Abstract
The Allium Bulbar Urethral Stent (BUS) is a fully covered, self-expandable, large caliber metal stent specially designed for the treatment of bulbar urethra strictures. The stent is intended for a long term use for the purpose of opening the occluded urethral passage and to allow spontaneous urination. This study objective was to evaluate the clinical efficacy of temporary placement of the Allium BUS stent. This was a prospective study in 54 men with recurrent benign urethral stricture conducted during 2009 to 2012. All men underwent an internal urethrotomy or dilatation procedure followed by an endoscopic stent placement. Clinical success was achieved in 44 (81.4%) of the 54 patients. No patient reported discomfort at the stent site. 2 stents migrated distally. 1 stent was occluded. All stents were removed in a mean time of 8.8 (range 3-18) months following implantation. This experience with the Allium BUS for treating urethral strictures suggests that it is safe and reliable treatment modality.
Keywords: Lower urinary tract symptoms, stents, urethral obstruction, urethral stricture, urehtroscopy
Introduction
Benign urethral stricture is relatively common and management remains a therapeutic challenge for urologists despite recent developments in endoscopic and reconstructive surgery. Most strictures are acquired from injury or infection. Blunt perineal trauma causes injury to the bulbar urethra, pelvic fractures result in urethral distraction defects in the posterior urethra, whereas iatrogenic causes including urologic instrumentation and placing indwelling catheter which result in strictures anywhere throughout the urethra is probably the most common cause [1]. Endoscopic internal urethrotomy is the most common procedure for the treatment of benign urethral stricture and has a 50-60% recurrence rate [2,3]. The urethral stent was first introduced in 1988 for the treatment of recurrent urethral stricture and at that time was indicated for bulbar urethral strictures only [4]. Since 1990, various reports have been published regarding the efficacy of urethral stents. However these devices have a reported long-term efficacy of 63% in patients with recurrent urethral strictures [5]. Retrievable and other types of temporarily placed stents have been also introduced [6,7].
The purpose of our study was to evaluate the clinical efficacy of temporary placement of new bulbar urethral stent (BUS) Allium (Allium, Allium LTD, Caesarea, Israel) in the management of recurrent urethral strictures.
Material and methods
During the period during 2009 to 2012, 57 Allium bulbar urethral stents (BUS) were endoscopically placed in 54 patients with a mean age of 42 (20-64) years with recurrent benign bulbar urethral strictures. Patients who had strictures in other parts of the urethra were excluded.
The causes of the stricture included trauma in 39 patients, surgery in 12 patients, infection in 1 and unknown causes in 2 cases. All patients had a history of one or more internal urethrotomy or dilatation procedure (Table 1). The patients were evaluated with retrograde urethrogram and uroflowmetry. Residual urine volume was estimated with ultrasonography.
Table 1.
Number of patients who had one or more previous internal urethrotomy or dilatation procedure
| Number of previous surgeries | 1 | 2 | 3 | > 3 |
| Number of patients | 6 (11%) | 17 (32%) | 11 (20%) | 20 (37%) |
All patients provided informed consent form and underwent urethral stent placement. Internal urethrotomy was performed prior to stent placement. Stricture length was documented, as well as stricture etiology and prior stricture treatment.
The Allium BUS is a fully covered; self-expandable, large caliber metal stent specially designed for the treatment of bulbar urethral strictures (Figure 1). The stent is comprised of a coiled super-elastic structure covered by a polymeric coating designed to prevent mucosal hyperplasia through the stent wires and into the lumen as well as to reduce encrustation, stone formation and calcification. Stent insertion was done using a gun-like delivery system on which the stent in mounted and is deployed gradually (Figure 2). The patients were placed in lithotomy position under general or spinal anesthesia, internal urethrotomy was first performed and after measuring the length of the stricture, the stent was placed endoscopically, under vision, just beneath the external sphincter. Once inserted into the urethra with the aid of its special inserter, the stent is released to allow its self-expansion. The BUS high radial force along the stent body and soft sphincteric ends were specially designed to fit and adapt to the shape and dimensions of the normal bulbar urethra. A special unraveling feature allows stent retrieval by unraveling it into a thread-like strip and enabling a non-traumatic removal (Figure 3).
Figure 1.

Specially designed Allium BUS; fully covered, self-expandable, large caliber metal stent.
Figure 2.

Gun-like delivery system of Allium BUS on which stent is mounted.
Figure 3.
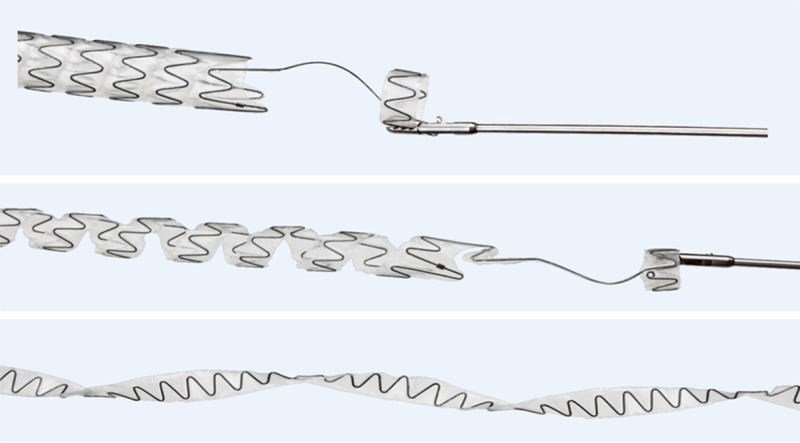
Special unraveling of Allium BUS into a thread-like strip during retrieval.
The indwelling time for the stents was planned to be 12 months. Progressive decreasing of urinary peak flow rate during this time period, recurrent urinary infection and stent migration were the removal criteria.
The success criteria after stent removal were no evidence of stricture on urethrogram or endoscopy, urinary peak flow greater than 15 ml/sec. and no recurrent urinary tract infection. Median follow-up was 8.3 (4-19) months after the last stent removal.
We also investigated the correlation between the success rate of stent indwelling period and stricture length. Mann-Whitney U test was used to compare the groups. A two-sided p < 0.05 was considered to indicate statistical significance.
Results
The mean length of the stricture was 2.44 (1.1-4.3) cm (Table 2). All stents were inserted successfully and positioned correctly just beneath the external sphincter. No adverse events related to the stent or the procedure was recorded.
Table 2.
Number of patients according to length of stricture
| Length of stricture | 1-2 cm | 2-3 cm | 3-4 cm | > 4 cm |
| Number of patients (%) | 39 (72) | 8 (15) | 5 (9) | 2 (4) |
None of the patients reported discomfort at the site of the stent. 3 patients complained of mild early urinary stress incontinence which resolved within one week. All patients reported good urinary flow after stent placement. Whereas the mean maximum urine flow rate was 4.3 ml/sec before the procedure, the mean flow rate five days after the stent placement was 20 ml/sec.
Stents were removed 3 to 18 months after implantation (mean 8.8 months) easily under local anesthesia. There was no post procedure complication. Median follow-up time after the removal of the stent was 8.3 (4-19) months.
Two stents migrated distally, one and three weeks after placement. Severe obstruction was observed in one stent after four weeks of implantation. We replaced these three stents immediately after removal of the previous ones.
The causes for early stent removal in the period of 3 to 11 months after implantation were migration in 6, chronic urinary infection in 4 and progressive decreasing of urinary peak flow rate in 3 patients. One stent remained in the body longer (18 months) than the planned period due to the patients’ late presentation.
Overall success was achieved in 44 (81.4%) of the 54 patients. Longer indwelling time (mean 10.6 months, (9-18)) was statistically related to higher clinical success compared to shorter period (mean 4.8 months (3-8)) (93% and 63.6%, respectively) (p < 0.001).
Clinical success was also related with the length of the stricture. 39 patients with a stricture length < 2 cm had a clinical success rate of 89.7% (35/39), while 15 patients with > 2 cm stricture length had a clinical success rate of 60% (p < 0.001).
Discussion
When urethral stents were first introduced for the management of recurrent urethral strictures, results suggested excellent outcomes [4,8]. The procedure was considered as an option for minimally invasive treatment. However, longer follow-up period began to cast doubt on the usefulness of urethral stenting as a primary treatment modality particularly for urethral strictures [9,10]. High rate of recurrent urethral strictures of up to 45% was observed in the follow-up period [9,11]. In our study, mean follow-up period was 8.8 months and the recurrence rate was 18.6%.
This is the first formal report about the use of this new Allium BUS stent. After longer follow-up period we will also be able to compare long term results. Previously several stents were used for the management of urethral strictures. Urolume (by American Medical Systems) was first introduced in 1988. Despite promising early results with the use of this self-expanding stent, long-term results were discouraging. Complication rates have been reported to be as high as 55%, mostly due to stent obstruction secondary to tissue hyperplasia [5,9,12,13]. These stents lack any coating or full cover, they incorporate into the urethra wall and allow tissue ingrowth, which contributes to a very problematic removal at a later stage. Because it is a permanent stent, it must be removed surgically if a complication necessitates removal [14]. The Allium stent that was used in our study has full polymeric cover to prevent mucosal hyperplasia. It is designed for temporary use and can be removed easily even after several months of implantation. The stent dilates the strictures to the full extent of stent diameter. The Allium BUS stent has an expansile force until it reaches its preset expanded diameter. Several types of temporarily stents have been introduced up to now including fully and non-covered stents all indicating what was describe above [6,7,15].
An important finding of the present study is the correlation between indwelling period of the stent and clinical success. Choi and coworkers reported similar result with the use of retrievable expandable nitinol urethral stent. Majority of the patients in their study needed at least 4 months of stent implantation for achieving successful clinical result [6].
If one reviews the literature critically and focuses on which reports demonstrate good results using urethral stents, it becomes obvious that primarily short strictures (less than 3 cm) have been treated successfully [12]. We have also similar results. Clinical success rate was statistically significantly higher in patients who had strictures less than 2 cm in our study. However, Choi and coworkers [6] reported that the mean stricture length in the patients successfully treated in their study (2.2 ± 0.08 cm) was not statistically significantly different from that of patients who experienced relapse (3.38 ± 2.25 cm) (p = 0.0715).
The most common complication necessitating premature stent removal was stent migration in our study. Stents of 6 patients were removed before 12 months because of migration. In 2 of the patients, stent migration was observed very early, in a few weeks after implantation and stents have been replaced with new ones immediately. Choi and coworkers also agreed that stent migration is a known problem associated with covered stents [6]. Stent encrustation, chronic urinary infection, urethral pain and restenosis are the other complications of stent placement in the longer follow-up period [9,14]. Decrease of urinary peak flow rate due to stent encrustation and chronic urinary infection were the reasons for stent removal in three and four patients respectively before 12 months in our study. Restenosis was also seen in 10 patients after the stent removal. Some authors mentioned that the bulbar urethra has uniform radial forces leading to a better stent fixation and epithelization and therefore, stents should be recommended only for bulbar urethral strictures. Another advantage of the bulbar urethra has been reported to be its rich spongio-fibrous structure that makes it less susceptible to erosion than other parts of the urethra [16]. All stents in our study were also placed in bulbar urethra. The device benefits the majority of our patients besides those with most common complications. When removal is required it can be easily done within a few minutes without any complication.
In conclusion, the main indication for Allium BUS is the management of bladder outlet obstruction caused by bulbar urethral strictures and to avoid restenosis after direct visual internal urethrotomy for urethral stricture. The nature of the biomaterial allows a 12 months indwelling period, flexibility of the metal alloy provides a good compromise between required radial force and patient tolerance, non-incorporation in the urethral wall, lack of overlay or encrustation and ease of removal make it an excellent solution for the treatment of bulbar strictures. Temporary placement of Allium covered stent for an extended duration is effective in inducing resolution of refractory urethral strictures according to this study results. A more extensive experience is necessary with a larger number of patients and a longer follow-up period to further confirm stents’ efficacy.
Disclosure of conflict of interest
None.
References
- 1.Peterson AC, Webster GD. Management of urethral stricture disease: developing options for surgical intervention. BJU Int. 2004;94:971–976. doi: 10.1111/j.1464-410X.2004.05088.x. [DOI] [PubMed] [Google Scholar]
- 2.Pansadoro V, Emiliozzi P. Internal urethrotomy in the management of anterior urethral strictures: long-term followup. J Urol. 1996;156:73–75. [PubMed] [Google Scholar]
- 3.Verges J, Desgrez JP, Claude JM, Cabane H. [Internal urethrotomy. Resection of urethral stricture (over 5 years follow-up)] . Ann Urol (Paris) 1990;24:73–75. [PubMed] [Google Scholar]
- 4.Milroy EJ, Chapple CR, Cooper JE, Eldin A, Wallsten H, Seddon AM, Rowles PM. A new treatment for urethral strictures. Lancet. 1988;1:1424–1427. doi: 10.1016/s0140-6736(88)92238-6. [DOI] [PubMed] [Google Scholar]
- 5.Milroy E, Allen A. Long-term results of urolume urethral stent for recurrent urethral strictures. J Urol. 1996;155:904–908. [PubMed] [Google Scholar]
- 6.Choi EK, Song HY, Shin JH, Lim JO, Park H, Kim CS. Management of recurrent urethral strictures with covered retrievable expandable nitinol stents: long-term results. AJR Am J Roentgenol. 2007;189:1517–1522. doi: 10.2214/AJR.07.2149. [DOI] [PubMed] [Google Scholar]
- 7.Na HK, Song HY, Yeo HJ, Park JH, Kim JH, Park H, Kim CS. Retrospective comparison of internally and externally covered retrievable stent placement for patients with benign urethral strictures caused by traumatic injury. AJR Am J Roentgenol. 2012;198:W55–61. doi: 10.2214/AJR.11.6792. [DOI] [PubMed] [Google Scholar]
- 8.Shaw PJ, Milroy EJ, Timoney AG, el Din A, Mitchell N. Permanent external striated sphincter stents in patients with spinal injuries. Br J Urol. 1990;66:297–302. doi: 10.1111/j.1464-410x.1990.tb14931.x. [DOI] [PubMed] [Google Scholar]
- 9.Hussain M, Greenwell TJ, Shah J, Mundy A. Long-term results of a self-expanding wallstent in the treatment of urethral stricture. BJU Int. 2004;94:1037–1039. doi: 10.1111/j.1464-410X.2004.05100.x. [DOI] [PubMed] [Google Scholar]
- 10.Masood S, Djaladat H, Kouriefs C, Keen M, Palmer JH. The 12-year outcome analysis of an endourethral wallstent for treating benign prostatic hyperplasia. BJU Int. 2004;94:1271–1274. doi: 10.1111/j.1464-410X.2004.05155.x. [DOI] [PubMed] [Google Scholar]
- 11.Konety BR, Phelan MW, O’Donnell WF, Antiles L, Chancellor MB. Urolume stent placement for the treatment of postbrachytherapy bladder outlet obstruction. Urology. 2000;55:721–724. doi: 10.1016/s0090-4295(00)00486-6. [DOI] [PubMed] [Google Scholar]
- 12.Sertcelik N, Sagnak L, Imamoglu A, Temel M, Tuygun C. The use of self-expanding metallic urethral stents in the treatment of recurrent bulbar urethral strictures: long-term results. BJU Int. 2000;86:686–689. doi: 10.1046/j.1464-410x.2000.00891.x. [DOI] [PubMed] [Google Scholar]
- 13.De Vocht TF, van Venrooij GE, Boon TA. Self-expanding stent insertion for urethral strictures: a 10-year follow-up. BJU Int. 2003;91:627–630. doi: 10.1046/j.1464-410x.2003.04200.x. [DOI] [PubMed] [Google Scholar]
- 14.Shah DK, Kapoor R, Badlani GH North American Study Group. Experience with urethral stent explantation. J Urol. 2003;169:1398–1400. doi: 10.1097/01.ju.0000049227.73112.3f. [DOI] [PubMed] [Google Scholar]
- 15.Yachia D, Beyar M. New, self-expanding, self-retaining temporary coil stent for recurrent urethral strictures near the external sphincter. Br J Urol. 1993;71:317–321. doi: 10.1111/j.1464-410x.1993.tb15950.x. [DOI] [PubMed] [Google Scholar]
- 16.Corujo M, Badlani GH. Epithelialization of permanent stents. J Endourol. 1997;11:477–480. doi: 10.1089/end.1997.11.477. [DOI] [PubMed] [Google Scholar]


