Abstract
As a special aphasia, the occurrence of crossed aphasia in dextral (CAD) is unusual. This study aims to improve the language ability by applying 1 Hz repetitive transcranial magnetic stimulation (rTMS). We studied multiple modality imaging of structural connectivity (diffusion tensor imaging), functional connectivity (resting fMRI), PET, and neurolinguistic analysis on a patient with CAD. Furthermore, we applied rTMS of 1 Hz for 40 times and observed the language function improvement. The results indicated that a significantly reduced structural and function connectivity was found in DTI and fMRI data compared with the control. The PET imaging showed hypo-metabolism in right hemisphere and left cerebellum. In conclusion, one of the mechanisms of CAD is that right hemisphere is the language dominance. Stimulating left Wernicke area could improve auditory comprehension, stimulating left Broca’s area could enhance expression, and the results outlasted 6 months by 1 Hz rTMS balancing the excitability inter-hemisphere in CAD.
Keywords: Crossed aphasia in dextral, diffusion tensor imaging, functional magnetic resonance imaging, positron emission tomography, repetitive transcranial magnetic stimulation
Introduction
Aphasia is the main neurological deficit after stroke, with the characteristics of increasing mortality, decreasing rates of functional recovery and probability to return to work, and the incidence rate of aphasia among stroke patients ranges from 21% to 38% [1,2]. Crossed aphasia in dextral (CAD) is defined as aphasia in a right-handed person due to right cerebral lesion. The estimated incidence of CAD reported to be about 3% of all aphasic cases [3,4]. Although the neural mechanisms have not yet been completely understood, there were several proposed explanations as the followings: ① a previous left hemisphere asymptomatic lesion that is symptomatic by a new lesion in the right side; ② ipsilateral control of the dominant hand; ③ bilateral representation of linguistic functions; ④ an arrested developmental stage in the lateralization of language function [5]. Therefore, the first aim of present study is to discover the underlying neural mechanisms for CAD patient by different neuroscience data.
Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive method of applying electromagnetic fields to the brain that can induce alterations of neuronal activity that outlast the stimulation period [6]. Low-frequency rTMS (1 Hz) can decrease the cortical excitability, while high frequency (5 Hz or > 5 Hz) can increase cortical excitability [7]. This potential change has been investigated as adjuvant strategies to neuron-rehabilitative interventions, especially to aphasia, such as CAD [8,9]. Thus, rTMS presents the opportunity to explore more effectively training for CAD patient with cortical stimulation.
Traditional language studies tend to focus on classical language areas like left inferior frontal and superior temporal cortex, which is Wernicke’s and Broca’s areas. Broca’s area is a key region with functions linked to speech production [10], which is now typically defined as the pars opercularis (Pop) and pars triangularis (PTr) of the inferior frontal gyrus of the dominant hemisphere (i.e., BA44 and 45) [11]. The recent neuron-imaging studies have shown that areas 44 and 45 respectively play different functional roles in language comprehension and action recognition [12]. Therefore, the functional connectivity map of CAD patient with BA44 and BA45 as seeds may reveal the dysfunction of language network.
In the present study, a CAD patient had no signal improving after ordinary language therapy for one month. Functional magnetic resonance imaging (fMRI), diffusion tensor imaging (DTI) and positron emission tomography (PET) were used to determine the neuron-cognitive data. We also try to improve language ability by applying 1 Hz rTMS.
Case report
Participants
A 54-year-old man was admitted to our center following the left-sided weakness and speech difficulties for two months. The language evaluation with Chinese version of the Western Aphasia Battery (WAB) [13] showed severe oral and text comprehension disturbances, reading and writing disability, spontaneous and fluent language (repeated and no meaning), word-finding disturbances and naming impossible (Table 1). MRI showed the right frontal, temporal and parietal cerebral infarction (Figure 1), the right side of the M2 segment of middle cerebral artery occlusion. The patient is strong dextromanuality according to the Chinese handness scale [14]. According to the criteria (aphasia, the right hemisphere lesion, strong right-handedness with no left-handedness in the family, no early brain damage, no tonal and ideographic languages, no bilingualism or illiteracy), the case was diagnosed as CAD [15]. Moreover, a control subject with equal age, education and normal language ability was recruited (age = 53, language ability is normal).
Table 1.
Cognition and language test pre and post rTMS
| Test | pre-rTMS | AQ | post-WrTMS | AQ | post-BrTMS | AQ | 6 months post-rTMS | AQ |
|---|---|---|---|---|---|---|---|---|
| WAB | ||||||||
| Fluency | 8 | 8 | 11 | 11 | 13 | 13 | 14 | 14 |
| Auditory | 45 | 2.25 | 82 | 4.1 | 97 | 4.85 | 126 | 6.3 |
| Comprehension | 86 | 8.6 | 100 | 10 | 100 | 10 | 100 | 10 |
| Repetition | 0 | 0 | 2 | 0.2 | 35 | 3.5 | 69 | 6.9 |
| Naming | 37.7 | 51.6 | 62.9 | 74.4 |
rTMS: repetitive transcranial magnetic stimulation; WAB: Western Aphasia Battery (Chinese Version); AQ: Aphasia quotient.
Figure 1.
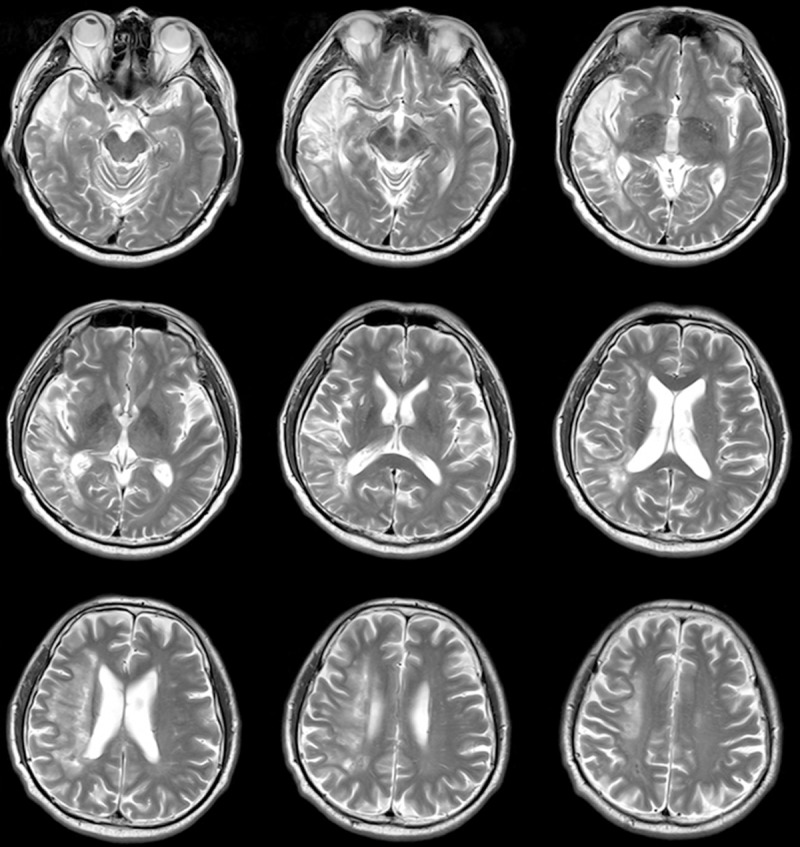
Distribution of the lesion areas. MRI images (T2) showed lesion at the right frontal-temple-parietal lobe infarction.
There is only one patient and one control subject in the present study. Therefore, we claimed here that any neuron-imaging changes observed are anecdotal.
Data acquisition
All data (DTI, fMRI and PET) were acquired using an integrated PET/MR scanner (Biograph mMR, Siemens). The PET (administration of 18F-FDG) scanner with an average spatial resolution of 4.2 mm and with a maximum sensitivity of 13.17 kcps/MBq at the center of FOV. Diffusion was measured in 30 isotropically distributed directions using echo-planar imaging (TE = 90 ms, TR = 9700 ms, FOV = 230 × 221, slice thickness is 3 mm) using a b-value of 1000 s/mm2. The blood oxygen level-dependent (BOLD) fMRI data were acquired using the same scanner (TE = 82 ms, TR = 4540 ms, FOV = 230 × 221, slice thickness = 5 mm).
DTI data preprocessing
The DTI data were corrected for eddy currents and head motion by affine registration to the non-weighted image (b0 image). Then, the skull was subsequently removed and the probabilistic connectivity distributions were derived with region of interest (ROI) as seeds using dMRI probabilistic tractography [16]. All these steps were performed by FSL [17].
Resting state fMRI data preprocessing
The fMRI data was pre-processed using steps of head motion correction, spatially smoothing (FWHM: 6 mm), temporally band-pass filtering (0.005 Hz-0.1 Hz), removal of linear and quadratic trends, regression out of a set of nuisance signals and linear registration to MNI space with a spatial resolution of 3 × 3 × 3 mm3.
Extraction of seed regions of interest
Bilateral BA44/45, two in each hemisphere, was selected as seed regions of interest (seed ROIs) from Juelich Histological Atlas with each voxel having probability above 25%.
Structural connectivity analysis
The probabilistic connectivity distributions were derived with BA44/45 as seeds using dMRI probabilistic tractography [16]. Then, structural connectivity pattern were identified based on connectivity distributions cut off by threshold of P < 0.0005.
Functional connectivity analysis
Mean time series of voxels in the seed ROI were used as seed signals for calculating functional connectivity pattern of the whole brain based on Pearson correlation [18], respectively. The resulting whole brain voxel-wise Pearson correlation coefficients were then transformed to z values using Fisher’s z transform for statistical test.
rTMS stimulation
rTMS was performed with a Magstim Rapid 2 stimulator (Magstim Company) equipped with an air-cooled figure-of eight coil (each loop 70 mm in diameter). The patient was seated in a chair with limbs relaxed and the head fixed and immobile in a comfortable position. Magnetic stimulation was applied at 90% of the resting motor threshold (RMT) at 1 Hz frequency, 600 trails each day for 5 days per week. There were totally 4 weeks for left homologous Wernicke area and another 4 weeks for left homologous Broca’s area. The coil was placed tangentially to the scalp [19].
Results
DTI results
By using bilateral BA44 or BA45 area as seed, the similar structural connectivity maps were obtained. For both of the CAD patient and the control subject, the BA44/45 area contains fibers linking the inferior frontal lobe with other prefrontal lobe areas. Compared with the normal right-handed control subject, the CAD patient showed significantly reduced structural connectivity, especially at the injury ipsilateral. Specifically in CAD patient, there were only few connections between the BA44/45 to superior-frontal, middle-frontal, inferior-frontal, pre-central, temporal lobe, putamen and callosum (Figures 2 and 3).
Figure 2.
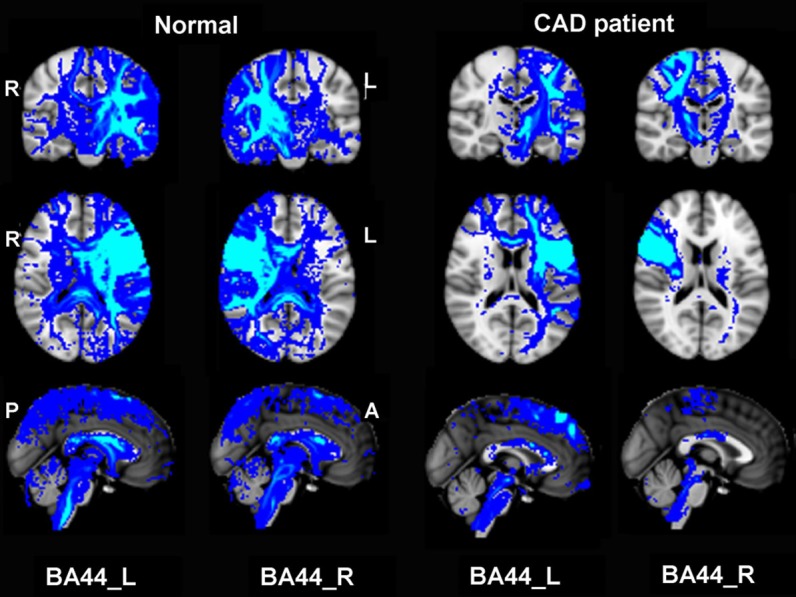
Structural connectivity patterns for BA44. Structural connectivity pattern in patient and normal was identified based on structural connectivity distributions cut off by threshold of P < 0.0005.
Figure 3.
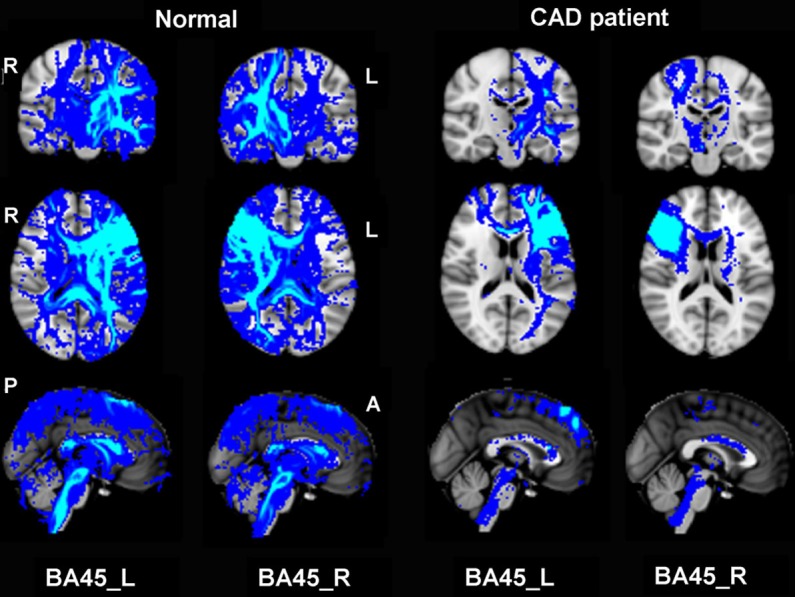
Structural connectivity patterns for BA45. Structural connectivity pattern in normal and patient was identified based on structural connectivity distributions cut off by threshold of P < 0.0005. CAD patient showed significantly reduced structural connectivity, especially at the injury ipsilateral.
Resting-fMRI results
Both patient and control subject showed brain areas positively or negatively correlated with BA44/45 (Figures 4 and 5). In general, for the normal right-handed case, the BA44/BA45 FC map indicated functional connectivity with widespread brain areas over bilateral frontal-sup, middle, sup-motor areas, temporal, parietal, occipital and cerebellum. However, for the CAD patient, there were only a few connectivity with frontal, temporal, parietal, occipital, and cerebellum. More specifically, both patient and normal subject indicated that BA44/45 activation was positively correlated to inferior frontal gyrus, precentral gyrus areas, superior frontal gyrus and negatively correlated to cingulum, temporal, cerebrum and occipital activation. Notably, the major functional connectivity difference between the two subjects was that, when treating brodmann 44, 45 areas of injury ipsilateral as seed areas, both of the positively and negatively functional connectivity to the contralateral hemisphere were obviously disrupted (Figures 4 and 5).
Figure 4.
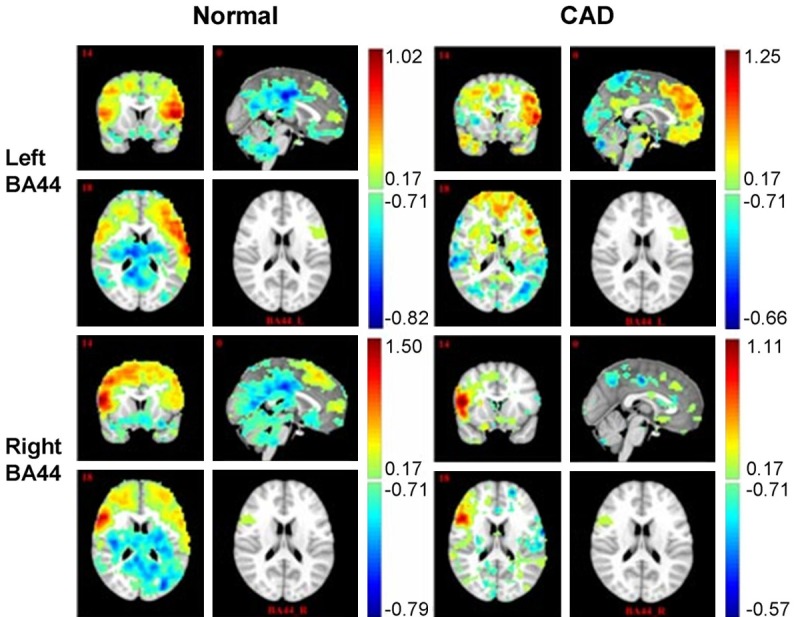
Functional analysis for BA44. CAD patient showed significantly reduced functional connectivity (both positive and negative connectivity), especially at the injury ipsilateral.
Figure 5.
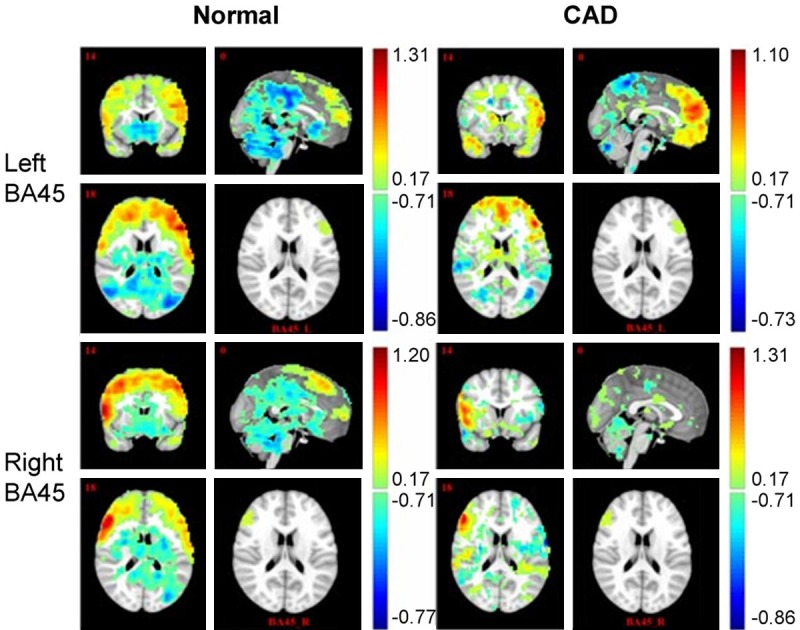
Functional analysis for BA45. Brain regions with statistically significant functional connectivity for BA44/45 were identified (P < 0.01 with cluster size > 50 voxels). CAD patient showed significantly reduced functional connectivity (both positive and negative connectivity), especially at the injury ipsilateral.
PET results
The PET image of the patient was presented in Figure 6 and it showed that hypo-metabolism in right hemisphere and left cerebellum, exceeding the injury range.
Figure 6.
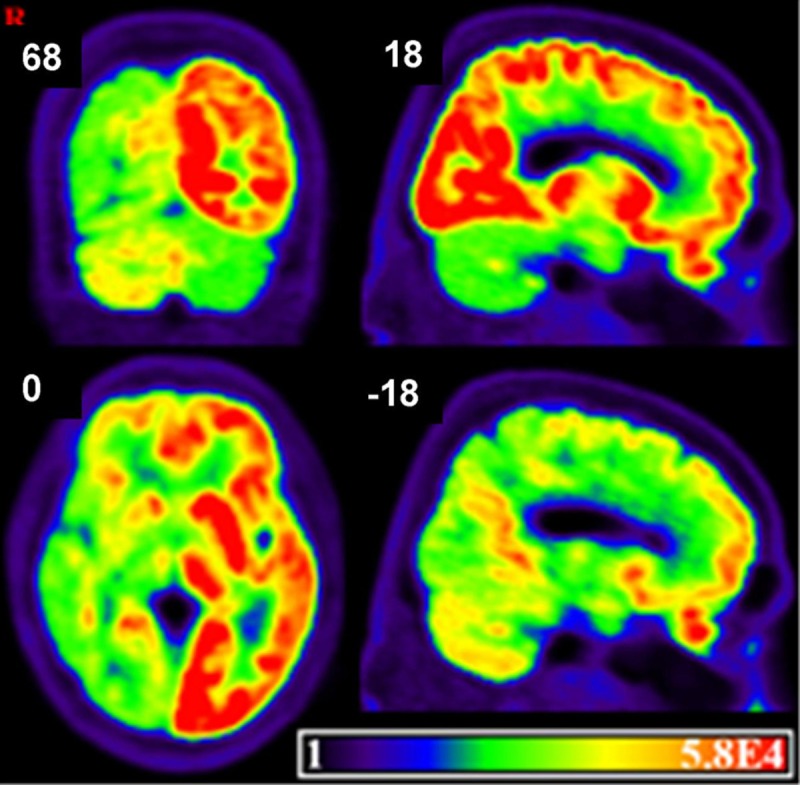
Metabolism of the brain in CAD patient. PET showed that hypo-metabolism in right hemisphere and left cerebellum, exceeding the injury range.
Clinical results
The clinical measurement results of language function are showed in Table 1. The results indicated that the language performance was improved after rTMS. Moreover, the ability of auditory comprehension was significantly improved by auditory comprehension apasia quotient (AQ) from 2.25 to 4.1 after stimulating left Wernicke area. Furthermore the naming ability was also improved by naming AQ from 0.2 to 3.5 after stimulating left Broca’s area. Additionally, the continually increasing of AQ from 62.9 to 74.4 was found during 6 months cessation of rTMS.
Discussion
Multiple modality image technology provides convenient approaches to study brain structure, function and metabolic networks, but we have not found its application in CAD. Hickok proposed the dual-stream model on the functional anatomy of speech processing [20,21]. The ventral stream, involving structures in the superior and middle portions of the temporal lobe, is involved in processing speech signals for comprehension (mapping from sound to meaning) and with bilaterally organized. The dorsal stream, involving brain areas in the posterior planum temporal region and posterior frontal lobe, is involved in translating acoustic speech signals into articulatory representations, which is essential for speech production (mapping from sound to action). From the DTI results of the normal case, the structural connectivity was basically in line with the dual-stream model. Especially, the fibers of BA44/45 areas linked to the temporal area and the extensive frontal areas. Furthermore, the fibers from left Broca’s area to right hemisphere reflect language connectivity between both hemispheres. For the patient, however, the fiber from Brodmann44/45 areas significant disrupted besides to frontal-sup, mid, inf and precentral. Such dysfunction can be interpreted by the good repetition ability and poor auditory comprehension according to the dual-stream model. The link of anatomy and language function also supported the viewpoint of Berthier, which agrees that the arcuate fasciculus (AF) is not the fundamental to the neural circuitry of repetition ability [22,23]. What’s important is that repetition can be done by translating sound into motor speech without linking to the conceptual system [21,24]. Considering the patient with the language function impediment, which may reveal the fact that right hemisphere is the language dominance for the CAD patient.
During resting states, there are many automatic activities of neurons, which constitute a default-mode network (DMN) [25,26]. Hampson et al. [15] found the positively functional connection between Broca’s area and Wernicke’s area, and the connection between Broca’s area and left premotor cortex. Still there was a negative correlation between activity in Broca’s area and the posterior cingulate cortex during a passive listening task [15]. Such result could be interpreted by the hypothesis of resources reallocation. The activated brain areas were responsible for performing the tasks, while the negative activated brain areas responsible for transferring and redistributing resources in the brain [27]. Furthermore, the functional connectivity abnormalities may reflect cognitive impairments of corresponding DMN regions, and improving integration is concurrent with language improvement [28]. For our case, active and deactivate areas, which are the part of language network, were seriously decreased in resting state fMRI imaging. We concluded that the seriously disrupted functional connectivity from right BA44/45 areas were the results of the injured dominant language areas and the exciting-inhibitory imbalance of inter-hemispheres after brain damage (transcallosal dis-inhibition) [29,30]. Because of different brain areas for specific functions and the lateralization of high-order functions, the neurons with specific tasks must inhibit neighboring neurons uninvolved in the task. Therefore, the change in excitability in adjacent and contralateral homotopic regions of a cortical lesion is a consequence of reduced collateral and transcallosal inhibition [29].
PET imaging showed that diffuse hypo-metabolism in the ipsilateral cerebrum and in the contralateral cerebellum, exceeding the size of the infarction, which is in line with the previous results of subcortical aphasia [31]. It is assumed that cerebellum is involved in the modulation of linguistic functions such as verbal fluency, word retrieval, syntax, reading, writing and meta-linguistic abilities [32,33]. And the fMRI study demonstrated that crossed cerebral and cerebellar language dominance is a typical characteristic of brain organization [34]. For our patient, both functional connectivity of cerebellum and left cerebellum hypo-metabolism present cerebellum linking to language processing, especially for left hemisphere because of the language dominance.
For ordinary aphasics, improvement is dependent on uninjured portions of the language network in left hemisphere and the over-activation in right side may represent a maladaptive strategy [29]. Nevertheless, normalizing the inter-hemispheric excitability within a bi-hemispheric language network and the reactivation of perilesional areas are the most efficient in regaining language functions [35]. As a noninvasive method, rTMS can restore the imbalance after brain injury and promote brain plasticity by changing the exciting-inhibitory communication of inter-hemisphere [36,37]. Naeser et al. [38] hypothesized that suppression of a cortical region of interest (ROI) in the right hemisphere with 1 Hz rTMS could decrease over-activation and modulate the bilateral neural network for naming. It was verified that applying low frequency rTMS in right Brodmann44/45 areas for acute or chronic stroke patients improved abilities of naming, repetition and description [7]. However, there is no research about improving sensory-dominant aphasia with rTMS except Kakuda’s, who applied 1 Hz rTMS to the Wernicke’s area for two sensory aphasics and comprehension deficits improved [39].
Compared with the normal case, both of the anatomy and function connectivity significant decreased and hypo-metabolism exceeding the focus can prove the language dominance in the right hemisphere. And, these changes may be caused by corpus callosum dis-inhibition, which indicated that rTMS could act as the potential treatment for this CAD. So far, there is only one case of CAD applyed rTMS and enhanced the naming function [8]. We established stimulating left Wernicke area to improve auditory comprehension, and stimulating left Broca’s area to enhance expression. And the results outlasted 6 months by 1 Hz rTMS balancing the excitability inter-hemisphere in CAD.
Although the current case study is the first to use resting-state fMRI, DTI and PET to investigate whether rTMS stimulation affects language performance in CAD, one limitation of our study is that this study failed to collect neuron-imaging data after rTMS. If we got that, the pre-post rTMS comparison could provide more information for our hypothesis. Therefore, more studies combing multiple neuron-imaging approaches and rTMS on CAD patient are needed to validate our conclusion.
In conclusion, one of the mechanisms of CAD is that right hemisphere is the language dominance. Stimulating left Wernicke area could improve auditory comprehension, and stimulating left Broca’s area could enhance expression. And the results outlasted 6 months by 1 Hz rTMS could balance the excitability inter-hemisphere in CAD.
Disclosure of conflict of interest
None.
References
- 1.Engelter ST, Gostynski M, Papa S, Frei M, Born C, Ajdacic-Gross V, Gutzwiller F, Lyrer PA. Epidemiology of aphasia attributable to first ischemic stroke: incidence, severity, fluency, etiology, and thrombolysis. Stroke. 2006;37:1379–1384. doi: 10.1161/01.STR.0000221815.64093.8c. [DOI] [PubMed] [Google Scholar]
- 2.Setoodeh R, Hakam A, Shan Y. Cerebral metastasis of cervical cancer, report of two cases and review of the literature. Int J Clin Exp Pathol. 2012;5:710–714. [PMC free article] [PubMed] [Google Scholar]
- 3.Marien P, Paghera B, De Deyn PP, Vignolo LA. Adult crossed aphasia in dextrals revisited. Cortex. 2004;40:41–74. doi: 10.1016/s0010-9452(08)70920-1. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Luo Q, Xiong Z, Liang W, Chen L, Xiong Z. Telmisartan counteracts TGF-beta1 induced epithelial-to-mesenchymal transition via PPAR-gamma in human proximal tubule epithelial cells. Int J Clin Exp Pathol. 2012;5:522–529. [PMC free article] [PubMed] [Google Scholar]
- 5.Bakar M, Kirshner HS, Wertz RT. Crossed aphasia. Functional brain imaging with PET or SPECT. Arch Neurol. 1996;53:1026–1032. doi: 10.1001/archneur.1996.00550100112020. [DOI] [PubMed] [Google Scholar]
- 6.Murdoch BE, Barwood CH. Non-invasive brain stimulation: a new frontier in the treatment of neurogenic speech-language disorders. Int J Speech Lang Pathol. 2013;13:234–244. doi: 10.3109/17549507.2012.745605. [DOI] [PubMed] [Google Scholar]
- 7.Wong IS, Tsang HW. A review on the effectiveness of repetitive transcranial magnetic stimulation (rTMS) on post-stroke aphasia. Rev Neurosci. 2013;24:105–114. doi: 10.1515/revneuro-2012-0072. [DOI] [PubMed] [Google Scholar]
- 8.Jung TD, Kim JY, Lee YS, Kim DH, Lee JJ, Seo JH, Lee HJ, Chang Y. Effect of repetitive transcranial magnetic stimulation in a patient with chronic crossed aphasia: fMRI study. J Rehabil Med. 2010;42:973–978. doi: 10.2340/16501977-0637. [DOI] [PubMed] [Google Scholar]
- 9.Sandrini M, Cohen LG. Noninvasive brain stimulation in neurorehabilitation. Handb Clin Neurol. 2013;116:499–524. doi: 10.1016/B978-0-444-53497-2.00040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantalupo C, Hopkins WD. Asymmetric Broca’s area in great apes. Nature. 2001;414:505. doi: 10.1038/35107134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dronkers NF, Plaisant O, Iba-Zizen MT, Cabanis EA. Paul Broca’s historic cases: high resolution MR imaging of the brains of Leborgne and Lelong. Brain. 2007;130:1432–1441. doi: 10.1093/brain/awm042. [DOI] [PubMed] [Google Scholar]
- 12.Skipper JI, Goldin-Meadow S, Nusbaum HC, Small SL. Speech-associated gestures, Broca’s area, and the human mirror system. Brain Lang. 2007;101:260–277. doi: 10.1016/j.bandl.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shewan CM, Kertesz A. Reliability and validity characteristics of the Western Aphasia Battery (WAB) J Speech Hear Disord. 1980;45:308–324. doi: 10.1044/jshd.4503.308. [DOI] [PubMed] [Google Scholar]
- 14.Zhang ZJ. The manual of behavioral medicine scale. Chin J Behav Med Sci. 2001;Special Issue:378. [Google Scholar]
- 15.Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002;15:247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45:S173–186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 18.Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- 19.Naeser MA, Martin PI, Treglia E, Ho M, Kaplan E, Bashir S, Hamilton R, Coslett HB, Pascual-Leone A. Research with rTMS in the treatment of aphasia. Restor Neurol Neurosci. 2010;28:511–529. doi: 10.3233/RNN-2010-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- 21.Hickok G. The cortical organization of speech processing: feedback control and predictive coding the context of a dual-stream model. J Commun Disord. 2012;45:393–402. doi: 10.1016/j.jcomdis.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berthier ML, Lambon Ralph MA, Pujol J, Green C. Arcuate fasciculus variability and repetition: the left sometimes can be right. Cortex. 2012;48:133–143. doi: 10.1016/j.cortex.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Epstein-Peterson Z, Vasconcellos Faria A, Mori S, Hillis AE, Tsapkini K. Relatively normal repetition performance despite severe disruption of the left arcuate fasciculus. Neurocase. 2012;18:521–526. doi: 10.1080/13554794.2011.633531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhai J, Yu Q, Chen M, Gao Y, Zhang Q, Li J, Wang K, Ji F, Su Z, Li W, Li X, Qiao J. Association of the brain-derived neurotrophic factor gene G196A rs6265 polymorphisms and the cognitive function and clinical symptoms of schizophrenia. Int J Clin Exp Pathol. 2013;6:1617–1623. [PMC free article] [PubMed] [Google Scholar]
- 25.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dariani S, Baluchnejadmojarad T, Roghani M. Thymoquinone attenuates astrogliosis neurodegeneration, mossy fiber sprouting, and oxidative stress in a model of temporal lobe epilepsy. J Mol Neurosci. 2013;51:679–686. doi: 10.1007/s12031-013-0043-3. [DOI] [PubMed] [Google Scholar]
- 27.McKiernan KA, D’Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: an fMRI investigation. Neuroimage. 2006;29:1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcotte K, Perlbarg V, Marrelec G, Benali H, Ansaldo AI. Default-mode network functional connectivity in aphasia: therapy-induced neuroplasticity. Brain Lang. 2013;124:45–55. doi: 10.1016/j.bandl.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006;98:118–123. doi: 10.1016/j.bandl.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Barwood CH, Murdoch BE, Whelan BM, Lloyd D, Riek S, JD OS, O’Sullivan JD, Coulthard A, Wong A. Improved language performance subsequent to low-frequency rTMS in patients with chronic non-fluent aphasia post-stroke. Eur J Neurol. 2011;18:935–943. doi: 10.1111/j.1468-1331.2010.03284.x. [DOI] [PubMed] [Google Scholar]
- 31.Kim YW, Kim HS, An YS. Statistical mapping analysis of brain metabolism in patients with subcortical aphasia after intracerebral hemorrhage: a pilot study of F-18 FDG PET images. Yonsei Med J. 2012;53:43–52. doi: 10.3349/ymj.2012.53.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murdoch BE. The cerebellum and language: historical perspective and review. Cortex. 2010;46:858–868. doi: 10.1016/j.cortex.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 33.De Smet HJ, Paquier P, Verhoeven J, Marien P. The cerebellum: Its role in language and related cognitive and affective functions. Brain Lang. 2013;127:334–342. doi: 10.1016/j.bandl.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Jansen A, Floel A, Van Randenborgh J, Konrad C, Rotte M, Forster AF, Deppe M, Knecht S. Crossed cerebro-cerebellar language dominance. Hum Brain Mapp. 2005;24:165–172. doi: 10.1002/hbm.20077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, Weiller C. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton RH, Sanders L, Benson J, Faseyitan O, Norise C, Naeser M, Martin P, Coslett HB. Stimulating conversation: enhancement of elicited propositional speech in a patient with chronic non-fluent aphasia following transcranial magnetic stimulation. Brain Lang. 2010;113:45–50. doi: 10.1016/j.bandl.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barwood CH, Murdoch BE, Whelan BM, Lloyd D, Riek S, O’Sullivan JD, Coulthard A, Wong A. Modulation of N400 in chronic non-fluent aphasia using low frequency Repetitive Transcranial Magnetic Stimulation (rTMS) Brain Lang. 2011;116:125–135. doi: 10.1016/j.bandl.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Helm-Estabrooks N, Cayer-Meade C, Kobayashi M, Theoret H, Fregni F, Tormos JM, Kurland J, Doron KW, Pascual-Leone A. Improved naming after TMS treatments in a chronic, global aphasia patient--case report. Neurocase. 2005;11:182–193. doi: 10.1080/13554790590944663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kakuda W, Abo M, Uruma G, Kaito N, Watanabe M. Low-frequency rTMS with language therapy over a 3-month period for sensory-dominant aphasia: case series of two post-stroke Japanese patients. Brain Inj. 2010;24:1113–1117. doi: 10.3109/02699052.2010.494587. [DOI] [PubMed] [Google Scholar]


