Abstract
The prognostic value of Interleukin 17 (IL-17) in cancer patients is currently under debate and remains inconclusive. We performed a systematic review and meta-analysis to evaluate the role of IL-17 as a prognostic marker in cancer. Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were combined to measure the effective value of IL-17 expression on prognosis. Nineteen eligible studies enrolling 2390 patients were identified. We found expression of IL-17 was not significantly correlated with overall survival (OS) in cancer (HR=1.29, 95% Cl: 0.94-1.76; P=0.12). Furthermore, compared to the data from our analysis that high expression of IL-17 predicted poor OS in both non-small cell lung carcinoma (NSCLC) (HR=2.30; 95% CI: 1.45-3.64; P<0.001; I2=0%) and hepatocellular carcinoma (HCC) (HR=2.02; 95% CI: 1.44-2.83; P<0.001; I2=0%), high expression of IL-17 was associated with favorable OS in esophageal squamous cell carcinoma (ESCC) (HR=0.63; 95% CI: 0.51-0.79; P<0.001; I2=0%). This meta-analysis showed that IL-17 has the potential to become a novel prognostic marker in HCC, NSCLC and ESCC. It could potentially help to monitor patients’ prognosis and assess therapeutic efficacy in clinical treatment.
Keywords: IL-17, prognosis, survival, meta-analysis
Introduction
Cancer is a class of diseases characterized by out-of-control cell growth. A total of 1,660,290 new cancer cases and 580,350 cancer deaths are projected to occur in the United States in 2013 [1]. Although, cancer death rates have decreased by 20% from their maximum in 1991 (215.1 per 100,000 population) to 2009 (173.1 per 100,000 population) [2], newer diagnostic methods with improved sensitivity and specificity are essential for the proper detection and prognosis of this fatal disease [3]. Recently, lots of biomarkers with potential prognostic value have been estimated in different types of cancers, such as osteopontin in hepatocellular carcinoma (HCC) [4], MET in gastric cancer [5] and CD44 in head and neck cancer [6]. There is still a great need for reliable and simple biomarkers to evaluate the prognostic significance of cancer.
IL-17 is a pro-inflammatory cytokine which is mainly produced by activated CD4+ T-helper cells (also known as Th17 cells), macrophages and CD8+ T cells [7]. As an essential pro-inflammatory cytokine, IL-17 induces a mass of cytokines and chemokines secretion, such as mesenchymal cells and myeloid cells, which recruit neutrophils and monocytes into the site of inflammation [8]. Furthermore, IL-17 correlates well with the graft-versus-host disease (GVHD), development of inflammation and autoimmune diseases [9-11].
In the recent time, accumulating evidence has shown that IL-17 has an influence on different kinds of cancer models [12], including prostate cancer [13], colorectal cancer [14], breast cancer [15], NSCLC [16], HCC [17], and ovarian cancer [18]. IL-17 promotes angiogenesis in tumor models [12,19,21] and granulopoiesis [20]. Moreover, some studies have shown that high expression of IL-17 correlates with tumor development and patient prognosis. A combined analysis on recent studies will provide a precise estimate on the prognostic relevance of IL-17 expression in cancer patients which has not yet been performed.
Previous studies have suggested that expression of IL-17 in cancer might serve as a prognostic factor but the direct association of IL-17 expression with survival of patient remains to be under debate. In this study, we performed the first meta-analysis to evaluate the role of IL-17 as a prognostic marker in cancer.
Materials and methods
Literature search
A comprehensive search of PubMed, EMBASE, OVID, Cochrane Library, Web of Science databases and China National Knowledge Infrastructure (CNKI) was done from database inception to July 12 2014 without language restriction. The search strategy was “interleukin-17 OR IL-17 OR IL17” AND “tumor OR neoplasm OR cancer OR carcinoma”. Furthermore, review articles and reference lists of retrieved articles were reviewed manually to complete our search. The database search was performed independently by X. Zhang and W. Weng. And disagreements were resolved through consensus by the review team.
Eligibility criteria
The studies included in this meta-analysis if the following conditions were met: (a) proven diagnosis of the IL-17 expression; (b) analyzed the correlation of IL-17 with survival outcome; (c) enrolled more than 30 patients (d) provided sufficient data to estimate the hazard ratio (HR) and 95% confidence intervals (CI) according to IL-17 expression; (e) when study patients overlapped with patients in other included studies, we selected the first study published. The two researchers (J. Wang and X. Zhang) independently read the titles and abstracts, and excluded the uncorrelated studies; then the full-texts were scrutinized by the review team. And we selected the studies for our meta-analysis according to the inclusion criteria.
Data abstraction
Two independent reviewers (X. Zhang and W. Weng) extracted the following information: authors, year of publication, country, tumor type, number of patients analyzed, distribution of age and gender, first-line therapies, tumor stage, method of IL-17 detection, cut-off level to consider IL-17 as highly expressed and HRs and their 95% CIs for OS, progression-free survival (PFS) in patients on palliative treatment or surgery and disease-free survival (DFS) for patients undergoing potentially curative resection. And we pooled the PFS of outcomes for patients on palliative treatment with DFS for patients undergoing surgery. We selected the multivariate analysis if univariate and multivariate analysis were both reported. Because the multivariate analysis has taken into consideration the confounding factor and is more accurate. If there were no HRs reported in the article, we used Engauge Digitizer version 4.1 (free software down-loaded from http://sourceforge.net) to read the Kaplane-Meier survival curves to get the HRs and their 95% CIs. If articles reported insufficient data (missing data, inconsistencies, or any other uncertainties), we asked corresponding authors for additional information.
Quality assessment
To identify high-quality studies, two independent investigators (X. Zhang and J. Wang) underlined with descriptive information and scored each publication based on the Newcastle-Ottawa Quality Assessment Scale [22] for cohort studies with moderate modifications (www.ncbi.nlm.nih.gov/pubmedhealth/PMH0015974). Scores were added up to compare study quality in a quantitative manner. Study with a score of 6 or higher was considered as a high quality study. We also searched for the impact factor of each study and calculated the mean value.
Statistical analysis
Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were combined to measure the effective value of IL-17 expression on prognosis. An HR >1 suggested poor prognosis in patients with high expression of IL-17. And P values <0.05 denoted statistical significance. If the study didn’t report the HRs, the Engauge Digitizer version 4.1 was used to read the kaplane-Meier curves to estimate the HRs and the 95% CIs. Three independent investigators (J. Wang, W. Weng and X. Zhang) read the curves in order to reduce reading variability. The heterogeneity among the studies was measured using the Q test and I2 test. The pooled HR for survival was calculated by random-effects models when heterogeneity among the studies was observed. Sensitivity analyses were carried out to test the robustness of the results of meta-analysis. Subgroup analyses were performed to investigate the value of IL-17 expression as a prognostic indicator for cancer patients in studies of tumor type, duration of follow-up, IL-17 expression detection methods and type of method used to obtain the HR. We also conducted tests of interaction to test for differences between subgroups. These analyses were performed by Review Manager Version 5.1 software (http://ims.cochrane.org/revman). The Begg’s and Egger’s test was performed by R (http://cran.r-project.org/bin/windows/base).
Results
Characteristics of identified studies
Following an initial search, 875 published studies were identified. However, after screening of the titles and abstracts and further reviewing in detail, nineteen studies were included in our meta-analysis [16,17,23-37,51,52]. The process of article identification, inclusion, and exclusion was summarized in Figure 1.
Figure 1.
Flow diagram summarizing the selection of eligible studies.
The baseline characteristics of the included studies were presented in Table S1. These studies concerned different cohorts of patients published between 2009 and 2014. The median sample size was 125 patients (range, 32-300 patients). Fourteen studies used immunohistochemistry (IHC) to detect the IL-17 positive cells [16,17,23-27,31,33-37,52]; three studies applied flow cytometry [28,29,32]; one study applied ELISA [51] and one study quantified the serum concentration of IL-17 [30]. Three studies assessed hepatocellular carcinoma (HCC) [17,30,36], three studies assessed gastric cancer [23,29,32], three studies assessed non-small cell lung cancer (NSCLC) [16,31,51], three studies assessed esophageal squamous cell carcinoma (ESCC) [26,37,52] and one each for pancreatic cancer [24], ovarian cancer [35], intrahepatic cholangiocarcinoma (ICC) [27], glioblastoma [34], colorectal cancer [25], chronic lymphocytic leukemia [28] and breast cancer [33]. Seventeen studies [16,17,23-27,51,52] were in China and the remaining two were in the United States [28] and Taiwan [33] respectively. Eighteen studies provided UICC stage [16,17,23-27,29-37,51,52] and the other one did not mention it. OS was obtained in 18 studies [16,17,23-33,35-37,51,52], and PFS/DFS was obtained in 6 studies [16,17,32-35].
Qualitative assessment
To evaluate the quality of studies included in our meta-analysis, we assessed representativeness of the exposed cohort, ascertainment of exposure, outcome of interest, comparability of cohorts, assessment of outcome and adequacy of follow up for each study [22]. All of the 16 inclusions were of high quality with scores ranging from 7 to 9 (Table S2). And the mean impact factor was 4.107 (Table 1).
Table 1.
Impact factors of studies included in the meta-analysis
| Study | Year | Impact factor |
|---|---|---|
| Chen [33] | 2013 | 2.857 |
| Chen [16] | 2010 | 3.392 |
| Chen [23] | 2011 | 3.168 |
| Cui [34] | 2013 | 3.168 |
| Gu [27] | 2012 | 4.12 |
| He [24] | 2011 | 2.464 |
| Jain [28] | 2012 | 5.935 |
| Lan [35] | 2013 | 3.677 |
| Liao [36] | 2013 | 3.066 |
| Liu [25] | 2011 | 2.406 |
| Liu [29] | 2012 | 3.382 |
| Lv [26] | 2011 | 3.73 |
| Wang [37] | 2013 | 3.637 |
| Wu [30] | 2012 | 3.73 |
| Zhang [31] | 2012 | 1.271 |
| Zhang [17] | 2009 | 9.858 |
| Zhuang [32] | 2012 | 12.821 |
| Xu [51] | 2014 | 1.879 |
| Lu [52] | 2013 | 3.463 |
| Mean value | 4.107 |
Meta-analysis
Overall, eighteen studies including 2390 tumor patients reported data on IL-17 expression and OS in solid tumors. The combined analysis of the 17 studies showed that expression of IL-17 was not significantly correlated with OS in cancer (HR=1.29, 95% Cl: 0.94-1.76; P=0.12). Furthermore, there was heterogeneity between studies (I2=80%, P<0.001) (Figure 2A), and a publication bias became obvious when visually inspecting the funnel plot (Figure 2B). The P-value of begg’s test was 0.302 and the P-value of egger’s test was 0.018 (Table S3). As for PFS, the HR was 1.78 (95% Cl 0.83-3.81; P=0.14; I2=84%) (Figure 3A) and a publication bias was not obvious (Figure 3B). No publication bias was tested in the Begg’s (P=1.000) and Egger’s test (P=0.213) (Table S3).
Figure 2.
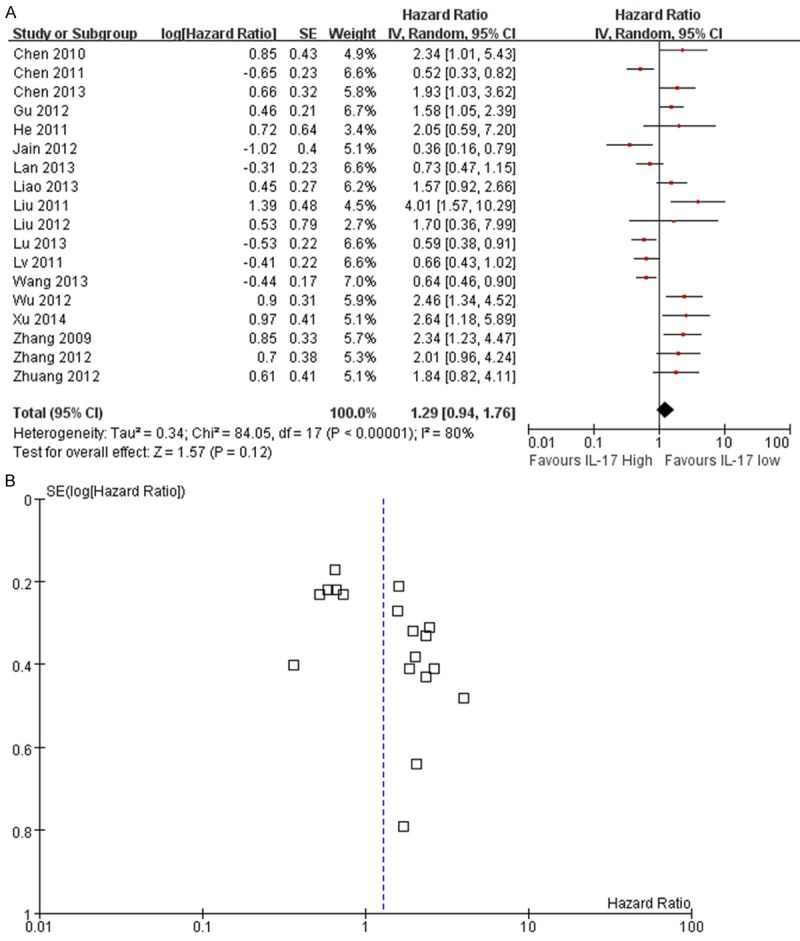
Meta-analysis comparing IL-17 expression and overall survival (OS) in cancer patients. A. The individual and pooled HR with 95% CIs was shown by forest blot. Heterogeneity was calculated by measuring the inconsistency (I2) and by the Cochrane Q test (Chi-squared test; Chi2). B. Funnel blot was used to reflect a potential publication bias.
Figure 3.
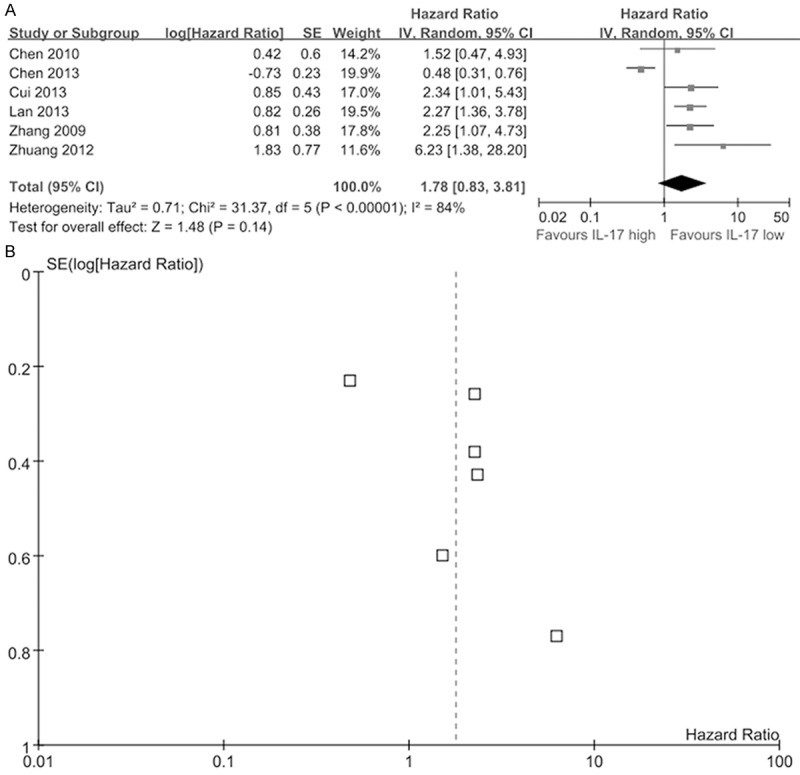
Meta-analysis comparing IL-17 expression and progression-free survival (PFS) in cancer patients. A. The individual and pooled HR with 95% CIs was shown by forest blot. Heterogeneity was calculated by measuring the inconsistency (I2) and by the Cochrane Q test (Chi-squared test; Chi2). B. Funnel blot was used to reflect a potential publication bias.
Tumor type
The results of the subgroup analyses were summarized in Table S3. Firstly, a subgroup analysis by tumor type was performed (Figure 4). We found high expression of IL-17 was significantly associated with poor OS in HCC (HR=2.02, 95% CI: 1.44-2.83; P <0.001), and slight heterogeneity was observed in the data (I2=0%; P=0.48). High expression of IL-17 in NSCLC also suggested a similar result (HR=2.30, 95% CI: 1.45-3.64; P<0.001) without heterogeneity (I2=0.0%; P=0.89). But high expression of IL-17 predicted improved OS in ESCC (HR=0.63, 95% CI: 0.51-0.79; P<0.001) and no heterogeneity was observed (I2=0.0%; P=0.92). In gastric cancer, high expression of IL-17 was not an obvious prognostic factor (HR=1.06, 95% Cl: 0.39-2.86; P=0.91). Moreover, there was only one study each evaluating the association between high expression of IL-17 and OS in pancreatic cancer, ovarian cancer, ICC, colorectal cancer, chronic lymphocytic leukemia and breast cancer. The pooled HR was 1.30 (95% Cl: 0.72-2.36; P=0.38; I2=80; P≤0.001). In chronic lymphocytic leukemia and ovarian cancer, higher expression of IL-17 was correlated with better OS and in other four types of cancer, higher expression of IL-17 was associated with worse OS (Figure 4).
Figure 4.
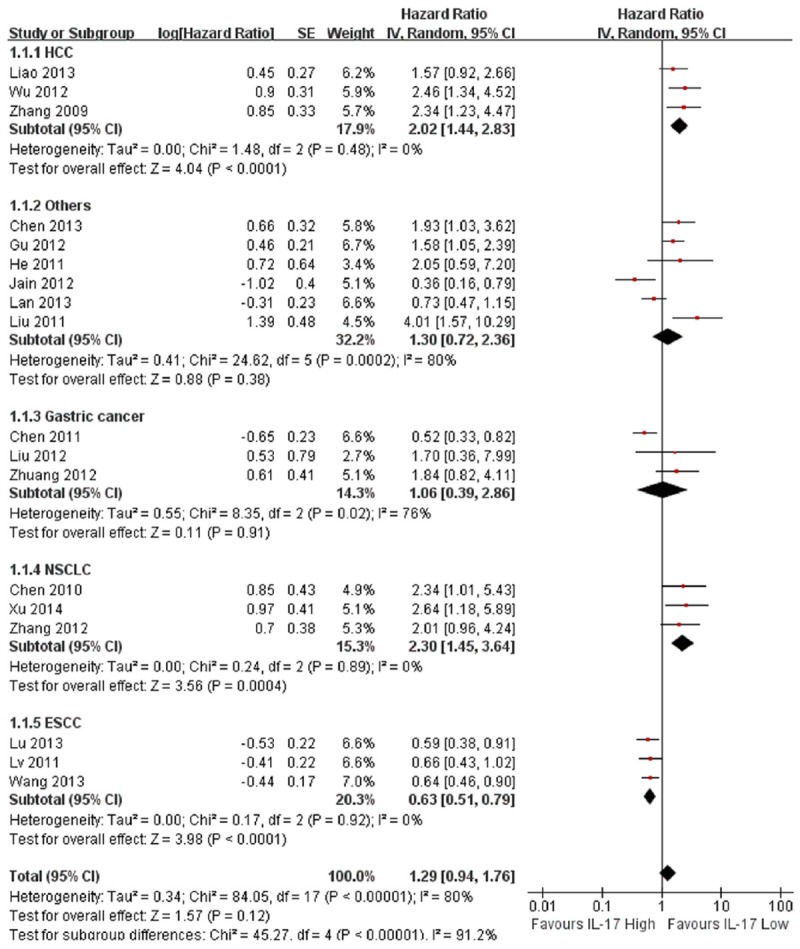
Meta-analysis of the association between high expression of IL-17 and overall survival (OS) in different tumor types.
Other subgroup analysis
As shown in Table S3, we performed other subgroup analysis including duration of follow-up, detection method and method to obtain HR. Studies with a median follow-up of more than 60 months showed favorable OS for high expression of IL-17 (HR=0.83, 95% Cl: 0.43-1.58; I2=83, P=0.003) and studies with a median follow-up of less than 60 months was associated with poor OS for high expression of IL-17 (HR=1.82, 95% Cl: 1.33-2.51; I2=0, P=0.71). Studies that applied IHC found higher expression of IL-17 suggested worse OS (HR=1.31, 95% Cl: 0.91-1.87; I2=80, P<0.001) and PFS (HR=1.84, 95% Cl: 0.77-4.39; I2=87, P<0.001). No significant associations between CD4+IL17+ T cells and prognostic effect were determined. There was only one study each using flow cytometry to detect CD8+IL17+ T cells and quantifying the serum concentration of IL-17, therefore the results were related entirely to the individual studies. Furthermore, both reported in text (OS: HR=1.44 95% Cl: 0.90-2.31; PFS: HR=1.78 95% Cl: 0.61-5.17) and estimated by us (OS: HR=1.28 95% Cl: 0.81-2.00; PFS: HR=2.01 95% Cl 1.07-3.77) did not show a significant association with OS, because some contradictory results were in presence (Table S3).
Sensitivity analyses
We performed sensitivity analyses to explore the heterogeneity among studies. However, with omitting 1 study at a time, there was still obvious heterogeneity among these studies (Table 2). So the heterogeneity was not generated by one individual study.
Table 2.
The influence of individual study on the pooled estimate (HR)
| Study omitted | Year | HR | 95% CI | Heterogeneity | |
|---|---|---|---|---|---|
|
|
|||||
| I2 | P value | ||||
| None | 1.29 | 0.94-1.76 | 80 | <0.001 | |
| Chen [33] | 2013 | 1.25 | 0.91-1.73 | 80 | <0.001 |
| Chen [16] | 2010 | 1.25 | 0.90-1.71 | 80 | <0.001 |
| Chen [23] | 2011 | 1.37 | 0.99-1.88 | 78 | <0.001 |
| Gu [27] | 2012 | 1.27 | 0.91-1.77 | 80 | <0.001 |
| He [24] | 2011 | 1.26 | 0.92-1.74 | 80 | <0.001 |
| Jain [28] | 2012 | 1.37 | 1.00-1.88 | 78 | <0.001 |
| Lan [35] | 2013 | 1.34 | 0.96-1.87 | 80 | <0.001 |
| Liao [36] | 2013 | 1.27 | 0.91-1.77 | 80 | <0.001 |
| Liu [25] | 2011 | 1.21 | 0.89-1.65 | 78 | <0.001 |
| Liu [29] | 2012 | 1.28 | 0.93-1.76 | 80 | <0.001 |
| Lv [26] | 2011 | 1.35 | 0.97-1.88 | 80 | <0.001 |
| Wang [37] | 2013 | 1.36 | 0.98-1.88 | 77 | <0.001 |
| Wu [30] | 2012 | 1.23 | 0.90-1.69 | 78 | <0.001 |
| Zhang [31] | 2012 | 1.25 | 0.91-1.73 | 79 | <0.001 |
| Zhang [17] | 2009 | 1.24 | 0.90-1.70 | 80 | <0.001 |
| Zhuang [32] | 2012 | 1.26 | 0.91-1.74 | 80 | <0.001 |
| Xu [51] | 2014 | 1.23 | 0.90-1.70 | 79 | <0.001 |
| Lu [52] | 2013 | 1.36 | 0.98-1.88 | 79 | <0.001 |
Discussion
Chronic inflammation plays an active role in cancer, IL-17 can promote cancer-elicited inflammation and prevent cancer cells from immune surveillance [38]. It can enhance T cell mediated anti-tumor responses by induction of MDSCs [39] and inhibit cytotoxic lymphocytes activities [40,41]. IL-17 can also enhance migration and recruitment of tumor cell [42,43]. However, IL-17 is associated with some anti-tumor mechanisms. It can reduce tumor growth and metastasis by promoting protective tumor immunity [44,45]. So we combined and investigated these data and performed a meta-analysis to obtain a further understanding of a potential association between IL-17 and prognosis in cancer patients. Moreover, it is the first meta-analysis regarding IL-17 in cancer prognosis features.
In our meta-analysis, expression of IL-17 was not significantly correlated with OS in cancer (HR=1.28, 95% CI: 0.94-1.76, P=0.12) (Figure 2A). So we did not regard IL-17 as a good prognostic marker since 6 of these 18 studies reported the opposite results and significant heterogeneity was observed among the studies (I2=80%, P<0.001). In our subgroup analysis for tumor type, higher expression of IL-17 predicted worse OS in HCC (HR=2.02, 95% CI: 1.44-2.83; P<0.001) with no heterogeneity in the data (I2=0%; P=0.48) (Figure 4). Thus, IL-17 may serve as a prognostic marker and therapeutic target for HCC. Recently, some studies reported that IL17A promoted HCC metastasis via NF-kB induced matrix metalloproteinases 2 and 9 expression [46] and tumor progression with AKT-dependent IL-6/JAK2/STAT3 activation [47]. Tumor metastasis and progression are usually correlated with poor prognosis [48]. Interleukin-17-activated monocytes were reported to suppress cytotoxic T-cell function through B7-H1 in hepatocellular carcinoma patients and IL-17 plays a contributing role in the induction of immune escape in HCC [53]. Furthermore, higher expression of IL-17 was also significantly related with worse OS in NSCLC (HR=2.3, 95%: CI 1.45-3.64; P<0.001) with no heterogeneity (I2=0%, P=0.89) (Figure 4). Studies also reported that IL-17 is related with the NSCLC invasion and increases lymph-angiogenesis in NSCLC by enhancing production of VEGF-C to promote tumor metastasis [49,50]. Numasaki et al [54] demonstrated that IL-17 increases the net angiogenic activity and in vivo growth of NSCLC via promoting CXCR-2-dependent angiogenesis. Li et al [55] found that IL-17 could directly promote the invasion of NSCLC cells both in vitro and in vivo and IL-6-Stat3 pathway was crucial for IL-17 to enhance the invasive potential of NSCLC cells. These findings all demonstrate that IL-17 is a pivotal cytokine involved in tumor progression of NSCLC. However, we found in ESCC, high expression of IL-17 suggested improved OS (HR=0.63, 95% Cl 0.51-0.79; P<0.001) without heterogeneity (I2=0%; P=0.91) (Figure 4). Lv et al [26] considered that IL-17 producing cells in ESCC might exert anti-tumor effects by enhancing cytotoxic T lymphocytes and NK cell responses. More mechanisms regarding IL-17 in ESCC need to be investigated in the future to confirm the speculation.
However, this study has several limitations. Firstly, the detection methods were different among these studies. Fourteen studies used IHC to detect IL-17 positive cells. Three studies performed flow cytometry. Wu et al [30] quantified serum IL-17 level. Although these studies were all correlated with IL-17 expression, they might cause statistical and clinical heterogeneity and the sensitivity of those methods is varied. Secondly, in the studies, cut-off values were also different, which might therefore account for the inconsistencies observed. Thirdly, most reports included in this meta-analysis come from one country, the representation is low. The HRs we estimated may be the source of heterogeneity because they are not the original clinical data. To account for heterogeneity, we used a random effects model, performing subgroup analyses to elucidate this heterogeneity. Finally, studies about each cancer type are not enough and in each subgroup, the sample size of patient case is low. We still need more studies in a certain cancer type to analyze the role of IL-17 in prognostic value features.
Despite these limitations, conclusive results are firstly provided in HCC, NSCLC, ESCC and patient survival by this meta-analysis. We still need more studies in different tumor types to assess the prognostic significance of IL-17. If the significance of IL-17 in cancer is better understood, we can generate a more efficient therapeutic strategy targeting IL-17 in cancer.
Conclusions
IL-17 was not significantly associated with overall survival in cancer patients. However, subgroup analysis showed that high expression of IL-17 predicted poor prognosis in both NSCLC and HCC, and high expression of IL-17 predicted favorable OS in ESCC. IL-17 may become a novel disease marker in different cancer and a more efficient therapeutic strategy targeting IL-17 can be generated with the further investigation of IL-17.
Acknowledgements
This study was supported by China National 973 projects (Grant Nos: 20111812 and 20110402), Natural science foundation of China (Grant Nos: 81272292 and 81301689), Climbing training program (assigned to Jiayi Wang) from Shanghai Tenth People’s Hospital.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Kuschel B, Auranen A, McBride S, Novik KL, Antoniou A, Lipscombe JM, Day NE, Easton DF, Ponder BA, Pharoah PD, Dunning A. Variants in DNA double-strand break repair genes and breast cancer susceptibility. Hum Mol Genet. 2002;11:1399–1407. doi: 10.1093/hmg/11.12.1399. [DOI] [PubMed] [Google Scholar]
- 2.Gansler T, Ganz PA, Grant M, Greene FL, Johnstone P, Mahoney M, Newman LA, Oh WK, Thomas CR Jr, Thun MJ, Vickers AJ, Wender RC, Brawley OW. Sixty years of CA: a cancer journal for clinicians. CA Cancer J Clin. 2010;60:345–350. doi: 10.3322/caac.20088. [DOI] [PubMed] [Google Scholar]
- 3.Paul D, Kumar A, Gajbhiye A, Santra MK, Srikanth R. Mass spectrometry-based proteomics in molecular diagnostics: discovery of cancer biomarkers using tissue culture. Biomed Res Int. 2013;2013:783131. doi: 10.1155/2013/783131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang CH, Xu GL, Jia WD, Ge YS, Li JS, Ma JL, Ren WH. Prognostic significance of osteopontin in hepatocellular carcinoma: a meta-analysis. Int J Cancer. 2012;130:2685–2692. doi: 10.1002/ijc.26301. [DOI] [PubMed] [Google Scholar]
- 5.Peng Z, Zhu Y, Wang Q, Gao J, Li Y, Li Y, Ge S, Shen L. Prognostic significance of MET amplification and expression in gastric cancer: a systematic review with meta-analysis. PLoS One. 2014;9:e84502. doi: 10.1371/journal.pone.0084502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Zhou J, Lu J, Xiong H, Shi X, Gong L. Significance of CD44 expression in head and neck cancer: a systemic review and meta-analysis. BMC Cancer. 2014;14:15. doi: 10.1186/1471-2407-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150:5445–5456. [PubMed] [Google Scholar]
- 8.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 11.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 12.Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol. 2009;183:4169–4175. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- 13.Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Gouvello S, Bastuji-Garin S, Aloulou N, Mansour H, Chaumette MT, Berrehar F, Seikour A, Charachon A, Karoui M, Leroy K, Farcet JP, Sobhani I. High prevalence of Foxp3 and IL17 in MMR-proficient colorectal carcinomas. Gut. 2008;57:772–779. doi: 10.1136/gut.2007.123794. [DOI] [PubMed] [Google Scholar]
- 15.Zhu X, Mulcahy LA, Mohammed RA, Lee AH, Franks HA, Kilpatrick L, Yilmazer A, Paish EC, Ellis IO, Patel PM, Jackson AM. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008;10:R95. doi: 10.1186/bcr2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Wan J, Liu J, Xie W, Diao X, Xu J, Zhu B, Chen Z. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer. 2010;69:348–354. doi: 10.1016/j.lungcan.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 18.Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci U S A. 2008;105:15505–15510. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du JW, Xu KY, Fang LY, Qi XL. Interleukin-17, produced by lymphocytes, promotes tumor growth and angiogenesis in a mouse model of breast cancer. Mol Med Rep. 2012;6:1099–1102. doi: 10.3892/mmr.2012.1036. [DOI] [PubMed] [Google Scholar]
- 20.Jovcic G, Bugarski D, Krstic A, Vlaski M, Petakov M, Mojsilovic S, Stojanovic N, Milenkovic P. The effect of interleukin-17 on hematopoietic cells and cytokine release in mouse spleen. Physiol Res. 2007;56:331–339. doi: 10.33549/physiolres.930944. [DOI] [PubMed] [Google Scholar]
- 21.Santos JI, Teixeira AL, Dias F, Mauricio J, Lobo F, Morais A, Medeiros R. Influence of peripheral whole-blood microRNA-7 and microRNA-221 high expression levels on the acquisition of castration-resistant prostate cancer: evidences from in vitro and in vivo studies. Tumour Biol. 2014;35:7105–13. doi: 10.1007/s13277-014-1918-9. [DOI] [PubMed] [Google Scholar]
- 22.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The newcastle-ottawa scale (nos) for assessing the quality of nonrandomized studies in meta-analyses. 2011. Available: www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 23.Chen JG, Xia JC, Liang XT, Pan K, Wang W, Lv L, Zhao JJ, Wang QJ, Li YQ, Chen SP, He J, Huang LX, Ke ML, Chen YB, Ma HQ, Zeng ZW, Zhou ZW, Chang AE, Li Q. Intratumoral expression of IL-17 and its prognostic role in gastric adenocarcinoma patients. Int J Biol Sci. 2011;7:53–60. doi: 10.7150/ijbs.7.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He S, Fei M, Wu Y, Zheng D, Wan D, Wang L, Li D. Distribution and clinical significance of th17 cells in the tumor microenvironment and peripheral blood of pancreatic cancer patients. Int J Mol Sci. 2011;12:7424–7437. doi: 10.3390/ijms12117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, Lin Z, Zhu B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun. 2011;407:348–354. doi: 10.1016/j.bbrc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Lv L, Pan K, Li XD, She KL, Zhao JJ, Wang W, Chen JG, Chen YB, Yun JP, Xia JC. The accumulation and prognosis value of tumor infiltrating IL-17 producing cells in esophageal squamous cell carcinoma. PLoS One. 2011;6:e18219. doi: 10.1371/journal.pone.0018219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu FM, Gao Q, Shi GM, Zhang X, Wang J, Jiang JH, Wang XY, Shi YH, Ding ZB, Fan J, Zhou J. Intratumoral IL-17(+) cells and neutrophils show strong prognostic significance in intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2012;19:2506–2514. doi: 10.1245/s10434-012-2268-8. [DOI] [PubMed] [Google Scholar]
- 28.Jain P, Javdan M, Feger FK, Chiu PY, Sison C, Damle RN, Bhuiya TA, Sen F, Abruzzo LV, Burger JA, Rosenwald A, Allen SL, Kolitz JE, Rai KR, Chiorazzi N, Sherry B. Th17 and non-Th17 interleukin-17-expressing cells in chronic lymphocytic leukemia: delineation, distribution, and clinical relevance. Haematologica. 2012;97:599–607. doi: 10.3324/haematol.2011.047316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu T, Peng L, Yu P, Zhao Y, Shi Y, Mao X, Chen W, Cheng P, Wang T, Chen N, Zhang J, Liu X, Li N, Guo G, Tong W, Zhuang Y, Zou Q. Increased circulating Th22 and Th17 cells are associated with tumor progression and patient survival in human gastric cancer. J Clin Immunol. 2012;32:1332–1339. doi: 10.1007/s10875-012-9718-8. [DOI] [PubMed] [Google Scholar]
- 30.Wu J, Du J, Liu L, Li Q, Rong W, Wang L, Wang Y, Zang M, Wu Z, Zhang Y, Qu C. Elevated pretherapy serum IL17 in primary hepatocellular carcinoma patients correlate to increased risk of early recurrence after curative hepatectomy. PLoS One. 2012;7:e50035. doi: 10.1371/journal.pone.0050035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang GQ, Han F, Fang XZ, Ma XM. CD4+, IL17 and Foxp3 Expression in Different pTNM Stages of Operable Non-small Cell Lung Cancer and Effects on Disease Prognosis. Asian Pac J Cancer Prev. 2012;13:3955–3960. doi: 10.7314/apjcp.2012.13.8.3955. [DOI] [PubMed] [Google Scholar]
- 32.Zhuang Y, Peng LS, Zhao YL, Shi Y, Mao XH, Chen W, Pang KC, Liu XF, Liu T, Zhang JY, Zeng H, Liu KY, Guo G, Tong WD, Shi Y, Tang B, Li N, Yu S, Luo P, Zhang WJ, Lu DS, Yu PW, Zou QM. CD8(+) T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology. 2012;143:951–962. e958. doi: 10.1053/j.gastro.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Chen WC, Lai YH, Chen HY, Guo HR, Su IJ, Chen HH. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology. 2013;63:225–233. doi: 10.1111/his.12156. [DOI] [PubMed] [Google Scholar]
- 34.Cui X, Xu Z, Zhao Z, Sui D, Ren X, Huang Q, Qin J, Hao L, Wang Z, Shen L, Lin S. Analysis of CD137L and IL-17 expression in tumor tissue as prognostic indicators for gliblastoma. Int J Biol Sci. 2013;9:134–141. doi: 10.7150/ijbs.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lan C, Huang X, Lin S, Huang H, Cai Q, Lu J, Liu J. High density of IL-17-producing cells is associated with improved prognosis for advanced epithelial ovarian cancer. Cell Tissue Res. 2013;352:351–359. doi: 10.1007/s00441-013-1567-0. [DOI] [PubMed] [Google Scholar]
- 36.Liao R, Sun J, Wu H, Yi Y, Wang JX, He HW, Cai XY, Zhou J, Cheng YF, Fan J, Qiu SJ. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. J Exp Clin Cancer Res. 2013;32:3. doi: 10.1186/1756-9966-32-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang B, Li L, Liao Y, Li J, Yu X, Zhang Y, Xu J, Rao H, Chen S, Zhang L, Zheng L. Mast cells expressing interleukin 17 in the muscularis propria predict a favorable prognosis in esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2013;62:1575–1585. doi: 10.1007/s00262-013-1460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu D, Wu P, Huang Q, Liu Y, Ye J, Huang J. Interleukin-17: a promoter in colorectal cancer progression. Clin Dev Immunol. 2013;2013:436307. doi: 10.1155/2013/436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 41.Novitskiy SV, Pickup MW, Gorska AE, Owens P, Chytil A, Aakre M, Wu H, Shyr Y, Moses HL. TGF-beta receptor II loss promotes mammary carcinoma progression by Th17 dependent mechanisms. Cancer Discov. 2011;1:430–441. doi: 10.1158/2159-8290.CD-11-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tartour E, Pere H, Maillere B, Terme M, Merillon N, Taieb J, Sandoval F, Quintin-Colonna F, Lacerda K, Karadimou A, Badoual C, Tedgui A, Fridman WH, Oudard S. Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev. 2011;30:83–95. doi: 10.1007/s10555-011-9281-4. [DOI] [PubMed] [Google Scholar]
- 43.Ersvaer E, Melve GK, Bruserud O. Future perspectives: should Th17 cells be considered as a possible therapeutic target in acute myeloid leukemia patients receiving allogeneic stem cell transplantation? Cancer Immunol Immunother. 2011;60:1669–1681. doi: 10.1007/s00262-011-1118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 45.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Lau GK, Chen L, Dong SS, Lan HY, Huang XR, Li Y, Luk JM, Yuan YF, Guan XY. Interleukin 17A promotes hepatocellular carcinoma metastasis via NF-kB induced matrix metalloproteinases 2 and 9 expression. PLoS One. 2011;6:e21816. doi: 10.1371/journal.pone.0021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gu FM, Li QL, Gao Q, Jiang JH, Zhu K, Huang XY, Pan JF, Yan J, Hu JH, Wang Z, Dai Z, Fan J, Zhou J. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol Cancer. 2011;10:150. doi: 10.1186/1476-4598-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang GQ, Han F, Fang XZ, Ma XM. [Expression of CD4(+) and IL-17, Foxp3 in non-small cell lung cancer and their correlation with microvessel density] . Zhonghua Zhong Liu Za Zhi. 2012;34:596–599. doi: 10.3760/cma.j.issn.0253-3766.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 50.Chen X, Xie Q, Cheng X, Diao X, Cheng Y, Liu J, Xie W, Chen Z, Zhu B. Role of interleukin-17 in lymphangiogenesis in non-small-cell lung cancer: Enhanced production of vascular endothelial growth factor C in non-small-cell lung carcinoma cells. Cancer Sci. 2010;101:2384–2390. doi: 10.1111/j.1349-7006.2010.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu C, Hao K, Yu L, Zhang X. Serum interleukin-17 as a diagnostic and prognostic marker for non-small cell lung cancer. Biomarkers. 2014;19:287–90. doi: 10.3109/1354750X.2014.908954. [DOI] [PubMed] [Google Scholar]
- 52.Lu L, Pan K, Zheng HX, Li JJ, Qiu HJ, Zhao JJ, Weng DS, Pan QZ, Wang DD, Jiang SS, Chang AE, Li Q, Xia JC. IL-17A promotes immune cell recruitment in human esophageal cancers and the infiltrating dendritic cells represent a positive prognostic marker for patient survival. J Immunother. 2013;36:451–458. doi: 10.1097/CJI.0b013e3182a802cf. [DOI] [PubMed] [Google Scholar]
- 53.Zhao Q, Xiao X, Wu Y, Wei Y, Zhu LY, Zhou J, Kuang DM. Interleukin-17-educated monocytes suppress cytotoxic T-cell function through B7-H1 in hepatocellular carcinoma patients. Eur J Immunol. 2011;41:2314–2322. doi: 10.1002/eji.201041282. [DOI] [PubMed] [Google Scholar]
- 54.Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze MT, Kolls JK, Sasaki H. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 55.Li Q, Han Y, Fei G, Guo Z, Ren T, Liu Z. IL-17 promoted metastasis of non-small-cell lung cancer cells. Immunol Lett. 2012;148:144–150. doi: 10.1016/j.imlet.2012.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


