Abstract
Purpose: This study is to investigate functional role of microRNA 96 (miR-96) in regulating cell survival and caspase-dependent apoptosis in rat retinal ganglion cell line RGC-5 cells. Method: RGC-5 cells were cultured in vitro. Lentiviral vectors of miR-96 inhibitor, miR-96 mimics and non-specific miRNA were applied. The effects of up-regulating or down-regulating miR-96 on cell survival, apoptosis and activation of apoptosis-related caspase proteins were assessed by MTT assay, TUNEL staining and western blotting analysis, respectively. Direct binding of miR-96 and its suspected target caspase protein, caspase 2 (Casp-2), was examined by luciferase reporter assay. Endogenous CASP2 gene was then silenced by siRNA in RGC-5 cells. The effect of down-regulating CASP2 on miR-96 mediated apoptosis in RGC-5 was assessed by western blotting and TUNEL staining. Results: Lentivirus mediated- miR-96 up-regulation significantly reduced RGC-5 cell viability 12 hours after transfection, whereas miR-96 down-regulation had no effect on RGC-5 cell survival up to 72 hours. Up-regulating miR-96 induced significant apoptosis in RGC-5 cells, whereas down-regulating miR-96 had little effect on RGC-5 apoptosis. Western blotting analysis demonstrated that RGC-5 apoptosis induced by overexpressing miR-96 is associated with activation of caspase proteins, including caspase-2, 3, 9. Luciferase reporter assay confirmed that Casp-2 was directly bound by miR-96 in RGC-5 cells, and siRNA-regulated silencing of CASP2 gene rescued the apoptosis in RGC-5 induced by over-expressing miR-96. Conclusion: miR-96 is an important regulator on RGC-5 cell survival and apoptosis, possibly through Casp-2.
Keywords: RGC-5, apoptosis, miR-96, caspase
Introduction
Retinal ganglion cells (RGCs) transmit visual signals into the brain through optic nerve. RGCs could be severely damaged in optic neuropathies, including glaucoma, a gradual but irreversible process of visual loss and blindness characterized by retinal ganglion degeneration [1,2]. RGC-5 is a clonal retinal ganglion cell line, originated from postnatal rat retina with characteristic retinal progenitor markers including Thy-1 Brn-3c and NMDA receptors [3,4]. It has since been established as a common model to study cellular and molecular mechanisms of glaucoma in a self-proliferative and morphologically undifferentiated in vitro system [5,6].
MicroRNAs (miRNAs) are groups of small-stranded, highly conserved, 17 to 23 nucleotides long noncoding RNAs, that mediate gene expression in various types of tissues and organs [7,8]. Studies have demonstrated that groups of miRNAs are differentially expressed in retina, than in other sensory organs, with distinct functions during retinal development either in human or animals [9,10]. Among them, miR-132 regulates neurotrophin-dependent retinal axonal branching and maturation [11], miR-124 is essential for the development of retinal axons and cone cells through targeting lhx2 [12], and miR-218 facilitates vascular patterning in retina by inhibiting Robo-slit pathways [13].
Interestingly, a sensory organ specific miRNA cluster, miR183/96/182, was shown to have synergic effect on retinal synaptogenesis and photoreceptor development [10,14,15], whereas individual member of the cluster, miR-182, is seemingly redundant or non-functional in the retina [16]. It thus prompts the question whether the other two members of the miRNA cluster, miR-183 and miR-96, would have any functional role, or if they do, what exact roles they play, in retina.
In the present study, in order to further elucidate the molecular mechanism of individual miRNA of the cluster, miR-96 in retina, we used lentiviral vector to specifically down-regulate or up-regulate endogenous miR-96 in RGC-5 cells. The corresponding effects of either miR-96 overexpression or knockdown on RGC-5 cell survival and apoptosis were then examined. More importantly, the direct molecular target of miR-96 in RGC-5 cells was probed by bioinformatic method and luciferase reporter assay, and then subsequently down-regulated by siRNA to further understand the associated molecular pathways involved in the regulation of miR-96 in retina.
Materials and methods
Cell culture
The RGC-5 cell line was generated by transformation of postnatal retinal cells from Sprague–Dawley rats at the Tissue & Culture Key Laboratory at Zibo Central Hospital, Zibo, Shandong Province, China, based on the method in previous study [4]. The cells were maintained in low-glucose Dulbecco’s modified Eagle’s medium (DMEM, Gibco, USA) supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin in a humidified tissue culture incubator with 5%/95% CO2/O2 at 37°C. Once reaching ~80% confluence, the cells were passaged by trypsinization in 0.25% Trypsin-EDTA every 2-3 days.
MicroRNA-96 inhibitor/mimics
In order to manipulate the endogenous expression of miR-96 in RGC-5 cells, the oligonucleotides of rno-miR-96-5p inhibitor, rno-miR-96-5p mimics and non-specific control were manufactured by RiboBio (RiboBio Co. Ltd., China). Those miRNA sequences were then coded into lentiviral constructs of pCDH-CMV-MCS-EF1-coGFP (System Biosciences, USA) and co-transfected with pPACK packaging lentiviral vector into HEK-293 cells, to produce miR-96 inhibitor vector (miR-96-Inhibitor), miR-96 mimics vector (miR-96-mimics) and control miRNA vector (miR-CTRL). RGC-5 cells were transfected with miR-96-Inhibitor, miR-96-mimics or miR-CTRL using Lipofectamine 2000 (Invitrogen, USA) per manufacturer’s recommendation.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from RGC-5 cells using the Trizol reagent (Invitrogen, USA) per manufacturer’s instructions. For assessing the expression level of microRNA, quantitative real-time PCR (qRT-PCR) was performed using the TaqMan MicroRNA Reverse Transcription Kit, TaqMan Universal PCR Master Mix (Applied Biosystems, USA), and the corresponding primers. U6 small nuclear was used as an internal control to normalize miR-96 levels. qRT-PCR reactions were conducted in triplicate on a RealPlex4 rt-PCR detection system (Eppendorf, Germany) with following conditions, 7 min at 94°C, and then 37 amplification cycles of 20 s at 95°C, 1 min at 65°C. The threshold cycle (Ct) was defined as the fractional cycle number, and the relative miRNA expression was calculated as 2-ΔΔCt.
MTT assay
RGC-5 cell viability was assessed using a MTT assay. Briefly, RGC-5 cells were cultured in a 96-well plate at a density of 5 × 103 cells/cm2. The lentiviruses of miR-96-inhibitor, miR-96-mimics and miR-CTRL were added to the culture for 2, 4, 6, 12, 48 and 72 hours. After removing the supernatant, 100 ml DMEM with 10% MTT 0.5 mg/mL (Sigma-Aldrich, USA) was added for additional 4 hours 37°C. Then, the formazan crystals were dissolved in 150 ml DMSO (Sigma-Aldrich, USA), and the optical absorption at 570 nm was measured with a Bio-Rad 400 microplate reader (Bio-Rad, USA). Measurements under each experimental condition was repeated at least 3 times, and normalized to the measurement under control condition of miR-CTRL.
Apoptosis assay
The apoptosis of RGC-5 cells was assessed using a terminal deoxynucleotidyl transferase-mediated fluoresceinated dUTP nick-end labeling (TUNEL) assay [17], with an in situ cell death detection kit (Roche, USA) per manufacturer’s instructions. Fluorescence images were collected digitally on an Axiovert 200 fluorescence microscope (Carl Zeiss, Germany), captured with a Photometrics SenSys cooled CCD camera (Roper Scientific, USA). DAPI was used to locate the nuclei of RGC-5 cells (blue). For each experiment, triplicate was conducted and five randomly selected fields were taken into analysis, and the apoptosis of RGC-5 cells was measured as the percentage of TUNEL-positive nuclei among DAPI positive nuclei.
Western blot
RGC-5 cells were lysed in 50 ml ice-cold lysis buffer (Beyotime, China). Lysates were then collected by centrifugation at 15,000 × g for 5 min, and protein concentration was determined by a BCA protein assay kit (Beyotime, China). The total protein were then dissolved in 10% SDS-PAGE gel and transferred to polyvinyli-dene fluoride (PVDF) membranes, which was subsequently blocked by 4% milk in tris-buffered saline containing 0.1% Tween (TBST) for 2 h at RT. They were then incubated over night at 4°C with primary antibodies, Thy-1 (1:2000, Santa Cruz Biotechnology, USA), Caspase-2 (1:2000, Santa Cruz Biotechnology, USA), Caspase-3 (1:2000, Santa Cruz Biotechnology, USA), Caspase-9 (1:2000, Santa Cruz Biotechnology, USA). Horseradish peroxidase-conjugated secondary antibodies (Bio-Rad, USA) were used on second day for 1 h at RT. Actin was used as loading control. The western blots were visualized with an ECL reaction kit (Beyotime, China) and chemiluminescence film (Beyotime, China) per manufacturer’s instruction.
Luciferase reporter assay
Total cDNA from RGC-5 cells was used to amplify the 3’UTR of Casp-2 by regular PCR. With restriction enzymes of Spe I and Hind III, the PCR product was cloned into a pMir-Report (Ambion, USA) to yield wild type luciferase vector Luc-CASP2-WT per manufacturer’s protocol. A mutant CASP-2 (Luc-CASP2-MUT) 3’UTR with inactivated miR-96 binding site (Figure 3A), and a control luciferase plasmid (Luc-Ctrl) were also constructed. Those vectors were than co-transfected with β-galactosidase and miR-96-mimics into HEK293 for 48 hours. Relative luciferase activities were measured using a Dual-Luciferase Reporter Assay System (Promega, USA) and detected by a GloMaxTM 20/20 detection system (Promega, USA).
Figure 3.
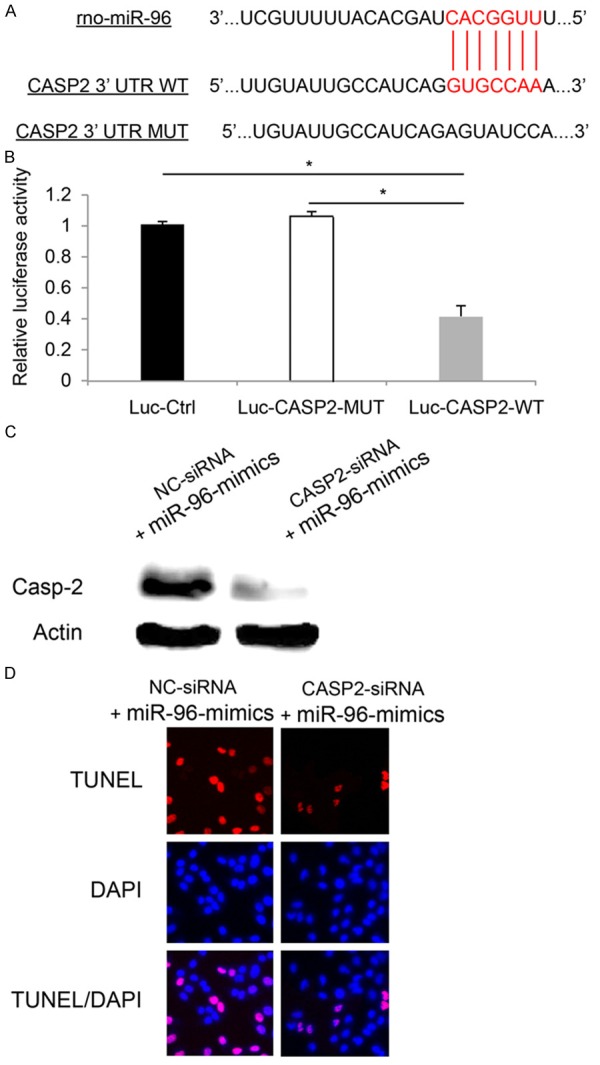
miR-96 targeted CASP2 gene in RGC-5 apoptosis. A. Online screening result from TargetScan showed predicted wild type (WT) 3’UTR binding site of CASP2 by miR-96. A mutant (MUT) CASP2 3’UTR was also shown. B. HEK 293T cells were transfected with control luciferase plasmid (Luc-Ctrl), luciferase plasmid containing mutant CASP2 3’UTR (Luc-CASP2-MUT), or plasmid containing wild type CASP2 3’UTR (Luc-CASP2-WT). Luciferase activities were measured 24 hours after transfection (*: P < 0.05). C. RGC-5 cells were transfected with 100 nM CASP2 specific siRNA (CASP2-siRNA), or 100 nM non-specific siRNA (NC-siRNA) for 24 hours, followed by lentiviral transfection of miR-96-mimics for another 24 hours. Western blotting was used to measure the protein level of Casp-2 in RGC-5 cells. D. TUNEL staining was performed to examine the apoptosis in miR-96 upregulated RGC-5 cells pre-treated with CASP2-siRNA or NC-siRNA.
CASP2 silencing
The silencing of CASP2 gene in RGC-5 cells was performed by siRNA transfection. CASP2 siRNA (CASP2-siRNA, 100 nM) and its control siRNA (NC-siRNA, 100 nM) were purchased from Ambion (Ambion, USA), and transfected into RGC-5 cells by Lipofectamine 2000 per manufacturer’s recommendation. The efficiency of siRNA silencing was confirmed by western blotting 48 hours after transfection.
Statistical analysis
All data are shown as the mean ± S.E.M. Statistical analyses were performed on SPSS software (version 16.0) using student’s t-test. A P-value of < 0.05 was considered statistically significance.
Results
Upregulation of miR-96 decreased RGC-5 cell viability
Recent studies had demonstrated that the miRNA cluster of miR-183/96/182 played important role in retinal development [14,15], yet the exact role of individual miRNA, miR-96, remains elusive. In the present study, we first investigated whether miR-96 had direct effect on the development of retinal precursor cell, RGC-5 cells in vitro. We cultured RGC-5 cells in 6-well plate, then used lentiviruses to either down-regulate or upregulate endogenous miR-96.
First, the efficiency of lentiviral transfection was checked by quantitative real-time PCR (qRT-PCR) in 24 hours. The results showed that, as compared to the miR-96 expression level in RGC-5 cells transfected with lentiviral vector of non-specific control miRNA (miR-CTRL), the expression level of miR-96 was significantly down-regulated in cells transfected with miR-96-inhibitor, and up-regulated in cells transfected with miR-96-mimics (Figure 1A). Thus, our results confirmed that lentiviral vectors were efficient in up-regulating or down-regulating the endogenous expression level of miR-96 in RGC-5 cells.
Figure 1.
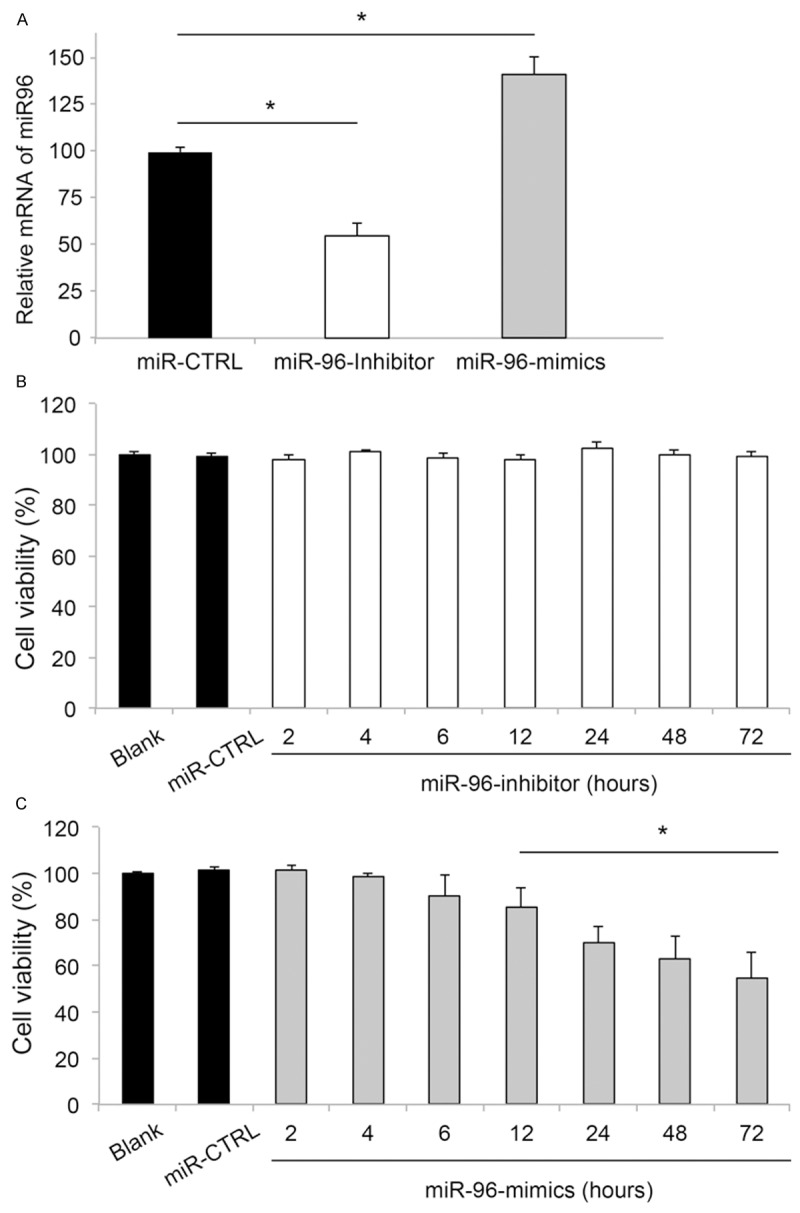
The effects of miR-96 down-regulation and up-regulation on RGC-5 viability. (A) RGC-5 cells were transfected with lentiviral vectors of miR-96 inhibitor, miR-96 mimics, non-specific control miRNA (miR-CTRL), or without lentiviral transfection (Blank). Twenty-four hours after transfection, the mRNA expression levels of miR-96 were examined by qRT-PCR (*: P < 0.05). Time-dependent cell viability was assessed by MTT assay for RGC-5 cells transfected with miR-96-inhibitor (B), or miR-96-mimics (C). Blank RGC-5 cells, or RGC-5 cells transfected with miR-CTRL were also examined (*: P < 0.05, vs. Blank).
We then examined the effect of changing miR-96 expression level on RGC-5 cell survival. We used a MTT assay and transfected RGC-5 cells with either miR-96-inhibitor or miR-96-mimics. For comparison, control RGC-5 cells were transfected with miR-CTRL or without lentiviral transfection (Blank). Cell viability was measured 2, 4, 6, 12, 24, 48 and 72 hours after transfection. The results showed that down-regulating miR-96 had no effect on RGC-5 cell viability (Figure 1B). However, up-regulating miR-96 resulted in significant decrease in RGC-5 cell viability, starting from 12 hours after transfection (Figure 1C).
Upregulation of miR-96 induced RGC-5 apoptosis
Since we found that upregulating miR-96 decreased RGC-5 cell viability, we wondered whether it also had effect on RGC-5 cell apoptosis. To examine this hypothesis, we transfected cultured RGC-5 cells with miR-96-mimics to up-regulate miR-96 and used a TUNEL assay to examine RGC-5 cell apoptosis 24 hours after transfection. For comparison, TUNEL assay was also applied in RGC-5 cells transfected with miR-96-inhibitor or miR-CTRL. The results demonstrated that, transfection of lentiviral vectors of miR-CTRL or miR-96-inhibitor had little effect on RGC-5 cell apoptosis, with little or no TUNEL positive cells observed in the culture (Figure 2A). On the other hand, considerable amount of TUNEL positive RGC-5 cells were observed in the culture transfection of miR-96-mimics (Figure 2A). Quantitative measurement confirmed that the percentage of apoptotic cells was significantly higher in RGC-5 cells with miR-96 up-regulation, as compared to the percentages of apoptotic cells in RGC-5 transfected with lentiviruses containing control vector or miR-96-inhibitor (Figure 2B, *: P < 0.05).
Figure 2.
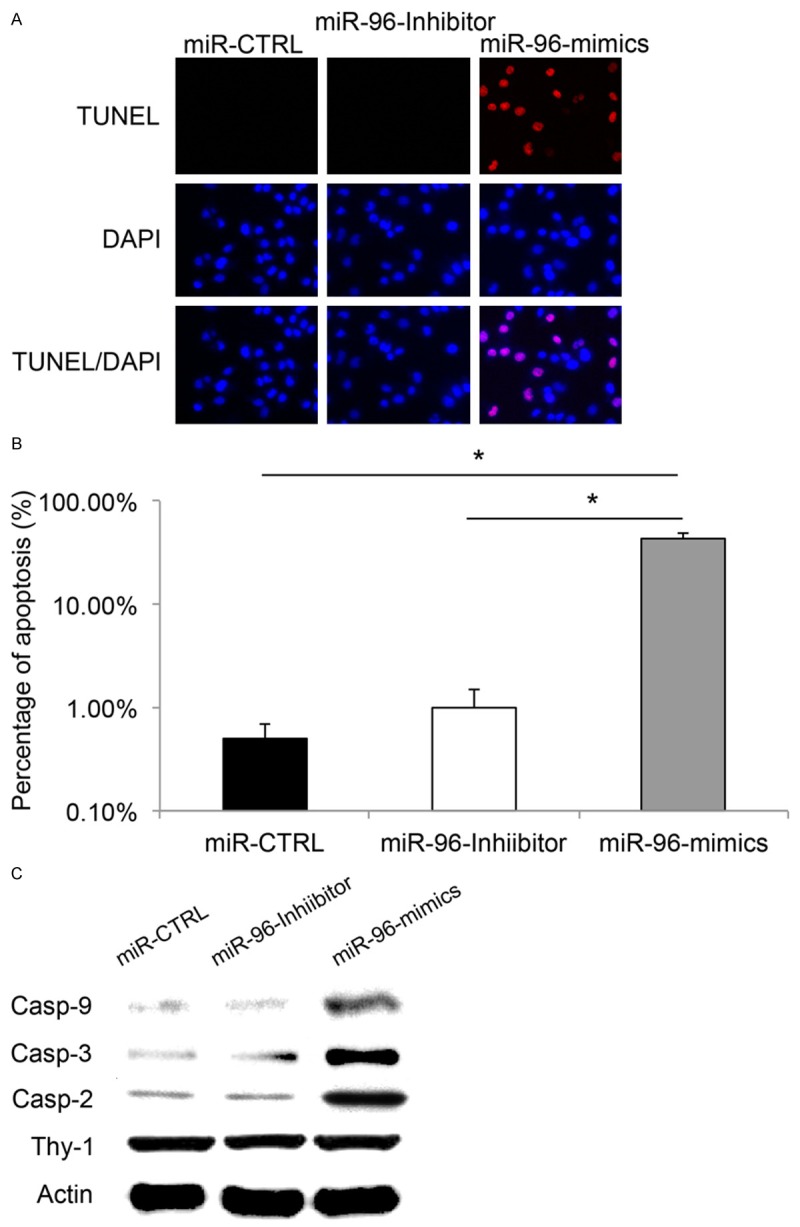
The effects of miR-96 down-regulation and up-regulation on RGC-5 apoptosis. RGC-5 cells were transfected with lentiviral vectors of miR-96 inhibitor, miR-96 mimics, or non-specific control miRNA (miR-CTRL) for 24 hours. A. TUNEL staining (Red) was used to identify the apoptotic RGC-5 cells. DAPI staining (Blue) was used to identify RGC-5 nuclei. B. Apoptosis was quantified by estimating the percentage of TUNEL positive cells against DAPI positive cells (*: P < 0.05). C. Apoptosis related caspases, including casp-2, casp-3 and casp-9 were examined by western blotting analysis 48 hours after transfection. Specific RGC marker, Thy-1 was also probed.
As caspase proteins are often associated with apoptosis in retina development, we then asked whether the regulation on miR-96 in RGC-5 would also induce the production of apoptosis-related caspases. Forty-eight hours after lentiviral transfection of miR-CTRL, miR-96-inhibitor or miR-96-mimics, RGC-5 cells were lysed and western blotting analysis was performed. The results showed that, casp-2, casp-3 and casp-9 were significantly up-regulated in RGC-5 cells transfected with miR-96-mimics, as compared to the expression levels in cells with miR-CTRL or miR-96-inhibitor, while the total proteins of RGC-5 cells (indicated by Thy-1 western blotting) were kept unchanged among all cells (Figure 2C, *: P < 0.05).
Caspase-2 was involved in miR-96 related RGC-5 apoptosis
Finally, we wondered whether caspases were directly involved in RGC-5 induced by miR-96 upregulation. Using online miRNA target prediction software TargetScan (http://www.targetscan.org), we found that casp-2 was a possible down-stream target of miR-96 in RGC-5 (Figure 3A). Using a luciferase reporter assay, we confirmed that in RGC-5 cells, CASP2 gene was the direct target of miR-96 (Figure 3B, *, P < 0.05).
We then used siRNA technology to genetically silence CASP2 gene in miR-96 up-regulated RGC-5 cells. The specificity of siRNA knockdown was examined by western blotting. The results showed that in miR-96 up-regulated RGC-5 cells, CASP2 specific siRNA (CASAP2-siRNA) significantly reduced the product of Casp-2 protein, as compared to the protein level in cell treated non-specific siRNA (NC-siRNA) (Figure 3C).
After that, we examined the effect of knocking down CASP2 gene on miR-96 mediated RGC-5 apoptosis. We pre-transfected RGC-5 cells with either CASP2-siRNA (100 nM) or NC-siRNA (100 nM) for 24 hours. After washing RGC-5 three times with normal cultured medium, we transfected RGC-5 cells with miR-96-mimics for another 24 hours to induced apoptosis (as shown in Figure 2). Our results showed that, significantly less apoptotic RGC-5 cells were observed while CASP2 gene was down-regulated in miR-96 over-expressed RGC-5 cells (Figure 3D), suggesting that inhibiting CASP2 reduced the apoptotic effect induced by miR-96 upregulation in RGC-5 cells.
Discussions
A sensory organ specific miRNA cluster miR182/96/183 has been demonstrated to play important role in retinal development [14,15]. However, the individual miRNA of the cluster, miR-182 seems to be redundant or non-functioning in retina [16]. In the present study, we examined whether miR-96, another member of the miRNA cluster, might have functional role in regulating retinal ganglion precursor cell line RGC-5 cells. We discovered that up-regulating miR-96 had an apoptotic effect to induce apoptosis and reduce cell viability in RGC-5 cells, thus highlighting a first ever report on the functional role of miR-96 in retina.
In the peripheral sensory system, miR-96 mutation has been show to associate with nonsyndromic progressive hearing loss [18,19]. Thus, our results of miR-96 regulating cell survival and apoptosis in RGC-5 cells are in line with those studies, confirming the functional role of miR-96 in sensory system. Intriguingly, unlike the previous study showing mutation of miR-96 in human patients did not yield specific visual impairment [18], our report did show a non-redundant role of miR-96 in rat retinal tissues. The explanation of the difference could be two fold. First, there might be a species difference in term of the exact mechanism of miR-96 in mammalian sensory systems, as human form of miR-96 is redundant and non-functional whereas rat miR-96 is essential and functional. Second, the redundancy of miR-96 could be just one dimensional as mutation (as shown in human), or down-regulation of miR-96 (shown in our study) had no effect on visual system function, whereas up-regulation of miR-96 (also shown in our study) is anti-proliferative or pro-apoptotic in visual system. Thus, further experiments of over-expressing miR-96 in animals in vivo, or screening human gene mutation of miR-96 overexpression would undoubtedly provide more information on the underlying mechanism of miR-96 regulation in visual system.
Also in the present study, we demonstrated that RGC-5 apoptosis was mediated by miR-96 and associated with caspase activation. We also showed that subsequent genetic knocking down of CASAP2 gene rescued the apoptotic effect of miR-96 overexpression. Inhibition of Casp-2 has been demonstrated to protect retinal ganglion from apoptosis [20], and activations of other caspase proteins have been shown to be responsible for retinal degeneration or diabetic retinopathy [21,22]. Our data clearly demonstrated that caspase proteins was the direct down-stream targets of miR-96 regulation in retina, though our conclusive results only limited to one caspase protein, casp-2. Since our results showed that not only Casp-2, but also Casp-3 and Casp-9 were activated by miR-96 upregulation in RGC-5 cells, it would be interesting to investigate the possible interactions between miR-96 and other caspase proteins to know if they are also the direct targets of miR-96 or indirectly involved through Casp-2 activation.
In conclusion, our study revealed a functional role of miR-96, possibly through caspase-dependent apoptosis, in regulating retinal ganglion cells. The results may help to develop new therapeutic strategies in treating patients with retinal disease.
References
- 1.Osborne NN, Melena J, Chidlow G, Wood JP. A hypothesis to explain ganglion cell death caused by vascular insults at the optic nerve head: possible implication for the treatment of glaucoma. Br J Ophthalmol. 2001;85:1252–1259. doi: 10.1136/bjo.85.10.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ju WK, Misaka T, Kushnareva Y, Nakagomi S, Agarwal N, Kubo Y, Lipton SA, Bossy-Wetzel E. OPA1 expression in the normal rat retina and optic nerve. J Comp Neurol. 2005;488:1–10. doi: 10.1002/cne.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal N, Agarwal R, Kumar DM, Ondricek A, Clark AF, Wordinger RJ, Pang IH. Comparison of expression profile of neurotrophins and their receptors in primary and transformed rat retinal ganglion cells. Mol Vis. 2007;13:1311–1318. [PubMed] [Google Scholar]
- 4.Krishnamoorthy RR, Agarwal P, Prasanna G, Vopat K, Lambert W, Sheedlo HJ, Pang IH, Shade D, Wordinger RJ, Yorio T, Clark AF, Agarwal N. Characterization of a transformed rat retinal ganglion cell line. Brain Res Mol Brain Res. 2001;86:1–12. doi: 10.1016/s0169-328x(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 5.Johnson TV, Tomarev SI. Rodent models of glaucoma. Brain Res Bull. 2010;81:349–358. doi: 10.1016/j.brainresbull.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison JC, Cepurna Ying Guo WO, Johnson EC. Pathophysiology of human glaucomatous optic nerve damage: insights from rodent models of glaucoma. Exp Eye Res. 2011;93:156–164. doi: 10.1016/j.exer.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 9.Ryan DG, Oliveira-Fernandes M, Lavker RM. MicroRNAs of the mammalian eye display distinct and overlapping tissue specificity. Mol Vis. 2006;12:1175–1184. [PubMed] [Google Scholar]
- 10.Xu S, Witmer PD, Lumayag S, Kovacs B, Valle D. MicroRNA (miRNA) transcriptome of mouse retina and identification of a sensory organ-specific miRNA cluster. J Biol Chem. 2007;282:25053–25066. doi: 10.1074/jbc.M700501200. [DOI] [PubMed] [Google Scholar]
- 11.Marler KJ, Suetterlin P, Dopplapudi A, Rubikaite A, Adnan J, Maiorano NA, Lowe AS, Thompson ID, Pathania M, Bordey A, Fulga T, Van Vactor DL, Hindges R, Drescher U. BDNF promotes axon branching of retinal ganglion cells via miRNA-132 and p250GAP. J Neurosci. 2014;34:969–979. doi: 10.1523/JNEUROSCI.1910-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanuki R, Onishi A, Koike C, Muramatsu R, Watanabe S, Muranishi Y, Irie S, Uneo S, Koyasu T, Matsui R, Cherasse Y, Urade Y, Watanabe D, Kondo M, Yamashita T, Furukawa T. miR-124a is required for hippocampal axogenesis and retinal cone survival through Lhx2 suppression. Nat Neurosci. 2011;14:1125–1134. doi: 10.1038/nn.2897. [DOI] [PubMed] [Google Scholar]
- 13.Small EM, Sutherland LB, Rajagopalan KN, Wang S, Olson EN. MicroRNA-218 regulates vascular patterning by modulation of Slit-Robo signaling. Circ Res. 2010;107:1336–1344. doi: 10.1161/CIRCRESAHA.110.227926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lumayag S, Haldin CE, Corbett NJ, Wahlin KJ, Cowan C, Turturro S, Larsen PE, Kovacs B, Witmer PD, Valle D, Zack DJ, Nicholson DA, Xu S. Inactivation of the microRNA-183/96/182 cluster results in syndromic retinal degeneration. Proc Natl Acad Sci U S A. 2013;110:E507–516. doi: 10.1073/pnas.1212655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Q, Sun W, Okano K, Chen Y, Zhang N, Maeda T, Palczewski K. Sponge transgenic mouse model reveals important roles for the microRNA-183 (miR-183)/96/182 cluster in postmitotic photoreceptors of the retina. J Biol Chem. 2011;286:31749–31760. doi: 10.1074/jbc.M111.259028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin ZB, Hirokawa G, Gui L, Takahashi R, Osakada F, Hiura Y, Takahashi M, Yasuhara O, Iwai N. Targeted deletion of miR-182, an abundant retinal microRNA. Mol Vis. 2009;15:523–533. [PMC free article] [PubMed] [Google Scholar]
- 17.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mencia A, Modamio-Hoybjor S, Redshaw N, Morin M, Mayo-Merino F, Olavarrieta L, Aguirre LA, del Castillo I, Steel KP, Dalmay T, Moreno F, Moreno-Pelayo MA. Mutations in the seed region of human miR-96 are responsible for nonsyndromic progressive hearing loss. Nat Genet. 2009;41:609–613. doi: 10.1038/ng.355. [DOI] [PubMed] [Google Scholar]
- 19.Lewis MA, Quint E, Glazier AM, Fuchs H, De Angelis MH, Langford C, van Dongen S, Abreu-Goodger C, Piipari M, Redshaw N, Dalmay T, Moreno-Pelayo MA, Enright AJ, Steel KP. An ENU-induced mutation of miR-96 associated with progressive hearing loss in mice. Nat Genet. 2009;41:614–618. doi: 10.1038/ng.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vigneswara V, Berry M, Logan A, Ahmed Z. Pharmacological inhibition of caspase-2 protects axotomised retinal ganglion cells from apoptosis in adult rats. PLoS One. 2012;7:e53473. doi: 10.1371/journal.pone.0053473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C, Li Y, Peng M, Laties AM, Wen R. Activation of caspase-3 in the retina of transgenic rats with the rhodopsin mutation s334ter during photoreceptor degeneration. J Neurosci. 1999;19:4778–4785. doi: 10.1523/JNEUROSCI.19-12-04778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohr S, Xi X, Tang J, Kern TS. Caspase activation in retinas of diabetic and galactosemic mice and diabetic patients. Diabetes. 2002;51:1172–1179. doi: 10.2337/diabetes.51.4.1172. [DOI] [PubMed] [Google Scholar]


