Abstract
Aim: Myeloid-derived suppressor cells (MDSCs) are a population of cells which negatively regulate immune response during tumor progression. In this study, we assessed the accumulation of MDSCs (CD33+CD11b+HLA-DR-CD14-) in patients with prostate cancer and its clinical relevance. Methods: We tested the frequency of MDSCs in the peripheral blood of patients with prostate cancer or benign prostate hyperplasia and healthy donors. Serumal interleukin-8, -6 and -10 were analyzed. Effects of MDSCs on the T cell response were determined. Results: MDSCs increased in cancer patients, and there was an association between MDSCs and cancer stages or overall survival. Elevated serumal interleukin-8 and -6 in cancer patients correlated with MDSCs. Moreover, accumulation of MDSCs was associated with defective T cell function. Conclusion: Our study showed an increased population of MDSCs in patients with prostate cancer. Interleukin-8 and -6 in serum may play a new important role companied with MDSCs in prostate cancer.
Keywords: IL-6, IL-8, MDSCs, prostate cancer
Introduction
Recent research has established that many cancers are characterized by the overproduction of a range of immature myeloid immunosuppressive cells [1,2], and these cells are defined as myeloid-derived suppressor cells (MDSCs). MDSCs represent a heterogeneous population comprised of progenitors and precursors of myeloid cells and its induction is an important immune-evading mechanism used by tumors [3]. In preclinical models, the phenotype of MDSCs consists of co-expression of the myeloid lineage differentiation antigens Gr-1 (Ly6G) and CD11b (CR3, Mac-1), including monocytic (Ly6G-Ly6Chigh) and granulocytic (Ly6G+Ly6Clow) cells [4]. In cancer patients, MDSCs express either or both of the common myeloid markers CD33 or CD11b [5], and are LIN- and/or HLA-DR- [6]. Thus, human MDSCs were initially defined as HLA-DR-CD33+ or CD14-CD11b+ cells [7], with both phenotypes identifying cell populations with T cell suppressive activity. They are also further classified as subset of the CD11b+CD14- polymorphonuclear granulocyte morphology distinct from the mononuclear CD11b+CD14+ monocytes. Previous work also established the presence of granulocytic CD15+ MDSCs in the circulation of human cancers including renal, lung cancer, breast, colon, and pancreatic cancers [8]. Even so, more and more markers have been associated with MDSCs function in recent year.
MDSCs play a pivotal role in cancer progression by suppressing immune response [9]. The increasing frequency and phenotype of circulating MDSCs in peripheral blood have been reported in many types of cancers, both in the preclinical models and human patients [10]. Several reports also have shown increased infiltration of MDSCs in cancers including breast cancer, lung cancer, and multiple myeloma, both in the primary tumor and metastatic sites [11-13]. However, due to their heterogeneity, the frequency, phenotype and suppressive function in patients with cancer are highly debated and it is still not clear whether one subset is predominant over the other, especially in prostate cancer.
In the present study, we investigated the clinical characteristics of circulating MDSCs in patients with prostate cancer. We isolated peripheral blood mononuclear cells (PBMCs) and measured percentges of MDSCs to determine the relationship between MDSCs levels and clinical cancer stages. We detected three subtypes of MDSCs, including granulocytic CD33+CD11b+HLA-DR-CD14-, monocytic CD33+CD11b+HLA-DR-CD14+, and granulocytic CD33+CD11b+HLA-DR-CD15+ MDSCs. Among these phenotypes, the major one is CD33+CD11b+HLA-DR-CD14-, so we defined the MDSCs as CD33+CD11b+ HLA-DR-CD14- in this study and further explore their correlation with T progression, nodal status and metastasis of prostate cancer. Levels of cytokine including Interleukin (IL)-8, -6 and -10 were also determined to explore their relationship with MDSCs.
Patients and methods
Patients and healthy donors
Peripheral blood specimens were collected from 80 patients with prostate cancer (mean age 67.8; range 38-84) with newly diagnosed and histologically confirmed tumors: stages I-II (n=20); stage III (n=29); stage IV (n=31) in accordance with the TNM classification of the American Joint Committee on Cancer Criteria. All the patients were hospitalized at Department of Urology, Inner Mongolia People’s Hospital, from 2009 to 2013. The study was carried out in accordance with the institutional ethical guidelines and the use of human tissues was approved by the Medical Ethics Committee of Inner Mongolia Medical University (IMMP study ID O13-332115). Every patient involved in the study was asked to sign a piece of written informed consent which has been approved by the ethics committee of Inner Mongolia Medical University, and all the consents were saved by the ethics committee. The study was conducted according to the principles expressed in the Declaration of Helsinki. Patient characteristics are detailed in Table 1. Twenty age-matched normal healthy volunteers served as normal controls. Additional twenty patients with benign prostatic hyperplasia (BPH) were included. Clinical follow-up data were available for 62 patients, while 18 patients were excluded for lack of information.
Table 1.
Clinicopathological parameters of patients
| Characteristic | N (%) |
|---|---|
| All cases | 80 (100) |
| Age | |
| ≤70 y | 35 (43.8) |
| >70 y | 45 (56.2) |
| Preoperative PSA | |
| <4 ng/mL | 33 (41.3) |
| 4-10 ng/mL | 25 (31.2) |
| >10 ng/mL | 22 (27.5) |
| Gleason score | |
| 4-6 | 32 (40.0) |
| 7 | 27 (33.8) |
| 8-10 | 21 (26.2) |
| TNM classification | |
| I-II | 20 (25.0) |
| III | 29 (36.3) |
| IV | 31 (38.8) |
| Depth of invasion | |
| T1 | 10 (12.5) |
| T2 | 12 (15.0) |
| T3 | 27 (33.8) |
| T4 | 31 (38.8) |
| Nodal status | |
| N0 | 30 (37.5) |
| N1 | 50 (62.5) |
| Distant metastasis | |
| M0 | 43 (53.8) |
| M1 | 37 (46.2) |
PBMCs isolation and flow cytometry analysis
Five milliliters of venous blood was collected into EDTA-coated evacuated tubes and processed within 2 hours. Blood collected from patients was obtained prior to surgery, radiation or any systemic chemotherapy. Blood was diluted with an equal volume of plain RPMI and then layered over Ficoll-Paque Plus. The solution was centrifuged at 1100× g for 20 minute by density centrifugation. PBMCs were isolated from the interface and washed in RPMI. Platelets were removed by an additional density centrifugation over cold PBS. Cells were counted and stored at liquid N2 for subsequent analysis.
For flow cytometry, PBMCs were incubated with specific antibodies including CD33-PE, CD11b-FITC, CD14-PerCP-Cy5.5, HLA-DR-APC and CD15-PE-Cy7 (BioLegend, CA, USA). PBMCs were also labeled with the appropriate isotype control antibodies as negative controls. After staining, cells were resuspended in 500 μL of FACS buffer and evaluated by multicolor flow cytometry in BD FACSCanto II flow cytometer (BD Bioscience). Data were analyzed with Flowjo software. MDSCs (CD33+CD11b+HLA-DR-CD14-) were calculated as a percentage of total live PBMCs.
Serum isolation and measurement of cytokines
Three milliliters of venous blood was collected into promoting coagulating tubes and centrifuged within 1 hour. The serum obtained was stored frozen in aliquots at -80°C for subsequent analysis. Serum inflammatory cytokines were analyzed by enzyme-linked immunosorbent assay in accordance to the manufacturer’s instructions.
Determination of T cell function
T cell function was determined as previously described [5]. PBMCs were incubated in plates with anti-CD3/CD28-coated beads at 37°C for 3 days, followed by determination of total viable cell number using a hemacytometer. Cell culture supernatant was assayed for IL-2 and IFN-γ concentration using the Searchlight™ multiplex assay system. Briefly, 50 μL supernatant was added to a 96-well plate pre-spotted with either IL-12 or IFN-γ antibodies. After several washes, biotinylated antibodies with different specificity within the same cytokines were added. Streptavidin conjugated to horse radish peroxidase and SuperSignal ELISA Femto Chemiluminescent Substrate was added successively to generate luminescent signal. The amount of signal was proportional to the amount of cytokine.
PBMCs were labeled with carboxyfluorescein diacetate succinimidyl ester (CSFE, 10 μM) and plated in 96-well plates. After 5 days, cells were harvested and stained for CD4 and CD8 markers; the data were acquired and detected by flow cytometry.
CD33+HLA-DR- myeloid cells and CD3+ T cells were purified using the Rosettesep™ kit according to the manufacturer’s instructions. Increasing ratios of CD33+HLA-DR- cells were added to T cells. For T cell activation, anti-CD3 and anti-CD28-coated beads were added to each well at a bead to T cell ratio of 1:1. Plates were cultured for 4 days, then pulsed with 1.0 μCi of (3H)-TdR (NEN, MA, USA) for 8 hours and lysed with distilled water. Thymidine incorporation was determined by detecting the amount of radioactivity using a β-counter.
Statistical analysis
Results are expressed as mean ± SD and processed using SPSS 13.0 statistical software. The statistical significance of differences between groups was determined by one-way ANOVA or Mann-Whitney U test. Spearman’s correlation analysis was used to analyze the relationship between MDSCs and cytokines. Survival curves were plotted according to the KaplanMeier method and compared using the Log-rank test. P<0.05 was considered to be statistically significant.
Results
Circulating CD33+CD11b+HLA-DR-CD14- cells are major subtype of MDSCs in prostate cancer patients which are elevated and associated with clinical stages
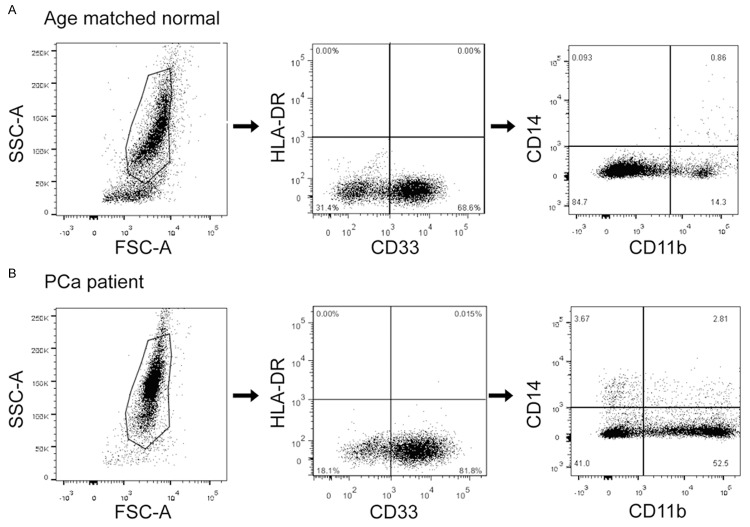
PBMCs from patients with newly diagnosed prostate cancer were analyzed for MDSCs and compared with healthy, age-matched normal controls and patients with BPH. Three subtypes of MDSCs, including granulocytic CD33+CD11b+HLA-DR-CD14-, monocytic CD33+CD11b+HLA-DR-CD14+, and granulocytic CD33+CD11b+HLA-DR-CD15+ MDSCs were detected. Figure 1 shows the representative dot plots for one of the patients and normal controls included in the study to illustrate the gating strategy. Acquired cells were first gated based on their expression of CD33 and HLA-DR and then cells expressing the markers CD11b and CD14 were determined. The level of all the three subtypes of MDSCs were significantly elevated in patients with prostate cancer compared with both normal controls and patients with BPH (P=0.001, Figure 2A-C). Moreover, among these phenotypes, the major one is CD33+CD11b+HLA-DR-CD14-, so we defined the MDSCs as CD33+CD11b+HLA-DR-CD14- in this study (Figure 2D). As shown in Figure 3, CD33+CD11b+HLA-DR-CD14- MDSCs levels increased along with the clinical stages (Figure 3A), including progression of T classifications (Figure 3B), N classifications (Figure 3C) and distant metastasis (Figure 3D).
Figure 1.
MDSCs FACS and scatter plots of cancer patients and healthy controls. CD33+CD11b+HLA-DR-CD14- MDSCs FACS gating of PBMC of a patient with prostate cancer (A) and an age-matched normal control (B). Dot plots represent live gated events. The forward and side scatter gate was analyzed for CD33+HLA-DR- cells. Then the CD33+HLA-DR- gate was analyzed for cells expressing CD11b and CD14. MDSCs were calculated as a percentage of live cells in PBMCs. Markers analyzed are indicated in the axis of each FACS plot.
Figure 2.
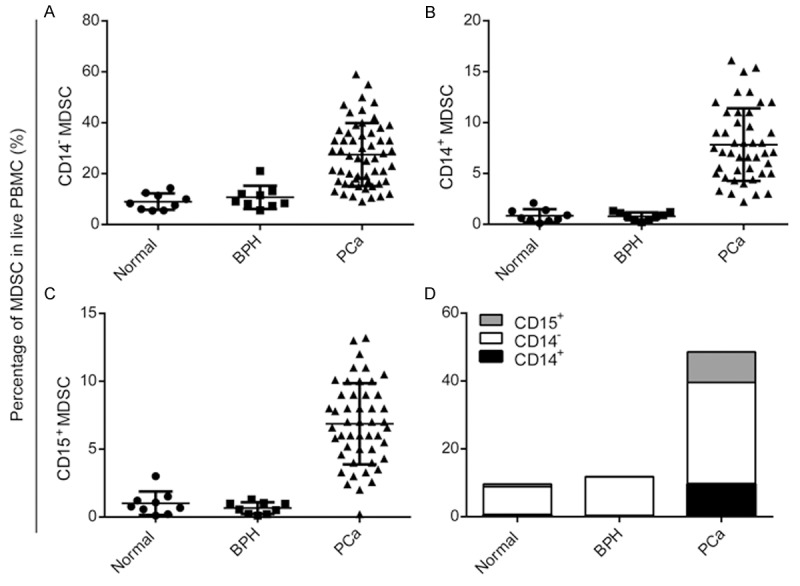
Significantly elevated MDSCs in patients with prostate cancer. A. Mean granulocytic CD33+CD11b+HLA-DR-CD14- MDSCs levels in patients with prostate cancer were significantly higher than age-matched controls and patients with BPH. B. Mean monocytic CD33+CD11b+HLA-DR-CD14+ MDSCs levels in patients with prostate cancer were higher than controls and patients with BPH. C. Mean granulocytic CD33+CD11b+HLA-DR-CD15+ MDSCs levels in patients with prostate cancer were higher than controls and patients with BPH. D. CD33+CD11b+HLA-DR-CD14- MDSCs is the the major phenotype of MDSCs in prostate cancer.
Figure 3.
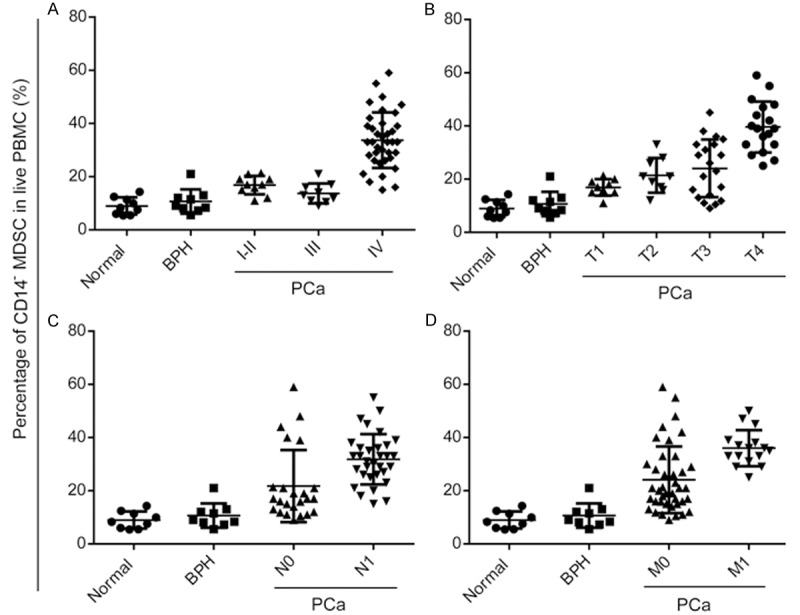
CD33+CD11b+HLA-DR-CD14- MDSCs correlated with clinical stages. Correlation between percentage of MDSCs and clinical stages (A), T classification (B), N classification (C) and distant metastasis (D) in patients with prostate cancer and normal controls (*P<0.05, **P<0.01).
Cytokines including IL-8, -6 and -10 are higher in patients with prostate cancer and correlate with clinical stages
Levels of inflammatory cytokines important for MDSCs expansion and function were evaluated in this cohort of patients with prostate cancer [14-16]. Serum levels of IL-8, -6 and -10 were all elevated in cancer patients compared with normal controls and patients with BPH (P<0.01, Figure 4, left lanes). While serum levels of IL-1b and TNF-α were up-regulated in only 13 and 7 patients respectively (data not shown). Besides, levels of IL-8, -6 and -10 were also correlated to the clinical stages of cancer (Figure 4, right lanes).
Figure 4.
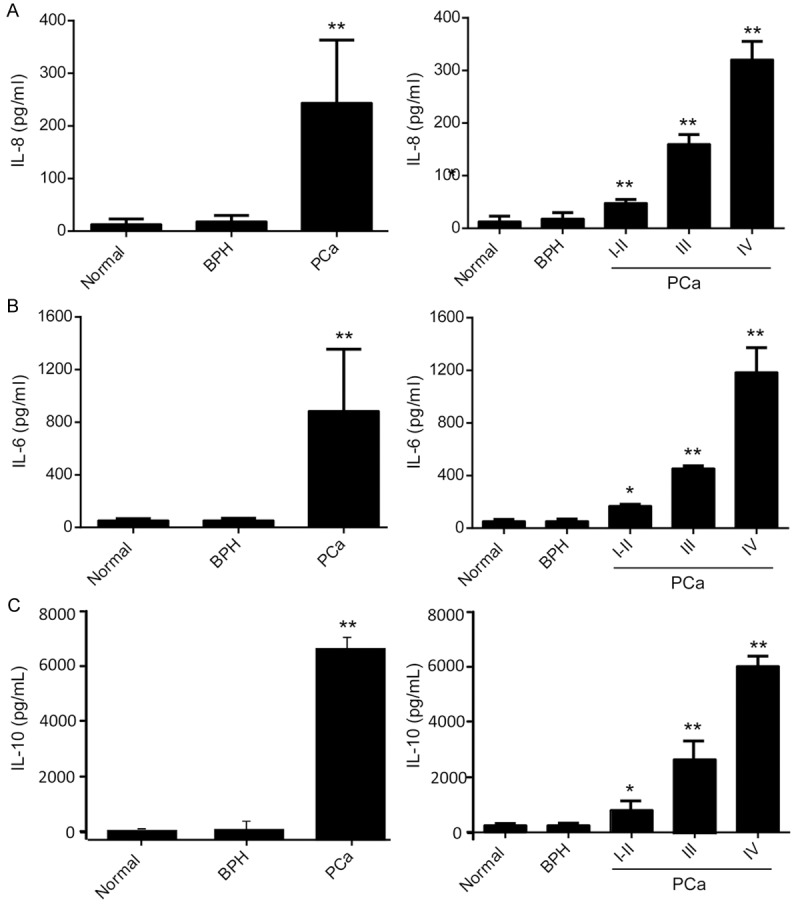
IL-8, -6 and -10 are significantly higher expressed in patients with prostate cancer and correlated with stages of cancer. Serum cytokines levels including IL-8 (A), IL-6 (B) and IL-10 (C) in age-matched normal controls and patients with prostate cancer were measured using ELISA assays. Serum samples from patients had a significantly higher level of IL-8, 6 and 10 than control samples (left lanes) and the levels increased with clinical stages (right lanes) (*P<0.05, **P<0.01).
MDSCs correlate with serum IL-8 and -6 in patients with prostate cancer
Analysis of MDSCs and cytokines revealed strong correlation between the percentage of MDSCs and IL-8 (Figure 5A, r=0.7149, P<0.0001) or IL-6 (Figure 5B, r=0.6392, P<0.0001), but not IL-10 (r=0.267, P=0.233).
Figure 5.
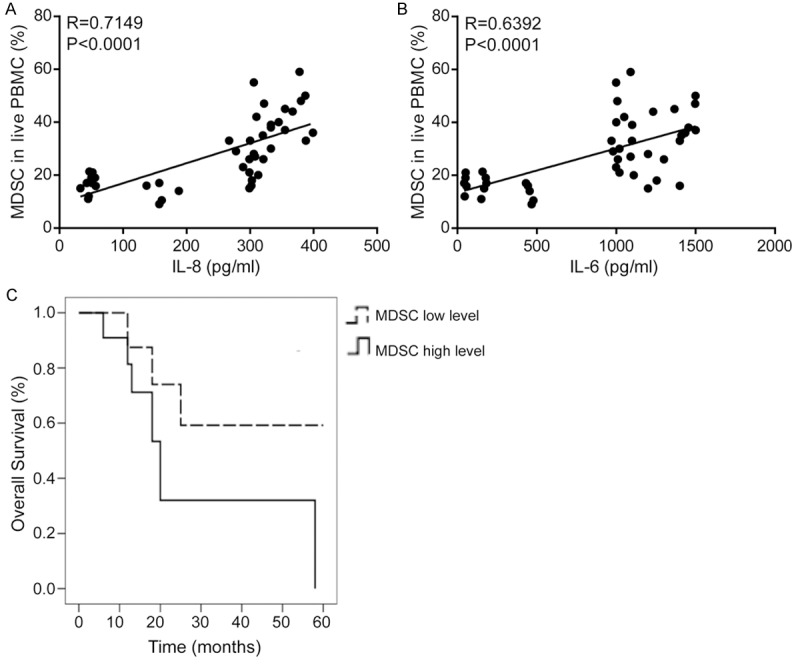
MDSCs correlated with serum IL-8 and IL-6 in patients with prostate cancer. MDSCs were associated with the poor prognosis of patients with prostate cancer. Spearman correlation analyses between percentages of MDSCs and serum levels of IL-8 (A, r=0.7149, P<0.0001) and IL-6 (B, r=0.6392, P<0.0001) in patients with prostate cancer. (C) Correlation between percentages of MDSCs and survival by Kaplan-Meier analysis of patients with the high (≥ the median) or low (< the median) MDSCs level.
MDSCs are associated with the poor prognosis of patients with prostate cancer
To investigate the prognostic value of MDSCs, its association with an overall survival was evaluated using Kaplan-Meier survival curves with the log-rank test. Sixty-two patients were enrolled for this analysis. The follow-up time ranged from 1 to 60 months. The median survival time of the group with higher level of MDSCs was 19.158 months, and the cumulative 1-, 3- and 5-year survival rates were 63%, 41% and 24%, respectively. The median survival time of the lower level group was 55.011 months, and the 1-, 3- and 5-year survival rates were 88%, 76% and 49%, respectively. The difference between the groups was significant (P<0.01). The univariate survival analysis indicated that the survival rates of patients with higher level of MDSCs was lower than that of patients with lower level (Figure 5C, P=0.000).
Immunosuppressive effect of tumor-derived MDSCs
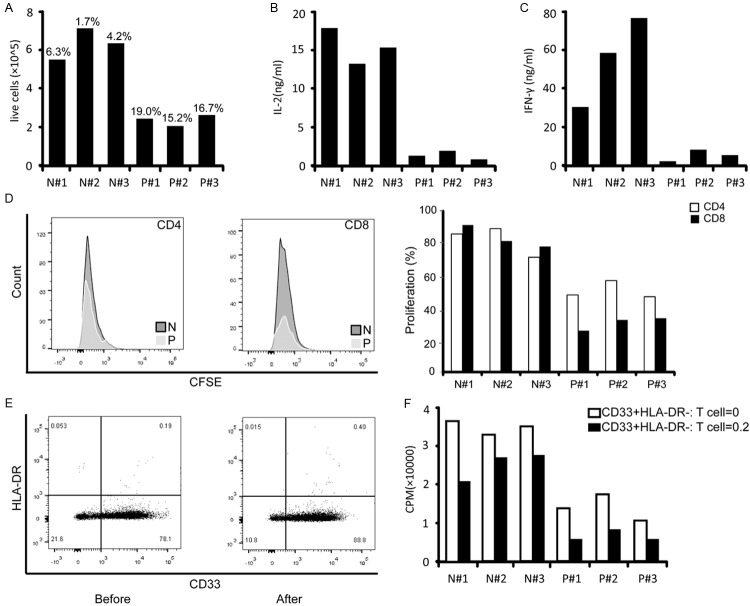
To determine if circulating MDSCs negatively impact the activity of T cells, PBMCs from three normal controls and three patients with stage IV cancer were cultured with anti-CD3/CD28-coated beads. As shown in Figure 6A-C, T cell activation in patients with advanced cancer was impaired compared to normal controls, as determined by decreased cell proliferation and secretion of IL-2 and IFN-γ. The proliferation of CD4+ T cells and CD8+ T cells were also significantly decreased (Figure 6D). To better understand the impact of MDSCs on T cell activity, increasing ratios of CD33+HLA-DR- cells were co-cultured with equal numbers of purified autologous T cells. After enrichment, purity of the CD33+HLA-DR- fraction was determined by flow cytometry. Approximately 80% of the cells showed positive CD33+ and negative HLA-DR (Figure 6E). Purified autologous T cells were activated in the presence of increasing ratios of CD33+HLA-DR- cells. Increasing numbers of CD33+HLA-DR- cells isolated from normal controls had a minimal impact on the proliferation of their autologous T cells. By contrast, a significant decrease in T cell proliferation was observed when CD33+HLA-DR- cells derived from cancer patients were added (Figure 6F).
Figure 6.
MDSCs decreased T cell responses. Equal numbers of PBMCs from normal controls (N) and three patients with stage IV cancer (P) were assayed for cell proliferation (A), IL-2 (B) and IFN-γ(C) secretion in response to activation with anti-CD3/CD28-coated beads. Corresponding percentages of circulating MDSCs are shown above in bold. D. PBMCs were stained with CFSE, cultured for 5 days, and then stained with monoclonal antibodies against CD4 and CD8; proliferation was quantified as the percentages of CFSElow cells. Left: Representative histogram of the FACS analysis. Right: Analysis of T cell proliferation. Myeloid cells (CD33+HLA-DR-) were isolated from freshly drawn blood from three normal controls (N) and three patients with stage IV cancer (P). Direct contact of T cells with isolated myeloid cells (CD33+HLA-DR-) from cancer patients inactivates T cell. E. Representative histograms of CD33+HLA-DR- fractions before and after enrichment. F. Proliferation of isolated autologous T cells in response to CD3/CD28 activation in the presence of the indicated ratios of purified autologous CD33+HLA-DR- cells.
Discussion
Prostate cancer is one of the major public health problems causing cancer-related deaths throughout the world with very poor prognosis and high possibilities of tumor invasion and migration [17]. It has been evident that prostate cancer is commonly infiltrated by a high number of immune cells, including T and B lymphocytes, macrophages, natural killer cells, dendritic cells and mast cell. All of these cells are irregularly scattered within the tumor and loaded with an assorted array of cytokines, chemokines, and inflammatory mediators [18]. Quite recently, studies on tumor and immune cells revealed the critical role of MDSCs, a novel and heterogeneous population of myeloid cells with specific inhibitory activity, in the process of tumorigenesis and metastasis [19]. MDSCs were significantly elevated in pancreatic, esophageal and gastric cancer compared with controls, and increasing percentage of MDSCs was associated with increased risk of death, and was an independent prognostic factor for survival [20]. MDSCs were also elevated in patients with metastatic renal cell carcinoma and patients with a relatively low proportion of MDSCs exhibited prolonged survival [21]. The high percentage of MDSCs in patients with advanced-stage melanoma correlated with disease progression and decrease overall survival partially by inhibiting the T cell activation [22]. Based on these findings, we speculated MDSCs might be correlated with prostate cancer development and progression. In this study, we demonstrated elevation of circulating MDSCs in patients with prostate cancer (defined as CD33+CD11b+HLA-DR-CD14-). Increasing MDSCs percentage was associated clinical stages and a prognostic factor for survival. Patients with prostate cancer also presented with increased serum levels of pro-inflammatory IL-8 and -6, which may be partly responsible for MDSCs accumulation.
MDSCs may exert their immunosuppressive effects against tumor partially through some cytokines [16,23]. In preclinical models, inflammatory cytokines including IL-8, CCL2 and CCL5 recruit MDSCs with pro-cancerous activities from the blood stream into the tumor site. These cytokines have potent angiogenic activities, promoting the motility of endothelial cells within tumor, sprouting and branching [24]. In parallel, TNF-α, which up-regulate expression of these cytokines, increases the release of matrix metalloproteinases and directly induces epithelial-to-mesenchymal transition and motility processes in cancer cells [16]. We found significant increases in serum levels of IL-8, -6 and -10 in patients with prostate cancer and their correlation with circulating MDSCs percentage. These data complement and provide clinical evidences for previously published data on intratumoral recruitment and differentiation of MDSCs and IL-8 production [25,26]. And it is also consistent with previous study that IL-6 was the most potent generator of MDSCs-like suppressor cells from normal donor PBMCs, and therefore a significant inducer of MDSCs [27].
Recently, MDSCs-centered therapeutic approach, characterized by inhibiting immune response against tumor in cancer patients and tumor-bearing mice, has drawn people’s attention. In patients with metastatic renal cell carcinoma, sunitinib significantly reduced accumulation of MDSCs in peripheral blood and reversed T cell suppression, thus provided a rationale for combining sunitinib with immunotherapy for treatment of certain tumors [28]. In murine models of lung cancer, depletion of MDSCs inhibited tumor growth, enhanced tumor cell apoptosis, reduced migration of tumors from the primary site to lung and enhanced therapeutic vaccination responses [29]. Treatment with IL-12 plus cyclophosphamide eliminated MDSCs in patients with colorectal cancer, which was essential to facilitate T cell infiltration and subsequent tumor elimination [30]. Our results showed that the elevated MDSCs in prostate patients were associated with clinical characteristics of patients and increased level of both IL-8 and -6. Moreover, higher level of MDSCs associated with poor prognosis of patients. Taken together, these results suggested MDSCs plus IL-8 or -6 may be a new potential prognostic factor and target of prostate cancer. Further investigation will be required to elucidate this question.
It is appreciated that MDSCs expressing arginase I deplete L-arginine and profoundly inhibit T cell function [5,22]. Inhibition of arginase I restores T cell function in vitro and induces an antitumor response in vivo [31]. Increased numbers of MDSCs in the peripheral blood of renal cancer patients correlated with a profound T cell dysfunction [7]. Our results, consistent with the previous study, demonstrated that increased circulating MDSCs were associated with decreased T cell activation in terms of proliferation and secretion of IL-2 and IFN-γ. Moreover, increasing numbers of MDSCs in direct contact with T cells was associated with greater inhibition of T cell proliferation. Therefore, MDSCs is a potential important mechanism of cancer-related T cell immunosuppression.
Conclusion
We demonstrated a marked increase in circulating CD33+CD11b+HLA-DR-CD14- MDSCs in patients with prostate cancer. Importantly, we demonstrated the elevation of MDSCs increased with stages of cancer and correlated with IL-8/IL-6 significantly. Abnormal accumulation of MDSCs is an important mechanism of T cell unresponsiveness in cancer patients. These data not only add to our understanding of the immunobiology of prostate cancer but also may be of importance in informing studies incorporating MDSCs inhibition strategies and IL-8/IL-6 directed therapies.
Acknowledgements
This study was financially supported by the Nature Science Foundation of Inner Mongolia Autonomous Region (2013MS1224), Scientific Project of Affiliated Hospital of Inner Mongolia Medical University (NYFY2010YB006), Youth Innovation Fund of Inner MongoliaMedical University (NY2010QN002), and Key Scientific Fund of Affiliated Hospital of Inner Mongolia Medical University (NYFYZD20130158).
Disclosure of conflict of interest
None.
Abbreviations
- BPH
benign prostatic hyperplasia
- IL
Interleukin
- MDSCs
Myeloid-derived suppressor cells
- PBMC
peripheral blood mononuclear cells
References
- 1.Ochando JC, Chen SH. Myeloid-derived suppressor cells in transplantation and cancer. Immunol Res. 2012;54:275–285. doi: 10.1007/s12026-012-8335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brusa D, Simone M, Gontero P, Spadi R, Racca P, Micari J, Degiuli M, Carletto S, Tizzani A, Matera L. Circulating immunosuppressive cells of prostate cancer patients before and after radical prostatectomy: profile comparison. Int J Urol. 2013;20:971–978. doi: 10.1111/iju.12086. [DOI] [PubMed] [Google Scholar]
- 3.Marigo I, Dolcetti L, Serafini P, Ostrand-Rosenberg S. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 4.Kong YY, Fuchsberger M, Xiang SD, Apostolopoulos V, Plebanski M. Myeloid derived suppressor cells and their role in diseases. Curr Med Chem. 2013;20:1437–1444. doi: 10.2174/0929867311320110006. [DOI] [PubMed] [Google Scholar]
- 5.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 7.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, Mier J, Ochoa AC. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 8.Khaled YS, Ammori BJ, Elkord E. Increased levels of granulocytic myeloid-derived suppressor cells in peripheral blood and tumour tissue of pancreatic cancer patients. J Immunol Res. 2014;2014:879897. doi: 10.1155/2014/879897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawant A, Ponnazhagan S. Myeloid-derived suppressor cells as osteoclast progenitors: a novel target for controlling osteolytic bone metastasis. Cancer Res. 2013;73:4606–4610. doi: 10.1158/0008-5472.CAN-13-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawant A, Deshane J, Jules J, Lee CM, Harris BA, Feng X, Ponnazhagan S. Myeloid-derived suppressor cells function as novel osteoclast progenitors enhancing bone loss in breast cancer. Cancer Res. 2013;73:672–682. doi: 10.1158/0008-5472.CAN-12-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawant A, Hensel JA, Chanda D, Harris BA, Siegal GP, Maheshwari A, Ponnazhagan S. Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J Immunol. 2012;189:4258–4265. doi: 10.4049/jimmunol.1101855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking infl ammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, Dreicer R, Bukowski R, Finke JH. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 16.Tartour E, Pere H, Maillere B, Terme M, Merillon N, Taieb J, Sandoval F, Quintin-Colonna F, Lacerda K, Karadimou A, Badoual C, Tedgui A, Fridman WH, Oudard S. Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev. 2011;30:83–95. doi: 10.1007/s10555-011-9281-4. [DOI] [PubMed] [Google Scholar]
- 17.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 18.Taverna G, Giusti G, Seveso M, Hurle R, Colombo P, Stifter S, Grizzi F. Mast Cells as a Potential Prognostic Marker in Prostate Cancer. Dis Markers. 2013;35:711–720. doi: 10.1155/2013/478303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochando JC, Chen SH. Myeloid-derived suppressor cells in transplantation and cancer. Immunol Res. 2012;54:275–285. doi: 10.1007/s12026-012-8335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Zhang Y, Liu Y, Wang L, Zhao L, Yang T, He C, Song Y, Gao Q. Association of Myeloid-derived Suppressor Cells and Efficacy of Cytokine-induced Killer Cell Immunotherapy in Metastatic Renal Cell Carcinoma Patients. J Immunother. 2014;37:43–50. doi: 10.1097/CJI.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 22.Jordan KR, Amaria RN, Ramirez O, Callihan EB, Gao D, Borakove M, Manthey E, Borges VF, McCarter MD. Myeloid-derived suppressor cells are associated with disease progression and decreased overall survival in advanced-stage melanoma patients. Cancer Immunol Immunother. 2013;62:1711–1722. doi: 10.1007/s00262-013-1475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mundy-Bosse BL, Young GS, Bauer T, Binkley E, Bloomston M, Bill MA, Bekaii-Saab T, Carson WE 3rd, Lesinski GB. Distinct myeloid suppressor cell subsets correlate with plasma IL-6 and IL-10 and reduced interferon-alpha signaling in CD4+ T cells from patients with GI malignancy. Cancer Immunol Immunother. 2011;60:1269–1279. doi: 10.1007/s00262-011-1029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben-Baruch A. The Tumor-Promoting Flow of Cells Into, Within and Out of the Tumor Site: Regulation by the Inflammatory Axis of TNF-α and Chemokines. Cancer Microenviron. 2012;5:151–164. doi: 10.1007/s12307-011-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rotondo R, Barisione G, Mastracci L, Grossi F, Orengo AM, Costa R, Truini M, Fabbi M, Ferrini S, Barbieri O. IL-8 induces exocytosis of arginase 1 by neutrophil polymorphonuclears in nonsmall cell lung cancer. Int J Cancer. 2009;125:887–893. doi: 10.1002/ijc.24448. [DOI] [PubMed] [Google Scholar]
- 26.Chikamatsu K, Takahashi G, Sakakura K, Ferrone S, Masuyama K. Immunoregulatory properties of CD44+ cancer stem-like cells in squamous cell carcinoma of the head and neck. Head Neck. 2011;33:208–215. doi: 10.1002/hed.21420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185:2273–2284. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol. 2011;11:856–861. doi: 10.1016/j.intimp.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srivastava MK, Zhu L, Harris-White M, Kar UK, Huang M, Johnson MF, Lee JM, Elashoff D, Strieter R, Dubinett S, Sharma S. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS One. 2012;7:e40677. doi: 10.1371/journal.pone.0040677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medina-Echeverz J, Fioravanti J, Zabala M, Ardaiz N, Prieto J, Berraondo P. Successful colon cancer eradication after chemoimmunotherapy is associated with profound phenotypic change of intratumoral myeloid cells. J Immunol. 2011;186:807–815. doi: 10.4049/jimmunol.1001483. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, Antonia S, Ochoa JB, Ochoa AC. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specifi c T-cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]