Abstract
Osteonecrosis (ON) of the jaw has previously been linked to the use of biphosphonates; however, new drugs, also shown similar conditions. This article presents a female patient with mandibular ON related to the use of denosumab. The 55-year-old presented with bone exposure with 8 months of evolution after a dental extraction. The patient began subcutaneous injections of 60 mg denosumab four months prior to the extraction and the lesion remained after the procedure. The patient, with 14 months of follow-up, show mandible ON with no favorable evolution. The clinical condition is presented and the literature of ON associated with denosumab is discussed.
Keywords: Denosumab, osteonecrosis, cancer therapy
Introduction
Osteonecrosis is a rare clinical condition characterized by a decreased blood supply with the inhibition of osteoblastogenesis, and an increase in the apoptosis of osteocytes [1].
The first cases of ON were published in 2003, exposing important alterations associated with the use of bisphosphonates [2]; the clinical presentation of ON is related to deficient oral hygiene and more dramatically to the manipulation of oral tissue like dental extractions and minor surgical procedures in the jaw [3].
Denosumab is a monoclonal antibody used in the treatment of osteoporosis, approved by the FDA and the European Medicines Agency in 2010 [4]. Recently it has been linked to some cases of ON; the following case presents mandibular ON associated with the use of denosumab.
Case report
A 55-year-old female patient presented with the initial diagnosis of invasive ductal carcinoma in the left breast. A partial mastectomy and ipsilateral lymph node dissection were performed in May 2012, and then chemotherapy was given in 4 cycles. In November 2012 a vesicle tumor was resected with a diagnosis of papillary urothelial carcinoma and tangential radiation therapy of 2.66 Gy up to a total of 42.56 Gy was delivered daily.
In May 2013 the patient came to the Division of Oral and Maxillofacial Surgery at the Universidad de La Frontera due to the presence of a mandibular bone lesion associated with an extraction of tooth 3.5 (Figure 1) performed in October 2012 without complications, relating an atraumatic procedure (first consultation 8 months after the extraction).
Figure 1.
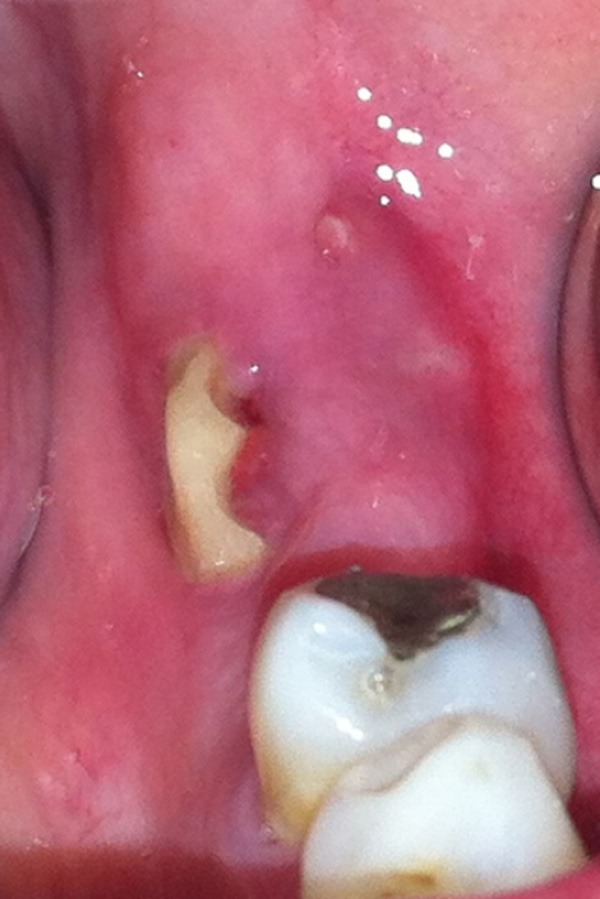
Clinical appearance of the lesion in May 2013, 8 months after the dental extraction.
Suspecting ON, the examination revealed that the patient had been undergoing treatment of subcutaneous injections of 60 mg denosumab from July 2012; thus, the therapy started 4 months prior to the dental extraction. Suspension of the drug was indicated and an ON management protocol was begun with local therapy with 0.12% chlorhexidine daily and follow-up of the case (AAOMS, 2007), where until November 2013 no important changes in the clinical presentation were observed (Figure 2).
Figure 2.
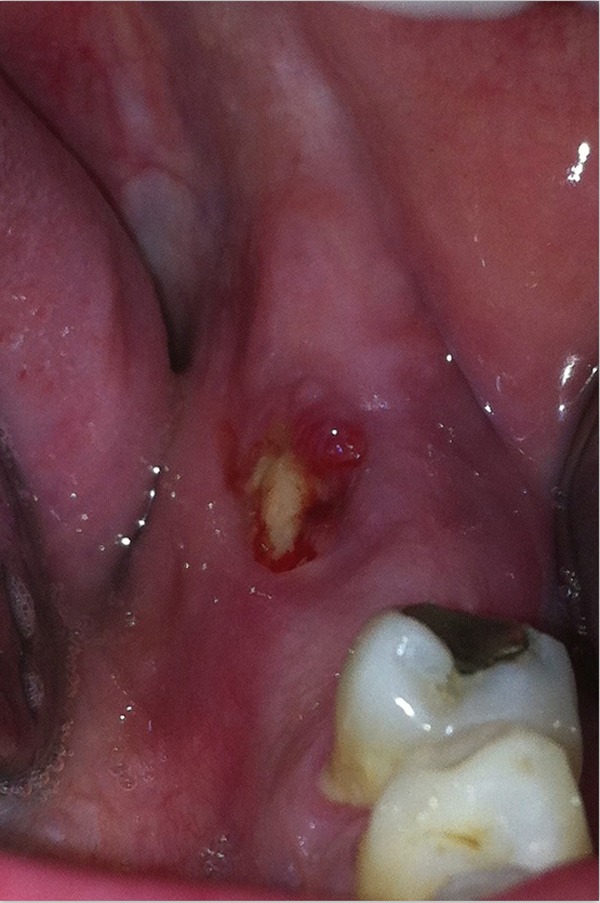
Clinical appearance of the lesion in November 2013, 14 months after the dental extraction.
The patient’s symptoms included localized pain, with no increase in volume or edema, with the functional impotence associated to pain for mandibular manipulation and eating. The case continues to evolve until the best therapeutic protocol for the patient can be determined.
Discussion
Denosumab has become an adequate option for managing such diseases as osteoporosis contributing to the constant increase in bone mineral density [6]. Recently its use in renal pathologies has been studied with promising results [7]. Denosumab is also gaining acceptance in the international market because it carries a much lower economical cost than the use of biphosphonates, which could lead to public health care systems choosing it due to its greater cost-effectiveness and clinical performance [8].
However, potential complications of denosumab have been described with ON. Pichardo [9] reported a case of a 74-year-old patient treated with denosumab after treatment for prostate cancer (patient in a system of phase III clinical trials), presenting intraoral fistula and symptoms associated with ON 7 months after the treatment began. In another report, an extensive group of 2207 female patients treated with denosumab (60 mg subcutaneously every 6 months for 2 years), 2 cases were described with ON in the final results [10].
Another case was reported by Neuprez [11], who presented a man with osteoporosis being treated with 60 mg denosumab administered subcutaneously every 6 months together with daily doses of calcium and vitamin D. Four months after therapy began, the patient had the third molar extracted and 4 months later was evaluated for the presence of oral lesions with bone exposure, manifesting mandibular ON.
Recently was published a meta-analysis on 7 randomized clinical trials of patients treated with denosumab, biphosphonates and placebo [12]; it was observed that in a total of 8963 patients there was an incidence of ON in subjects using denosumab of 1.7%, presenting in addition an increased risk of developing ON compared to biphosphonates, although this increase was not statistically significant. This situation makes it possible to clarify the potential of denosumab in the genesis of ON.
The use of denosumab and its potential complications such as ON must be incorporated into the same protocols applied to the use of biphosphonates: maintaining good oral hygiene, limiting alcohol consumption and ceasing the use of cigarettes [13]. It can be concluded that denosumab is linked to ON in different conditions of oral health, and the best protocol for managing this clinical condition must be determined.
Disclosure of conflict of interest
None.
References
- 1.Sambrook PN, Ebeling P. Osteonecrosis of the jaw. Curr Rheumatol Rep. 2008;10:97–101. doi: 10.1007/s11926-008-0018-5. [DOI] [PubMed] [Google Scholar]
- 2.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 3.Jaimes M, Chaves Netto HDM, Olate S, Chaves MMGA, Albergaria-Barbosa JR. Biphosphonate and jaws osteonecrosis. Considerations about of treatment. Int J Morphol. 2008;26:681–688. [Google Scholar]
- 4.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:33–41. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 5.Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on biphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007;65:369–376. doi: 10.1016/j.joms.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Iolascon G, Napolano R, Gioia M, Moretti A, Riccio I, Gimmigliano F. The contribution of cortical and trabecular tissues to bone strenght: insights from denosumab studies. Clin Cases Miner Bone Metab. 2013;10:47–51. doi: 10.11138/ccmbm/2013.10.1.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sulková SD, Horácek J, Safránek R, Gorun P, Viklicky O, Palicka V. Denosumab associated with bone density increase and clinical improvement in a long-term hemodyalisis patient. Case report and review of the literatura. Acta Medica. 2014;57:30–4. doi: 10.14712/18059694.2014.6. [DOI] [PubMed] [Google Scholar]
- 8.Lothgren M, Ribnicsek E, Schmidt L, Habacher W, Lundkvist J, Pfeil A, Biteeva I, Vrouchou P, Bracco A. Cost per patient and potential budget implications of denosumab compared with zoledronic acid in adults with bone metastases from solid tumours who are at risk of skeletal-related events: an analysis for Austria, Sweden and Switzerland. Eur J Hosp Pharm Sci Parct. 2013;20:227–231. doi: 10.1136/ejhpharm-2012-000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pichardo SE, Kuypers SC, van Merkesteyn JP. Denosumab osteonecrosis of the mandible: a newentity? A case report. J Craniomaxillofac Surg. 2013;41:e65–e69. doi: 10.1016/j.jcms.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Papapoulos S, Chapurlat R, Libanati C, Brandi ML, Brown JP, Czerwinski E, Krieg MA, Man Z, Mellström D, Radominski SC, Reginster JY, Resch H, Roman Ivorra JA, Roux C, Vittinghoff E, Austin M, Daizadeh N, Bradley MN, Grauer A, Cummings SR, Bone HG. Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extension. J Bone Miner Res. 2012;27:694–701. doi: 10.1002/jbmr.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neuprez A, Coste S, Rompen E, Crielaard JM, Reginster JY. Osteonecrosis of the jaw in a male osteoporotic patient treated with denosumab. Osteoporos Int. 2014;25:393–395. doi: 10.1007/s00198-013-2437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qui WX, Tang LN, He LN, Yao Y, Shen Z. Risk of osteonecrosis of the jaw in cáncer patients receiving denosumab: a meta-analysis of seven randomized controlled trials. Int J Clin Oncol. 2014;19:403–410. doi: 10.1007/s10147-013-0561-6. [DOI] [PubMed] [Google Scholar]
- 13.Sivolella S, Lumachi F, Stellini E, Favero L. Denosumab and anti-angiogenic drug-related osteonecrosis of the jaw: an uncommon but potentially severe disease. Anticancer Res. 2013;33:1793–1797. [PubMed] [Google Scholar]


