, and numbers of TB and NTB. Then, true positive (TP), true negative (TN), false positive (FP) and false negative (FN) were calculated.
Inclusion and exclusion criteria
Two reviewers independently reviewed and evaluated all the studies. Disagreement was resolved following a discussion between them. Studies were included if they met following criteria: 1) The diagnosis of TP and non-TP was confirmed by histological and pathological examinations. 2) Information about the samples, sensitivity, specificity (95% confidence intervals [CI]) and number of patients was complete. 3) They were original articles. 4) They were published in English. Exclusion criteria: 1) It was no a case-control study. 2) They were case reports, letters to editor, reviews and Meta-analyses. 3) There were English in language. 4) Information was incomplete. Figure 1 shows the processes for the inclusion of studies using above criteria.
Figure 1.

Processes for study selection.
Data extraction
Two investigators independently extracted following information from the included studies: the name of the first author, the year of publication, the country of origin, the proportion of men and women, the number of patients and controls, gender, age, assay methods, sensitivity and specificity data, cut-off values, study design, and sample detection (Tables 1 and 2). All the data were collected from the published studies.
Table 1.
Characteristics of included studies for meta-analysis
| NO | Author | Country | Year | Method | Blind | Consecutive | QUADAS |
|---|---|---|---|---|---|---|---|
| 1 | Denise Duprat Neves | Brazil | 2006 | Diagnostic test | YES | Yes | 13 |
| 2 | Yoshiko Ogata | Japan | 2011 | Diagnostic test | NO | Yes | 11 |
| 3 | Lesley J.Burgess | Tygerberg | 1995 | Diagnostic test | YES | Yes | 14 |
| 4 | Khalid Hassanein | Egypt | 2010 | Diagnostic test | YES | Yes | 11 |
| 5 | Pınar Birsen Yıldız | Turkey | 2011 | Diagnostic test | YES | Yes | 11 |
| 6 | Hongxiu Wang | China | 2011 | Diagnostic test | YES | Yes | 12 |
| 7 | Nariman A. Helmy | Egypt | 2012 | Diagnostic test | Not clear | Yes | 13 |
| 8 | Alberto Garcia-Zamalloa | Spain | 2012 | Diagnostic test | NO | Yes | 12 |
| 9 | Mo-Lung Chen | China | 2003 | Diagnostic test | NO | NO | 12 |
| 10 | Fahmi Yousef Khan | Doha-Qatar | 2013 | Diagnostic test | YES | Yes | 13 |
| 11 | Yung-Ching Liu | China | 2011 | Diagnostic test | NO | Yes | 12 |
| 12 | Li-Ta Keng | China | 2013 | Diagnostic test | YES | Yes | 14 |
Table 2.
Characteristics of included studies for meta-analysis
| NO | First author | Age | Men % | Assay method | Source | Reference standard |
|---|---|---|---|---|---|---|
| 1 | Denise Duprat Neves | 33.8 | 73 | Giusti method | pleural fluid | Radiological and Histopathological |
| 2 | Yoshiko Ogata | 69 | 76.6 | auto analyzer | pleural fluid | Histological |
| 3 | Lesley J. Burgess | 49 | 58 | Giusti method | pleural fluid | Radiological and Microbiology |
| 4 | Khalid Hassanein | 35 | 76 | Giusti method | serum and BALF | Radiological and laboratory |
| 5 | Pınar Birsen Yıldız | 45 | 73.5 | Giusti method | pleural fluid | histopathology |
| 6 | Hongxiu Wang | 44.1 | 78.2 | Giusti method | pleural fluid | histopathology |
| 7 | Nariman A. Helmy | 29.2 | 45 | auto analyzer | pleural fluid | histopathology |
| 8 | Alberto Garcia-Zamalloa | 66.2 | 62.3 | automated ultraviolet kinetic assay | pleural fluid | Radiological and Microbiology |
| 9 | Mo-Lung Chen | 57.7 | 68 | automated ultraviolet kinetic assay | pleural fluid | histopathology or cytopathol ogy |
| 10 | Fahmi Yousef Khan | 38.9 | 84.5 | automated ultraviolet kinetic assay | pleural fluid | Thoracocentesis and histopathology |
| 11 | Yung-Ching Liu | 61 | 75 | Giusti method | pleural fluid | histopathology |
| 12 | Li-Ta Keng | 63.9 | 74 | Giusti method | pleural fluid | Radiological |
Note: BALF: bronchoalveolar lavage fluid.
Assessment of quality of included studies
Two reviewers independently assessed the quality of included studies by using the QUADAS (Quality Assessment of Diagnosis Accuracy Studies) (UK and Netherland) criteria [12], which were developed as a validated instrument for diagnostic studies. All criteria were classified as “YES”, “NO” or “Not clear” based on available information in the included studies. Furthermore, following information was also obtained: (1) consecutive or random samples of patients; (2) blind design (single or double). Disagreement was resolved by discussion between two investigators or the third-party adjudication.
Data synthesis and statistics analysis
The sensitivity, specificity, and number of TB patients and NTB patients were obtained from the retrieved articles, and the TP, FP, TN and FN were calculated according to the following formula: sensitive = TP/TP+FN, specificity = TN/FP+TN, TB+NTB = TP+FP+TN+FN, reach a 2*2 tables (Table 3). The pooled sensitivity (true positive rate, TPR), specificity (true negative rate, TNR or 1-false positive rate, FPR), positive likelihood ratio (LR+), negative likelihood ratio (LR-) and diagnostic odds ratio (DOR) of ADA in diagnosing TP were calculated using the Meta-Disc1.4 software (XI Cochrane Colloquium, Barcelona, Spain). These parameters were pooled using the random effect model [13].
Table 3.
Summary of included studies
| NO | Author | TB/NTB | ADA(U/L) | TP | FP | TN | FN | Se | Sp |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Denise Duprat Neves | 104/111 | 39 | 99 | 19 | 92 | 5 | 95.2 | 82.9 |
| 2 | Yoshiko Ogata | 124/311 | 36 | 106 | 42 | 269 | 42 | 85.5 | 86.5 |
| 3 | Lesley J. Burgess | 143/104 | 50 | 130 | 20 | 84 | 13 | 91 | 81 |
| 4 | Khalid Hassanein | 20/30 | 26.2 | 19 | 5 | 25 | 1 | 95 | 83.3 |
| 5 | Pınar Birsen Yıldız | 114/82 | 55 | 99 | 11 | 71 | 15 | 86.6 | 86.6 |
| 6 | Hongxiu Wang | 78/44 | 40 | 73 | 4 | 40 | 5 | 93.6 | 90.9 |
| 7 | Nariman A. Helmy | 19/21 | 30 | 16 | 6 | 15 | 3 | 84.2 | 71.4 |
| 8 | Alberto Garcia-Zamalloa | 73/399 | 40 | 65 | 29 | 370 | 8 | 89 | 92.7 |
| 9 | Mo-Lung Chen | 63/147 | 55.8 | 55 | 12 | 135 | 8 | 87.3 | 91.8 |
| 10 | Fahmi Yousef Khan | 72/31 | 16.65 | 62 | 8 | 23 | 10 | 86 | 74 |
| 11 | Yung-Ching Liu | 24/42 | 30 | 17 | 2 | 40 | 7 | 70.8 | 95.2 |
| 12 | Li-Ta Keng | 31/57 | 15.5 | 26 | 7 | 50 | 5 | 83.9 | 87.7 |
Data were analyzed using Meta-Disc 1.4 software. Forest plots were used to determine the pooled sensitivity, specificity, DOR, LR+ and LR- and the corresponding 95% CI. A summary receiver operating characteristic curve (SROC) [14] was delineated and the area of SROC was calculated to evaluate the diagnostic accuracy of ADA. The heterogeneity among these studies was assessed using the chi square test.
The random-effect model was used for meta-analysis. Moreover, analysis of diagnostic threshold effects was quantified by the Spearman correlation coefficient and suggested the absence of heterogeneity caused by threshold effect. The non-threshold effect was evaluated by the Cochran-Q method and the test of inconsistency index (I2). A low P value (<0.005) and a high I2 (>50%) suggested the presence of heterogeneity caused by the non-threshold effect. Stratified analyses were used to evaluate study design, golden standard and test-related factors responsible for heterogeneity caused by non-threshold effects [15].
Results
Detailed information of the included literature and quality assessment
Figure 1 displays the processes in the selection of eligible studies. A total of 110 studies were identified from the Medline, Google scholar and Web of Science database, 42 studies were excluded due to repeated publication, and 15 studies were excluded because they were reviews, evaluation studies, retrospective studies or published in other languages. The abstract and full texts of remained studies were screened, and 51 studies were excluded due to incomplete original information. Thus, 12 potentially eligible studies were included for meta-analysis. Included studies had information about the sensitivity, specificity, number of TP and non-TP patients, which were extracted for the calculation of TP, FP, TN and FN. Of 12 studies, there were 865 TP patients and 1379 non-TP patients. The quality of included studies was evaluated using the QUADAS criteria [12] and they were graded from 1 to 14 as shown in Table 1.
Determination of diagnostic accuracy
The sensitivity, specificity, LR+, LR- and DOR of ADA in the diagnosis of TP are presented in the forest plot (Figures 2, 3 and 4). The overall diagnostic sensitivity (SEN) and specificity (SPE) were 0.86 (95% CI 0.84-0.88) and 0.88 (95% CI 0.86-0.90), respectively. The LR+, LR- and DOR were 6.32 (95% CI 4.83-8.26), 0.15 (95% 0.11-0.22) and 45.25 (95% CI 27.63-74.08), respectively. Almost all the studies showed favorable sensitivity and specificity. Chi square test showed the chi square value of the sensitivity, specificity, PLR, NLR and DOR was 43.99 (P = 0.0000), 31.80 (P = 0.0008), 31.77 (P = 0.0008), 43.65 (P = 0.0000) and 31.31 (P = 0.0010) respectively, suggesting a substantially high heterogeneity for sensitivity, specificity, PLR, NLR and DOR among included studies. The SROC plot can be used to evaluate the effects of different thresholds on the sensitivity and specificity in a study. SROC curve can display the cutoff value between sensitivity and specificity. Our results showed that the AUC was 0.934, suggesting a high accuracy of ADA in the diagnosis of TP.
Figure 2.

Forest plot for estimation of sensitivity and specificity of ADA in the diagnosis of tuberculosis pleurisy. Point estimates from all studies are displayed as solid circles and show sensitivity and specificity of each study. Error bars: 95% CI. Pooled estimates for ADA are as follows: A. Sensitivity, 0.86 (95% CI 0.84-0.88). B. Specificity, 0.88 (95% CI 0.86-0.90).
Figure 3.

Forest plot for estimation of positive likelihood ratio and negative likelihood ratio of ADA in the diagnosis of tuberculosis pleurisy. Pooled estimates for ADA are as follows: A. Positive likelihood ratio (PLR), 6.32 (95% CI 4.83-8.26). B. Negative likelihood ratio (NLR), 0.15 (95% CI 0.11-0.22
Figure 4.
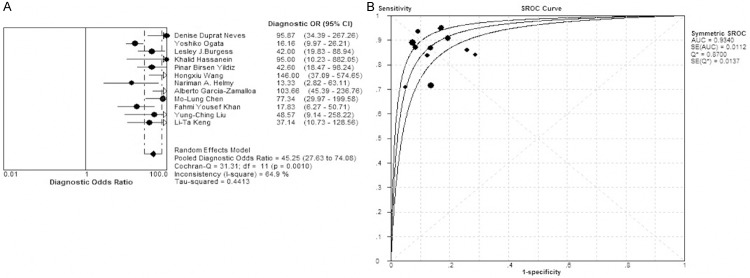
Forest plot for the diagnosis odds ratio (DOR) and Summary receiver operating characteristic curves (SROC). A. DOR: 45.25 (95% CI 27.63-74.08). B. AUC: 0.9340.
).
Heterogeneity and threshold effect
In the diagnosis test, included studies using different diagnostic cut-off values may cause heterogeneity. Therefore, it is important to explore the heterogeneity before data pooling. When there is a threshold effect, the sensitivity and specificity may correlate with each other negatively (sensitivity and 1-specificity correlate with each other positively) and the SROC is shoulder-shaped. The threshold effect was evaluated with the SROC, sensitivity and specificity, and results showed that SROC was not shoulder–shaped (Figure 4) and Spearman correlation coefficient was 0.175 (P = 0.587>0.05). Above findings proved that there was no threshold effect among included studies.
Non-threshold effect
In the diagnosis meta-analysis, the heterogeneity among included studies is due to the threshold effect and the non-threshold effect. The non-threshold effect contains the population (the severity of disease and sex); experiment test (such as different technology, operator, reagent and instrument); reference standard, etc. Therefore, the Cochran-Q of DOR is usually used to detect if there is heterogeneity due to non-threshold effect in diagnosis methods.
The heterogeneity due to non-threshold effect was evaluated with sensitivity, specificity, and DOR. Results showed the chi square value of sensitivity, specificity and DOR was 43.99 (P-0.0000), 31.8 (P = 0.0008), and 31.31 (P = 0.001). It indicates the heterogeneity due to non-threshold effect.
Meta-regression and subgroup analysis
Because of the non-threshold effect due to heterogeneity among included studies, the random effect model was used to assess the overall accuracy of ADA in the diagnosis TP. To investigate the reasons for heterogeneity, meta-regression was performed. According to materials provided by the literature, blind method (blind design: 0, not blind design: 1, not clear: 2), test method (Giusti method: 0, automated ultraviolet kinetic assay: 1), source of samples (pleural fluid: 0, other sources: 1), gold standard (pathological diagnosis is 0, others are 1) and consecutiveness (YES: 0, NO: 1)were set for ADA assay. Nine factors were included in meta-analysis according to P value from big to small to remove above factors gradually (QUADAS, consecutive, age, source, blind, assay, men and standard). Results showed that the source of heterogeneity in included studies was correlated with ADA. Due to the heterogeneity in the included studies, a subgroup analysis was performed. On the basis of results from meta-regression analysis, 12 studies were divided into two subgroups according to the ADA level. When the ADA was higher than 50 U/L (n = 3), analysis of diagnosis threshold showed the spearman correlation coefficient of sensitivity and 1-specificity was 0.5 (P = 0.667>0.05), indicating no threshold effect. The non-threshold effect was also evaluated. Results showed the absence of non-threshold effect (Figures 5 and 6). Therefore, the homogeneity is favorable in subgroups (ADA≥50 U/L).
Figure 5.

Forest plots for subgroup analysis of sensitivity and specificity. Subgroup analysis reveals studies (ADA≥50 U/L) have a good homogeneity. A. The pooled sensitivity of subgroup (ADA≥50 U/L) is 0.89 (95% CI 0.85-0.92). B. The pooled specificity of subgroup (ADA≥50 U/L) is 0.87 (95% CI 0.83-0.90).
Figure 6.

Forest plot for the diagnosis odds ratio (DOR) and Summary receiver operating characteristic curves (SROC) in a subgroup (ADA≥50 U/L). A. DOR: 49.38 (95% CI 30.53-79.89). B. AUC: 0.9421. SROC of ADA shows the diagnostic performance in a subgroup (ADA≥50 U/L).
Discussion
TP is an ordinary extra-pulmonary formation of tuberculosis all over the world and also the most common manifestation of tuberculosis [16]. Mycobacterium affects approximately 30% of the world’s populations and causes about 1.7 million deaths every year. Although, there are several methods used to diagnose the tuberculosis, such as tuberculin skin test, interferon-γ release assay and imaging method, but they are non-mandatory in clinical practice. In addition, there is still difficulty in the diagnosis of TP. Thus, it is imperative to develop a new method used to conveniently and effectively diagnose TP [17]. At present, the methods used to diagnose TP mainly include pleural biopsy, X-ray and ultrasonography. In addition, other assistant examinations may be done if necessary, such as diagnostic pleural puncture, routine thoracic fluid inspection, biochemical examination and bacterial culture.
Moreover, it is important and useful to identify some biochemical markers for the diagnosis of TP. There are some target biomarkers (such as interferon-γ, C-reactive protein (CRP), LDH, ADA, carcinoembryonic antigen, IL-6, TNF-α and VEGF) used in the diagnosis of TPE. ADA is a classical and highly sensitive biomarker for the diagnosis of TP, and can be used to distinguish TPE from non-TPE. In recent years, numerous studies [18] have shown that ADA provides a favorable diagnostic value in TP [17,18]. In the present study, we searched three databases and a total of 110 studies were identified. Finally, 12 studies were included for Meta-analysis of the diagnostic value of ADA in TP.
In this systematic review, the included 12 studies demonstrated that ADA plays an important role in the diagnosis of TP and the quality of these 12 articles was high. However, there washeterogeneity among these studies. Thus, Moses-Shapiro-Littenber model was used for statistical analysis. Results showed that non-threshold effect caused the heterogeneity among these included studies. Subgroup analysis revealed that the cut-off value of ADA was a source of non-threshold effect. Homogeneity was relatively favorable when ADA was ≥50 U/L. The value of ADA is quite significant in diagnosing tuberculosis pleurisy especially when ADA≥50 U/L. In conclusion, ADA can serve as a biomarker for the diagnosis of TP, especially when ADA is higher than 50 U/L.
The present meta-analysis had several limitations. First, studies were excluded using following criteria: insufficient original data, non-English language, incomplete patient number and unlisted test method, and so on, which may cause potential selection bias and affect the evaluation of diagnostic accuracy. Second, significant heterogeneity was observed in the included studies. The heterogeneity may influence the systemic evaluation. Third, there might be misclassification bias, and the qualities of included studies had inconformity. In addition, only 12 studies met the inclusion criteria and used for meta-analysis. It also limits the expansion of our findings.
In conclusion, the present meta-analysis demonstrates that ADA is a promising marker for the diagnosis of TP (especially when ADA is ≥50 U/L) with high sensitivity and specificity. This may be useful in clinical findings and traditional measurements including microbiological examination and pleural biopsy.
However, due to the limitations of our study, more studies with large sample size are required to confirm our findings.
Disclosure of conflict of interest
None.
References
- 1.Dabernat H, Theves C, Bouakaze C, Nikolaeva D, Keyser C, Mokrousov I, Geraut A, Duchesne S, Gerard P, Alexeev AN, Crubezy E, Ludes B. Tuberculosis epidemiology and selection in an autochthonous Siberian population from the 16th-19th century. PLoS One. 2014;9:e89877. doi: 10.1371/journal.pone.0089877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller R, Roberts CA, Brown TA. Genotyping of ancient Mycobacterium tuberculosis strains reveals historic genetic diversity. Proc Biol Sci. 2014;281:20133236. doi: 10.1098/rspb.2013.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su SB, Qin SY, Guo XY, Luo W, Jiang HX. Assessment by meta-analysis of interferon-gamma for the diagnosis of tuberculous peritonitis. World J Gastroenterol. 2013;19:1645–1651. doi: 10.3748/wjg.v19.i10.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tay TR, Tee A. Factors affecting pleural fluid adenosine deaminase level and the implication on the diagnosis of tuberculous pleural effusion: a retrospective cohort study. BMC Infect Dis. 2013;13:546. doi: 10.1186/1471-2334-13-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu YB, Ye ZJ, Qin SM, Wu C, Chen YQ, Shi HZ. Combined detections of interleukin 27, interferon-gamma, and adenosine deaminase in pleural effusion for diagnosis of tuberculous pleurisy. Chin Med J (Engl) 2013;126:3215–3221. [PubMed] [Google Scholar]
- 6.Ogata Y, Aoe K, Hiraki A, Murakami K, Kishino D, Chikamori K, Maeda T, Ueoka H, Kiura K, Tanimoto M. Is adenosine deaminase in pleural fluid a useful marker for differentiating tuberculosis from lung cancer or mesothelioma in Japan, a country with intermediate incidence of tuberculosis? Acta Med Okayama. 2011;65:259–263. doi: 10.18926/AMO/46851. [DOI] [PubMed] [Google Scholar]
- 7.Daniil ZD, Zintzaras E, Kiropoulos T, Papaioannou AI, Koutsokera A, Kastanis A, Gourgoulianis KI. Discrimination of exudative pleural effusions based on multiple biological parameters. Eur Respir J. 2007;30:957–964. doi: 10.1183/09031936.00126306. [DOI] [PubMed] [Google Scholar]
- 8.Shen Y, Yang T, Jia L, Wang T, Chen L, Wan C, Wang L, Yan Y, Yi Q. A potential role for D-dimer in the diagnosis of tuberculous pleural effusion. Eur Rev Med Pharmacol Sci. 2013;17:201–205. [PubMed] [Google Scholar]
- 9.Chung JH, Kim YS, Kim SI, Park K, Park MS, Kim SK, Chang J. The diagnostic value of the adenosine deaminase activity in the pleural fluid of renal transplant patients with tuberculous pleural effusion. Yonsei Med J. 2004;45:661–664. doi: 10.3349/ymj.2004.45.4.661. [DOI] [PubMed] [Google Scholar]
- 10.Liang QL, Shi HZ, Wang K, Qin SM, Qin XJ. Diagnostic accuracy of adenosine deaminase in tuberculous pleurisy: a meta-analysis. Respir Med. 2008;102:744–754. doi: 10.1016/j.rmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Greco S, Girardi E, Masciangelo R, Capoccetta GB, Saltini C. Adenosine deaminase and interferon gamma measurements for the diagnosis of tuberculous pleurisy: a meta-analysis. Int J Tuberc Lung Dis. 2003;7:777–786. [PubMed] [Google Scholar]
- 12.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 14.Chen XZ, Zhang WH, Yang K, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. Quantitative comparisons of summary receiver operating characteristics (sROC) curves among conventional serological tumor biomarkers for predicting gastric cancer in Chinese population. Tumour Biol. 2014;35:9015–22. doi: 10.1007/s13277-014-1986-x. [DOI] [PubMed] [Google Scholar]
- 15.Little MP. Do non-targeted effects increase or decrease low dose risk in relation to the linear-non-threshold (LNT) model? Mutat Res. 2010;687:17–27. doi: 10.1016/j.mrfmmm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang JY, Rhee CK, Kang NH, Kim JS, Yoon HK, Song JS. Clinical Utility of Two Interferon-gamma Release Assays on Pleural Fluid for the Diagnosis of Tuberculous Pleurisy. Tuberc Respir Dis (Seoul) 2012;73:143–150. doi: 10.4046/trd.2012.73.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruan SY, Chuang YC, Wang JY, Lin JW, Chien JY, Huang CT, Kuo YW, Lee LN, Yu CJ. Revisiting tuberculous pleurisy: pleural fluid characteristics and diagnostic yield of mycobacterial culture in an endemic area. Thorax. 2012;67:822–827. doi: 10.1136/thoraxjnl-2011-201363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keng LT, Shu CC, Chen JY, Liang SK, Lin CK, Chang LY, Chang CH, Wang JY, Yu CJ, Lee LN. Evaluating pleural ADA, ADA2, IFN-gamma and IGRA for diagnosing tuberculous pleurisy. J Infect. 2013;67:294–302. doi: 10.1016/j.jinf.2013.05.009. [DOI] [PubMed] [Google Scholar]


