Abstract
The prognostic value of the HPV status in ESCC is much controversial, this study aimed to determine the prognostic importance of high-risk HPV and p16 in patients with ESCC. A total of 105 consecutive patients who underwent esophagectomy in 2008 were included in this study. All specimens with ESCC were tested by in situ hybridization for HPV16/18 and immunohistochemistry for p16 expression. Kappa values were calculated using Cohen’s kappa test. The 5-year overall survival (OS) and progression-free survival (PFS) were calculated in relation to the two markers and the Cox proportional hazards model was used to determine the hazard ratio (HR) of variables. Thirty-nine (37.1%) of 105 were p16-positive, and HPV was detected in 29 of the 105 patients (27.6%) with ESCC. P16 was detected in 25 of the 29 patients (86.2%) who were HPV-positive, and only 14 of 76 patients (18.4%) who were HPV-negative (P < 0.001). Cohen’s kappa coefficient revealed an agreement in two researchers (kappa = 0.61). The 5-year OS rate and PFS rate in the p16-positive group were 64.1% and 58.7%, respectively, and the rates in the p16-negative group were 45.5%, 37.9%, respectively. The difference of survival rate between the two groups remained statistically significant. P16-positive patients had better 5-year rates of OS and PFS than p16-negative group (P = 0.02 and P = 0.007 by the Log-rank test, respectively). Using HPV status as a stratification factor, we found differences in OS and PFS that were consistent with those based on p16 expression. P16 is a very good marker of HPV infection for ESCC. HPV-positive or p16-positive ESCC is a distinct entity with a favorable prognosis compared with HPV-negative or p16-negative ESCC.
Keywords: Human papillomavirus, p16INK4A, squamous cell carcinoma, esophageal cancer, prognosis
Introduction
Esophageal cancer (EC) is one of the most common gastrointestinal malignancies with a strong aggression in the developing countries [1]. China is located in the famous “Asian esophageal cancer belt”, the regions around the Taihang Mountain in northern China is well known as one of the highest incidence areas for EC [2]. EC is the fifth most common cancer and the fourth most common cause of death from cancer in China [3], and more than 90% of cases are esophageal squamous cell carcinoma (ESCC) [4]. Despite increasing rates of esophageal adenocarcinoma in many western countries, ESCC remains the dominant histological type of EC worldwide [5]. Due to the absence of early symptoms, invasiveness of the disease, and its late diagnosis, it is generally associated with a poor prognosis and the 5-year survival is only about 10% [6].
Worldwide, more than 550,000 new cases of human cancer are linked with HPV infection annually [7]. According to previous studies, HPV-16 is the most prevalent type in squamous cell carcinoma, followed by HPV-18 [8], while other high-risk HPV types are rare [9]. HPV is causally linked with majority of cervical cancer [10], and is strongly associated with the head and neck cancers, particularly the oropharyngeal squamous cell carcinoma [11]. The histologic similarities between the oropharyngeal squamous epithelium and upper esophagus would suggest that HPV can infect esophagus along the route. On the basis of the discovery of HPV-associated koilocytes in EC, HPV infection in EC was suggested as early as 1981 [12]. Since then, HPV has been suggested as a risk factor for ESCC. The role of HPV in the development of ESCC is still controversial.
Once HPV infection has taken place, viral DNA synthesis occurs. Additional accumulating changes can lead to transformation [13]. These changes are a multistage complex interaction of the host immune system in combination with the expression of two viral oncogenes: E6 and E7, followed by a series of epigenetic changes occurring in dysplastic lesions. HPV-positive cancer is associated with wild-type p53 and downregulation of cyclin D and the retinoblastoma tumor suppressor protein named pRb [14]. Inactivation of pRb lead to upregulation of CDKN2A and overexpression of of p16INK4A protein hereafter denoted as p16. Thus HPV-positive squamous cell carcinoma are strongly correlate with high expression of p16 [15].
In some previous clinical studies, patients with HPV-associated squamous cell carcinoma have an improved prognosis than those with HPV-negative tumors [16-20]. With the present study, we sought to determine the infection of HPV and p16 expression in ESCC, we also sought to evaluate the prognostic significance of p16 and HPV in patients with ESCC treated with surgery in northern China. Our hope is to find a more rational treatment modalities and our biggest goal is to make use of the existing and developing HPV-vaccines to prevent and treat these HPV-associated malignancies through mass vaccination.
Patients and methods
Patients and tissues samples
We searched the EC database in the Oncology Center, Qilu Hospital of Shandong University to find 273 consecutive patients from January 2007 to December 2007 who had undergone surgery with or without adjunctive radiotherapy and/or chemotherapy. Patients treated with neoadjuvant therapy, which could potentially interfere with the prevalence of HPV were excluded, as were patients who died within 30 days after surgery. The additional exclusion criteria comprised the non-squamous cell subtype and uncooperative patients unable to answer questions or who could not be contacted. All eligible patients were aged ≥ 18 years. The Ethics Committee of Qilu Hospital of Shandong University approved this analysis (documentation No. 2013085) and all patients provided their written informed consent. 105 patients met the protocol study criteria and had sufficient ESCC tumor tissue to detect HPV16/18 and p16. Serial 5-μm sections were cut from each patient’s tumor tissue. All slides were reviewed by a pathologist specializing in gastrointestinal pathology. Staging was determined based on the AJCC (American Joint Committee on Cancer) TNM staging system [21]. Alcohol intake cut-off point was 0.025 kg/day. The cut-off value was based on the 2011 Chinese Inhabitant Dietary Guideline. The demarcation point of anemia was 12 g/L for men and 11 g/L for women.
Surgery
Types of esophagectomy included Ivor-Lewis and the three-stage (right thoracotomy, midline laparotomy and left cervical incisions) esophagectomy. The majority of patients underwent an Ivor-Lewis esophagectomy (N = 92).
Follow up
Patients were followed until death or for at least 5 years. Follow-up time was calculated from the date of surgery to death or the date of the last contact. All patients had a regular follow-up schedule; physical examinations and imaging studies were performed every 3 months during the first 2 years after surgery and every 6 months during years 3 through 5. Routine radiological examinations and esophagoscopy were performed when necessary. Additional parameters were obtained from the database for inpatients or tumor registry for outpatients of Qilu Hospital of Shandong University.
Laboratory studies
Formalin-fixed, paraffin-embedded tumor specimens were evaluated for high-risk HPV subtypes 16 and 18 using the in situ hybridization-catalyzed signal-amplification method for biotinylated probes (GenPoint, Dako). A HPV-positive tumor was defined as a tumor for which there was specific staining of tumor-cell nuclei for HPV in cervical squamous cell carcinoma analysis, whereas the negative control was obtained by omitting the HPV probe. All slides were scored as positive or negative. Carcinomas were classified as HPV positive when brown signal was seen localized to the nuclei of tumor cells [22]. All scorings were conducted with no knowledge of p16 immunohistochemistry status.
Processions of p16 immunohistochemical detection were carried out by the Dako Envision-System method (Code: GK500705) using a primary antibody against p16 (monoclonal mouse anti-human p16INK4A protein, Clone G175-405, Dako). A p16-positive tumor was used as a positive control, negative controls were obtained by omitting the primary antibody. P16-positive was defined as ≥ 70% of cells showing strong nuclear or/and cytoplasm immunolabeling. All scorings were conducted with no knowledge of clinical characteristics or outcome.
Study end points
The primary end point was overall survival (OS), defined as the time from date of surgery to death or the last date of follow-up. Secondary end points included progression-free survival (PFS), defined as the time from date of surgery to local or distant recurrence. Local recurrence refers to regional lymph node metastasis or tumor recurrence at the primary site. Death from the primary cancer without a documented site of recurrence or death from an unknown cause was considered death from local-regional disease.
Statistical analysis
According to p16 status or HPV status patients included in our study were divided into two groups. The differences between the two groups were tested for significance using the Mann-Whitney test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables and the Kruskal-Wallis test for ranked data. The Cohen’s kappa test was used to analyze the consistency between p16 expression by immunohistochemistry and HPV status by in situ hybridization. The Kaplan-Meier method and Log-rank test were used for analysis and comparison of survival curves. The Cox proportional hazards model was used to determine the hazard ratio (HR) of variables on 5-year OS and PFS in univariate and multivariate analysis. The results were given as HRs with their 95% confidence interval (CI). P values less than 0.05 were considered statistically significant. All analyses were performed using SPSS 16 (SPSS Inc., Chicago, IL).
Results
Patient characteristics
A total of 105 patients (81 males and 24 females) met the protocol study criteria for analysis. The median age of the patients was 60 (range, 42 to 78) years at the date of surgery. Thirty-nine (37.1%) of 105 patients were p16 positive. Baseline characteristics of p16-positive and p16-negative patients are shown in Table 1. Patients who were p16 positive had higher differentiation grade, better performance status, and they were less likely to be current smokers.
Table 1.
Baseline characteristics of the study patients and their tumors, according to p16-expression
| Total | p16-positive | p16-negative | ||
|---|---|---|---|---|
|
|
||||
| (N = 105) | (N = 39) | (N = 66) | ||
|
|
||||
| Characteristics | No. (%) | No. (%) | No. (%) | P Value |
| Gender | ||||
| Male | 81 (80.0) | 30 (79.5) | 51 (80.3) | 0.97 |
| Female | 24 (20.0) | 9 (20.5) | 15 (19.7) | |
| Age | ||||
| Median (range) | 60 (42-78) | 61 (45-76) | 62 (41-78) | 0.25Δ |
| Tumor location | ||||
| Cervical/Upper | 11 (10.5) | 6 (15.4) | 5 (7.8) | 0.34 |
| Middle | 49 (46.7) | 19 (48.7) | 30 (45.5) | |
| Low | 45 (42.9) | 14 (35.9) | 31 (46.7) | |
| pT status | ||||
| pT1 | 20 (19.0) | 9 (23.1) | 11 (16.7) | 0.59* |
| pT2 | 22 (21.0) | 9 (23.1) | 13 (19.7) | |
| pT3 | 58 (55.2) | 20 (51.3) | 38 (57.6) | |
| pT4 | 5 (4.8) | 1 (2.6) | 4 (6.1) | |
| pN status | ||||
| pN0 | 68 (64.8) | 27 (69.2) | 41 (62.1) | 0.76* |
| pN1 | 25 (23.8) | 8 (20.5) | 17 (25.8) | |
| pN2 | 10 (9.5) | 3 (7.7) | 7 (10.6) | |
| pN3 | 2 (1.9) | 1 (2.6) | 1 (2.6) | |
| TNM stage (AJCC) | ||||
| I | 23 (21.9) | 9 (27.3) | 14 (21.2) | 0.35 |
| II | 49 (46.7) | 21 (53.8) | 28 (42.4) | |
| III | 33 (31.4) | 9 (23.1) | 24 (36.4) | |
| Differentiation grade | ||||
| Well | 27 (25.7) | 14 (35.9) | 13 (19.7) | 0.034 |
| Moderate | 44 (41.9) | 18 (46.2) | 26 (39.4) | |
| Poor | 34 (32.4) | 7 (17.9) | 27 (40.9) | |
| Hemoglobin | ||||
| High | 71 (67.6) | 22 (56.4) | 49 (74.2) | 0.06 |
| Low | 34 (32.4) | 17 (43.6) | 17 (25.8) | |
| Alcohol intake (kg/day) | ||||
| < 20 | 53 (50.5) | 19 (48.7) | 34 (51.5) | 0.78 |
| ≥ 20 | 52 (49.5) | 20 (51.3) | 32 (48.5) | |
| Current smoker | ||||
| Yes | 42 (40.0) | 9 (23.1) | 33 (50.0) | 0.007 |
| No | 63 (60.0) | 30 (76.9) | 33 (50.0) | |
| Adjuvant therapy | 0.28 | |||
| Yes | 44 (41.9) | 19 (48.7) | 25 (37.9) | |
| No | 61 (58.1) | 20 (51.3) | 41 (62.1) | |
| ECOG performance status | ||||
| 0 | 53 (50.5) | 26 (66.7) | 27 (40.9) | 0.01 |
| 1~2 | 52 (49.5) | 13 (33.3) | 39 (59.1) | |
AJCC: American Joint Commission on Cancer Staging; Pt: pathological tumor stage; pN: pathological node stage; ECOG: Eastern Cooperative Oncology Group.
P values were calculated with the use of the Kruskal-Wallis test.
P values were calculated with the use of the Mann-Whitney test.
Analysis of HPV and p16
Thirty-nine (37.1%) of 105 patients were p16-positive (Figure 1A). Twenty five (64.1%) of 39 p16-positive tumors were stained positive for HPV by in situ hybridization (Figure 1B), only four (6.1%) of 66 p16-negative tumors were stained positive for HPV. The presence of HPV and p16 expression in tumors had a good agreement (kappa = 0.61; 95% CI = 0.45 to 0.77). P16 expression was strongly associated with HPV positivity (86.2% in HPV-positive tumors vs. 18.4% in HPV-negative tumors, P < 0.001) (Table 2).
Figure 1.
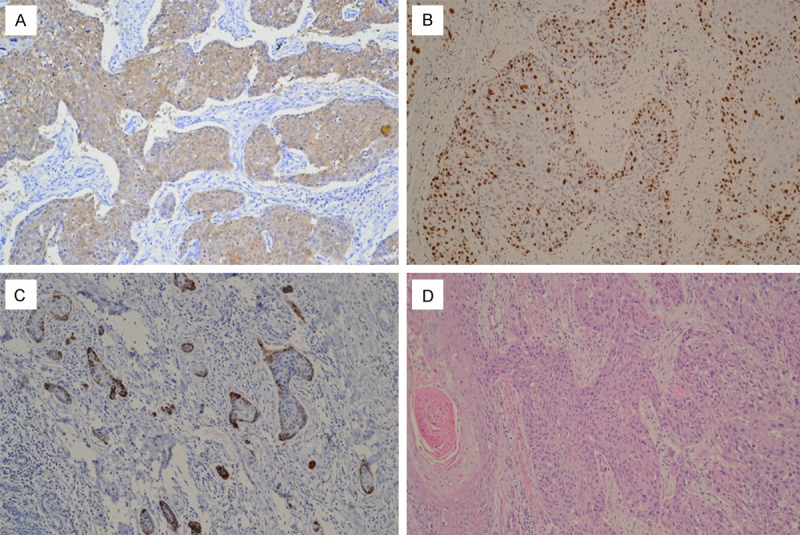
A. Immunohistochemical staining of p16INK4A in esophageal squamous cell carcinomas. 70% or more than 70% of tumor cells showing strong nuclear and cytoplasm immunolabeling. B. In situ hybridization signal of HPV-positive esophageal squamous cell carcinomas. Numerous tumor cells show positive nuclear signals. C. P16INK4A expression by immunohistochemistry. Less than 70% of tumor cells showing immunolabeling, such staining was defined negativity. D. Hematoxylin and eosin staining in esophageal squamous cell carcinomas. (Original magnification × 200).
Table 2.
Correlation between p16 immunohistochemistry and HPV in situ hybridization esophageal squamous cell carcinoma
| Total | p16-positive | p16-negative | |||
|---|---|---|---|---|---|
|
|
|||||
| (N = 105) | (N = 39) | (N = 66) | |||
|
|
|||||
| HPV Status | No. (%) | No. (%) | No. (%) | P Value | Kappa Value |
| Positive | 29 (27.6) | 25 (64.1) | 4 (6.1) | < 0.001 | 0.61 |
| Negative | 76 (72.4) | 14 (35.9) | 62 (93.9) | ||
P and Kappa values were calculated with the use of Pearson’s chi-square test and Cohen Kappa test, respectively.
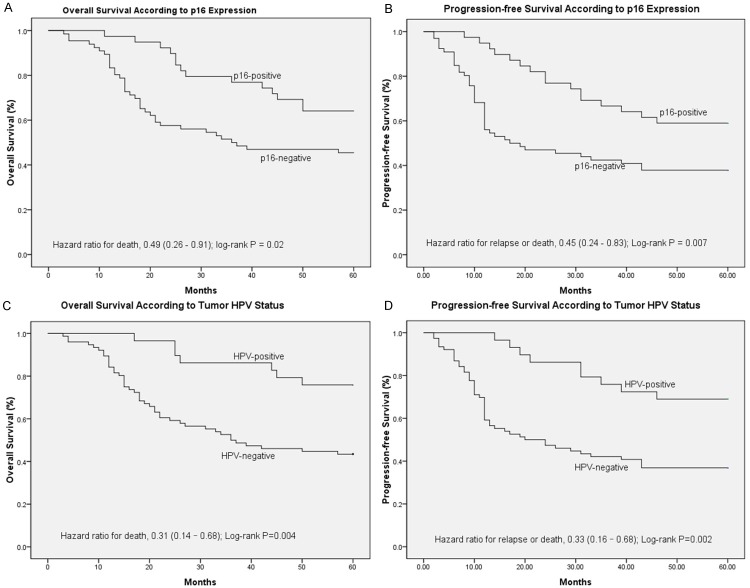
Survival analysis
Based on Kaplan-Meier analysis, OS was superior in the p16-positive group compared with that in the p16-negative group, with 5-year survival rates of 64.1% and 45.5%, respectively (HR = 0.49; 95% CI = 0.26 to 0.91; P = 0.02; Figure 2A). P16-positive patients also had statistically significantly better PFS than P16-negative patients, the 5-year rates of PFS were 58.7% and 37.9%, respectively (HR = 0.45; 95% CI = 0.24 to 0.83; P = 0.007; Figure 2B). Using HPV status as a stratification factor, the survival results were consistent with the results based on p16 expression. OS was superior in the HPV-positive group compared with that in the HPV-negative group, with 5-year survival rates of 65.9% and 43.4%, respectively (HR = 0.31; 95% CI = 0.14 to 0.68; P = 0.004; Figure 2C). The 5-year rates of PFS were 61.8% and 36.8%, respectively (HR = 0.33; 95% CI = 0.16 to 0.68; P = 0.002; Figure 2D).
Figure 2.
Kaplan-Meier estimates of survival among the study patients, according to tumor HPV status or p16-expression. For 5-year overall survival rate (A) and 5-year progression-free survival rate (B), p16-expression was significantly associated with improved outcomes (P = 0.021; P = 0.007; respectively). For 5-year OS rate (C) and 5-year progression-free survival rate (D), HPV was significantly associated with improved outcomes (P = 0.004; P = 0.002; respectively).
Cox regression analysis of OS and PFS, including the prognostic factors of hemoglobin, T category, N category, smoking, and ECOG performance status, demonstrated that p16 status and ECOG performance status were the significant factors in multivariable analysis (Table 3) for OS and p16 status was the only significant factor in multivariable analysis for PFS (Table 4). Patients with p16-positive tumors had a 58% lower risk of death than patients with p16-negative (adjusted HR = 0.42, 95% CI = 0.26 to 0.91, P = 0.03). Patients with p16-positive tumors had a risk of progression that was 61% lower than that of patients with p16-negative tumors (adjusted HR = 0.39, 95% CI = 0.27 to 0.77, P = 0.02) (Table 4).
Table 3.
Multivariate analysis of overall survival
| Factors | Levels | 95% CI | HR | P Value |
|---|---|---|---|---|
| T category | T1/T2 vs. T3/ T4 | 0.58-2.12 | 1.04 | 0.69 |
| N category | N0 vs. N1/N2/N3 | 0.85-3.78 | 1.79 | 0.13 |
| Current smoker | Yes vs. No | 0.46-1.51 | 0.81 | 0.43 |
| Hemoglobin | High vs. Low | 0.54-6.29 | 1.71 | 0.39 |
| ECOG performance status | 0 vs. 1/2 | 1.08-9.04 | 3.08 | 0.04 |
| p16 status | positive vs. negative | 1.75-8.54 | 4.23 | 0.03 |
HR: hazard ratio; CI: confidence interval; ECOG: eastern cooperative oncology group.
Table 4.
Multivariate analysis of progression-free survival
| Factors | Levels | 95% CI | HR | P Value |
|---|---|---|---|---|
| T category | T1/T2 vs. T3/T4 | 0.89-9.63 | 2.04 | 0.13 |
| N category | N0 vs. N1/N2/N3 | 0.91-3.78 | 1.63 | 0.07 |
| Current smoker | Yes vs. No | 0.63-1.68 | 0.63 | 0.26 |
| Hemoglobin | High vs. Low | 0.16-1.31 | 0.51 | 0.49 |
| ECOG performance status | 0 vs. 1/2 | 0.86-3.42 | 1.78 | 0.08 |
| p16 status | positive vs. negative | 1.12-5.71 | 2.52 | 0.02 |
HR: hazard ratio; CI: confidence interval; ECOG: eastern cooperative oncology group.
Discussion
HPV has recently been established as a risk factor for oropharyngeal squamous cell carcinoma and HPV-associated cancers has been associated with an improved prognosis [11]. The histologic similarities between the oropharyngeal squamous epithelium and upper esophagus would suggest that HPV can infect esophagus along the route. Based on the presence of HPV in the oropharyngeal squamous epithelium and the association with carcinoma of oropharynx, we inferred that HPV is strongly associated with ESCC.
The present study demonstrates that ESCC tissues had HPV infection with an incidence of 27.6% in 105 patients, and all cases were HPV-16 positive. The observation was consistent with the previous studies in high-risk areas for ESCC in China [23,24]. The study patients were identified from Qilu Hospital of Shandong University located in Shandong Province, which was a high-incidence area for ESCC in China [25]. There are more than 130 HPV types identified and these have been classified into low- or high-risk groups according to their potential for oncogenesis [26]. HPV has a specific tropism for squamous epithelium cells where it can cause hyperproliferative lesions, and then cause carcinogenesis [26]. HPV with potential for oncogenesis based on persistent infection is known as the high risk HPV. HPV type 16 and 18 are known to cause the majority of squamous cell carcinomas [8,9].
HPV-positive tumors are characterized by high expression of p16 and p16 is widely considered a surrogate marker for HPV infection in the context of squamous cell carcinoma [15]. In our study, Thirty-nine (37.1%) of 105 were p16-positive. Twenty five (86.2%) of the 29 HPV-positive ESCC cases expressed p16, while fourteen (18.4%) of 76 HPV-negative subgroup (P < 0.001). P16 was minimally detectable in HPV-negative tumors [27] and was an established biomarker for the function of the HPV E7 oncoprotein which is a standard for defining a tumor as being effected with HPV [28]. The presence of HPV by in situ hybridization and p16 expression by immunohistochemistry in tumors had a good agreement (kappa = 0.61; 95% CI = 0.45 to 0.77).
Retrospective studies have reported improved outcomes in HPV-associated squamous cell carcinoma, and many of these studies was head and neck cancer, especially oropharyngeal cancer [16-20]. In this study, we have demonstrated the prognostic significance of p16 and HPV status in patients with primary esophageal carcinoma treated surgery. The improved survival in p16-positive patients was observed. OS was superior in the p16-positive group with 5-year survival rates of 64.1%, however 45.5% in the p16-negative group (HR = 0.49; 95% CI = 0.26 to 0.91; P = 0.021). The 5-year rates of PFS were 58.7% and 37.9%, respectively (HR = 0.45; 95% CI = 0.24 to 0.83; P = 0.007). Using HPV status as a stratification factor, the survival results were consistent with the results based on p16 expression. Based on Kaplan-Meier analysis, OS and PFS were superior in the HPV-positive group compared with that in the HPV-negative group, 5-year survival rates were 65.9% and 43.4%, respectively (HR = 0.31; 95% CI = 0.14 to 0.68; P = 0.004), and 5-year rates of PFS were 61.8% and 36.8%, respectively (HR = 0.33; 95% CI = 0.16 to 0.67; P = 0.002).
Cox regression analysis of OS and PFS, patients with p16-positive tumors had a risk of progression that was 61% lower than that of patients with p16-negative tumors (adjusted HR = 0.39, 95% CI = 0.27 to 0.77, P = 0.02). P16 status was an independent prognostic factor for OS and PFS among patients with ESCC. Our study has examined p16 in conjunction with HPV by in situ hybridization, support p16 by immunohistochemistry as an effective surrogate for HPV. Immunohistochemical technique is operated simply, reproducible and lower cost than in situ hybridization, suitable for use in routine clinical care and clinical trials [19].
Our study has clearly shown that p16-positive cancer was associated with higher differentiation grade and better performance status, and they were less likely to be current smokers. Significant differences between the three aspects may be the potential causes leading to the superior prognosis in patients with p16-positive. Another hypothesis could also explain the results. Integration of HPV result in higher expression of the oncoproteins E6/E7, thereby abrogating the p53 and Rb protein functions, promoting genomic rearrangements [29], rearranged DNA is theoretically more sensitive to radiation and chemotherapy [30], providing an explanation for the indication of higher survival rates for patients with HPV-positive tumors. Our sample was too small to exclude confounding by infection of HPV and adjuvant therapy, analysis of a larger study could more thoroughly evaluate the possibility of confounding by HPV and adjuvant therapy via analysis of Different adjuvant treatment patterns.
The link between HPV positivity in ESCC and smoking was still under investigation, Some studies have suggested synergistic effect [31], genetic alterations induced by tobacco-associated carcinogens may be strengthened by HPV and lead to increased DNA mutation, unstable genome causes the disorder of cell cycle, not surprisingly, p16-positive patients were much less likely to be current smokers.
In the present study, fourteen (51.9%) of 27 well differentiation grade tumors were stained positive for p16 with immunohistochemistry, 40.9% (18) of 44 moderate differentiation grade tumors were positive. The lowest incidence was found in poor differentiation grade tumors, only 20.1% (7 of 34) were stained positive for p16. These results suggested that p16 protein expression in esophageal carcinoma was related to differentiation grade, can be used as a reference index of the degree of tumor differentiation. In our study, patients with better performance status had higher incidence of p16-positive than patients with not so good ECOG performance status. The link between p16 positivity in ESCC and ECOG performance status was still under investigation. The ECOG performance status, although reported in previous studies, have not been consistently identified, probably because of the small sample size in many of these studies [32].
In conclusion, our study clearly demonstrates that HPV-associated ESCC treated with surgery has a superior outcome compared with HPV-negative ESCC. Furthermore, HPV (or p16) is the most important prognostic variable in multivariable analysis. Although statistically significant differences in survival were observed between HPV-positive and -negative, definitive conclusions cannot be drawn from this study due to its small sample size and retrospective nature, larger confirmatory studies are needed to provide definitive evidence.
Acknowledgements
The authors thank Dr. Wei Ma (oncologist from the Department of Radiation Oncology, Cancer Hospital, General Hospital of Ningxia Medical University, Yinchuan 750000, China.) for his expert suggestions and technical assistance. This work was supported by China Postdoctoral Science Fund (No. 2011M500531).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Tran GD, Sun XD, Abnet CC, Fan JH, Dawsey SM, Dong ZW, Mark SD, Qiao YL, Taylor PR. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113:456–463. doi: 10.1002/ijc.20616. [DOI] [PubMed] [Google Scholar]
- 3.Mao WM, Zheng WH, Ling ZQ. Epidemiologic risk factors for esophageal cancer development. Asian Pac J Cancer Prev. 2011;12:2461–2466. [PubMed] [Google Scholar]
- 4.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 5.Ries L, Melbert D, Krapcho M, Stinchcomb D, Howlader N, Horner M, Mariotto A, Miller B, Feuer E, Altekruse S. SEER Cancer Statistics Review, 1975-2005. National Cancer Institute; Bethesda, MD: 2008. Available at: seer. cancer. gov/csr/1975-2001 2007. [Google Scholar]
- 6.Klint Å, Engholm G, Storm HH, Tryggvadóttir L, Gislum M, Hakulinen T, Bray F. Trends in survival of patients diagnosed with cancer of the digestive organs in the Nordic countries 1964-2003 followed up to the end of 2006. Acta Oncol. 2010;49:578–607. doi: 10.3109/02841861003739330. [DOI] [PubMed] [Google Scholar]
- 7.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, Rosenberg PS, Bray F, Gillison ML. Worldwide Trends in incidence rates for oral cavity and oropharyngeal cancers. J. Clin. Oncol. 2013;31:4550–4559. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munoz N. Human papillomavirus and cancer: the epidemiological evidence. J Clin Virol. 2000;19:1–5. doi: 10.1016/s1386-6532(00)00125-6. [DOI] [PubMed] [Google Scholar]
- 9.Moberg M, Gustavsson I, Gyllensten U. Real-time PCR-based system for simultaneous quantification of human papillomavirus types associated with high risk of cervical cancer. J Clin Microbiol. 2003;41:3221–3228. doi: 10.1128/JCM.41.7.3221-3228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naucler P, Chen HC, Persson K, You SL, Hsieh CY, Sun CA, Dillner J, Chen CJ. Seroprevalence of human papillomaviruses and Chlamydia trachomatis and cervical cancer risk: nested case-control study. J Gen Virol. 2007;88:814–822. doi: 10.1099/vir.0.82503-0. [DOI] [PubMed] [Google Scholar]
- 11.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Syrjänen K. Histological changes identical to those of condylomatous lesions found in esophageal squamous cell carcinomas. Arch Geschwulstforsch. 1981;52:283–292. [PubMed] [Google Scholar]
- 13.Rietbergen MM, Leemans CR, Bloemena E, Heideman DA, Braakhuis BJ, Hesselink AT, Witte BI, Baatenburg de Jong RJ, Meijer CJ, Snijders PJ. Increasing prevalence rates of HPV attributable oropharyngeal squamous cell carcinomas in the Netherlands as assessed by a validated test algorithm. Int J Cancer. 2013;132:1565–1571. doi: 10.1002/ijc.27821. [DOI] [PubMed] [Google Scholar]
- 14.El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: A guide for interpretative relevance and consistency. Head Neck. 2012;34:459–461. doi: 10.1002/hed.21974. [DOI] [PubMed] [Google Scholar]
- 15.Shi W, Kato H, Perez-Ordonez B, Pintilie M, Huang S, Hui A, O’Sullivan B, Waldron J, Cummings B, Kim J. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J. Clin. Oncol. 2009;27:6213–6221. doi: 10.1200/JCO.2009.23.1670. [DOI] [PubMed] [Google Scholar]
- 16.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 17.Reimers N, Kasper HU, Weissenborn SJ, Szer H, Preuss SF, Hoffmann TK, Speel EJ, Dienes HP, Pfister HJ, Guntinas-Lichius O. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer. 2007;120:1731–1738. doi: 10.1002/ijc.22355. [DOI] [PubMed] [Google Scholar]
- 18.Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, Sasaki C, Joe J, Camp RL, Rimm DL. Molecular classification identifies a subset of human papillomavirus-associated oropharyngeal cancers with favorable prognosis. J. Clin. Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 19.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J. Clin. Oncol. 2009;27:1992–1998. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]
- 20.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: Review and meta-analysis. Int J Cancer. 2007;121:1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 21.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 22.Berumen J, Unger ER, Casas L, Figueroa P. Amplification of human papillomavirus types 16 and 18 in invasive cervical cancer. Hum Pathol. 1995;26:676–681. doi: 10.1016/0046-8177(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 23.Gao Y, Hu N, Han X, Giffen C, Ding T, Goldstein A, Taylor P. Family history of cancer and risk for esophageal and gastric cancer in Shanxi, China. BMC Cancer. 2009;9:269. doi: 10.1186/1471-2407-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen D, Shan B, Wang S, Zhang L, Wei L, Zhou W, Peng Q. A positive family history of esophageal/gastric cardia cancer with gastric cardia adenocarcinoma is associated with a younger age at onset and more likely with another synchronous esophageal/gastric cardia cancer in a Chinese high-risk area. Eur J Med Genet. 2010;53:250–255. doi: 10.1016/j.ejmg.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Zheng S, Vuitton L, Sheyhidin I, Vuitton DA, Zhang Y, Lu X. Northwestern China: a place to learn more on oesophageal cancer. Part two: gene alterations and polymorphisms. Eur J Gastroenterol Hepatol. 2011;23:1087–1099. doi: 10.1097/MEG.0b013e32834a14d9. [DOI] [PubMed] [Google Scholar]
- 26.zur Hausen H. Papillomaviruses in the causation of human cancers-a brief historical account. Virology. 2009;384:260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 27.Reed AL, Califano J, Cairns P, Westra WH, Jones RM, Koch W, Ahrendt S, Eby Y, Sewell D, Nawroz H. High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res. 1996;56:3630–3633. [PubMed] [Google Scholar]
- 28.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 29.Dahlgren L, Mellin H, Wangsa D, Heselmeyer-Haddad K, Bjornestal L, Lindholm J, Munck-Wikland E, Auer G, Ried T, Dalianis T. Comparative genomic hybridization analysis of tonsillar cancer reveals a different pattern of genomic imbalances in human papillomavirus-positive and -negative tumors. Int J Cancer. 2003;107:244–249. doi: 10.1002/ijc.11371. [DOI] [PubMed] [Google Scholar]
- 30.Kahla S, Kochbati L, Maalej M, Oueslati R. Situation of HPV16 E2 gene status during radiotherapy treatment of cervical carcinoma. Asian Pac J Cancer Prev. 2014;15:2869–2873. doi: 10.7314/apjcp.2014.15.6.2869. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz SM, Daling JR, Madeleine MM, Doody DR, Fitzgibbons ED, Wipf GC, Carter JJ, Mao EJ, Huang S, Beckmann AM, McDougall JK, Galloway DA. Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection. J Natl Cancer Inst. 1998;90:1626–1636. doi: 10.1093/jnci/90.21.1626. [DOI] [PubMed] [Google Scholar]
- 32.Kong CS, Narasimhan B, Cao H, Kwok S, Erickson JP, Koong A, Pourmand N, Le QT. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2009;74:553–561. doi: 10.1016/j.ijrobp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]