Abstract
In addition to original role of lowering cholesterol, statins display multiple neuroprotective mechanisms. In this study, 6-Hydroxydopamine (6-OHDA)-treated pheochromocytoma-12 (PC12) cells were used to investigate the neuroprotective nature of lovastatin. After incubation with 6-OHDA and/or lovastatin, test kits were used to detect the levels of LDH and glutamate, which were released from PC12 cells exposed to different culture media. The mRNA levels of TNF-α, and NMDAR1 were determined by RT-PCR and the protein levels were analyzed by western blot. Our results show that lovastatin significantly decreased both the mRNA and the protein levels of TNF-α and NMDAR1. ELISA assays revealed increased lactate dehydrogenase (LDH) and glutamate binding activity in 6-OHDA-lesioned PC12 cells, and this increase could be prevented by lovastatin. Our results suggest that lovastatin induces neuroprotection by inhibiting NMDAR1 and TNF-α. The data provide direct evidence of the potential application of lovastatin for the treatment of parkinson’s diseases.
Keywords: Parkinson’s disease, lovastatin, NMDA receptor1, TNF-α
Introduction
Statins are cholesterol-lowering agents, but more and more effects of statins have been found, including neuroprotection, immunomodulation, and anti-inflammation. It had been demonstrated that lipophilic statin can decrease the incidence of PD [1,2]; however, the exact mechanisms are still unclear. If statins are potentially beneficial for PD, they may present an interesting challenge.
N-methyl-D-aspartate (NMDA) receptors are widespread in the nucleus accumbens, cerebral cortex, striatum, and hippocampus [3,4]. Changes of NMDA receptors are closely associated with many brain functions. Mishizen found that the NMDA receptor binding levels were obviously decreased in the striatum and hippocampus of aged mice and AD patients [5]. In addition, activation of NMDA receptors can increase neuronal excitotoxicity and cause cell death. All these findings strongly suggest that NMDA receptors may play very important roles in neuropsychiatric and movement-related disorders in the brain.
PD is the second neurodegenerative disorder following AD. The dopamine and glutamatergic NMDA receptors have been implicated in many neuropsychiatric disorders. The dopaminergic disturbance may cause glutamatergic NMDA receptor imbalances in the brain [6], and vice versa [7]. In the 6-OHDA-lesioned rat model of PD, D1/NMDA receptor expression was obviously decreased in the lesioned striatum [8]. NMDA receptor antagonists increased dopaminergic neuronal survival and prevented the levodopa-induced abnormal motor response [9]. It will be interesting to find whether the application of Lovastatin can inhibit inflammatory responses by modulating the expression of NMDA receptors in the PD models.
Accordingly, in vitro study was applied to investigate the effect of lovastatin on 6-OHDA-stimulated PC12 cells. This study observed a possible correlation between lovastatin and NMDA receptor1 by using in vitro Parkinsonian cell models.
Materials & methods
Cell culture and treatments
The PC12 cell culture was routinely maintained in RPMI-1640 medium with 5% fetal bovine serum, 10% horse serum, 100 U/ml of benzyl penicillin, and 100 mg/L of streptomycin (Gibco). The cells were grown at 37°C in a humidified atmosphere containing 5% CO2. The cells were fed every 2-3 days and subcultured once they reached 80-90% confluence. For all experiments, the cells were seeded on 96-well plates or 6-well plates at a density of 1.0 × 105 cells/ml for 24 h. The culture cells were treated with 6-OHDA (100 μM, Sigma Chemical Co.), and/or lovastatin (2 μM, Merck & Co Inc) at the same time for 24 h.
MTT cell viability assay
MTT assay was performed to evaluate the PC12 cell viability after 6-OHDA and lovastatin treatment. Briefly, the PC12 cells were grown in 96-well plates (100 μl/well) at a density of 1 × 105 cells/ml. After incubation, the cells were washed once with PBS before adding 100 μl of free serum medium containing MTT (final concentration, 0.5 mg/ml) to each well. After incubation for 4 h, the supernatant was removed, and the cells were washed twice with PBS and the formazan product was dissolved in 100 ml of dimethylsulfoxide (DMSO). The absorbance was read at a wavelength of 570 nm in a microplate reader. The results were expressed as percentage of the control group.
LDH assay and glutamate measurement
LDH assay
The cell viability was also measured by determining the activity of LDH released into the medium when cellular membranes were damaged [10]. The total LDH activity was measured following specifications of In vitro Toxicology Assay Kit LDH based Tox-7 (Sigma-Aldrich, USA). The absorbance was measured in an automatic microplate reader at 490 nm. The data were presented as the percentage of LDH in 6-OHDA group, which was designated as 100%.
Glutamate assay
The concentration of glutamate was measured according to Glutamate Assay Protocol (BioVision, USA). Briefly, 10 μl of the 0.1 M glutamate standard solution was diluted using 990 μl of the assay buffer to generate 1 mM standard glutamate. Subsequently, 0, 2, 4, 6, 8, and 10 μl of the diluted glutamate standard solution were added to a 96-well plate in duplicate to generate 0, 2, 4, 6, 8, and 10 nmol/well standard. The volume was brought to 50 μl with assay buffer. The cells (1 × 106) were homogenized in 100 μl of assay buffer and centrifuged at 13,000 g to remove the insoluble material. Then, 50 μl of the sample was added to well of the 96-well plate. The optical density was measured at 450 nm using a microplate reader. The background was corrected by subtracting the value derived from the 0 glutamate control from all sample readings, and then the concentration of glutamate was calculated according to the instruction.
Quantitative real-time RT-PCR
For quantitative real-time RT-PCR, the total RNA was extracted using TRIzol (Invitrogen), according to the manufacturer’s protocol, and the cDNA was synthesized using primers with the Advantage RT for PCR kit (BD Biosciences). We quantified the PCR amplifications using SYBR Green PCR Master Mix (Applied Biosystems), and the results were normalized to GAPDH gene expression. All the experiments were performed in triplicate and repeated at least three times.
Western blotting analysis
The cells were pretreated with simvastatin and/or 6-OHDA/SiRNA and were rinsed thrice with ice-cold PBS, and incubated in ice-cold lysis buffers for 20 min on ice. Cells were sonicated and centrifuged at 14000 g for 15 minutes. The supernatants were collected for protein determination by BCA assay. The samples were mixed with 5 × Laemmli buffer and heated for 5 min at 100°C. Then, samples of equivalent total protein (30 μg) were run in NuPage Bis-Tris 10% gels (Invitrogen) and transferred to PVDF membrane. The PVDF membranes were incubated with the following primary antibodies: rabbit anti-TNF-α (1:1000), rabbit anti-NMDAR1 (1:800), and rabbit anti-β-actin (1:2000) overnight at 4°C. The next day, the PVDF membranes were washed thrice and horseradish peroxidase-conjugated secondary antibodies were added. Negative controls were prepared by excluding the primary antibodies. The images were analyzed using the alphaview software. The results were expressed as folds of the control group.
Statistical analysis
The data were expressed as mean ± SEM. MTT, LDH, and different protein quantifications with western blot were analyzed using one-way ANOVA, followed by Tukey’s post hoc analysis (SPSS 15.0 program, Chicago, IL). P values of less than 0.05 were regarded as statistically significant.
Results
Effects of 6-OHDA and lovastatin on PC12 cell viability
The cytotoxic effects of Lovastatin were evaluated with the MTT assay by measuring the viability of PC12 cells that were incubated with Lovastatin (0, 1, 2, 3, 4 μM) for 24 h in the absence of 6-OHDA (Figure 1A). We also examined the viability of PC12 cells treated with 2 μM Lovastatin for 6, 12, 24 or 48 h in absence of 6-OHDA. Interestingly, no significant differences in cell viability were found between normal PC12 cells and PC12 cells treated with 2 μM Lovastatin for 24 h (Figure 1B), which indicates that the inhibitory effect that we observed was not due to cytotoxicity.
Figure 1.
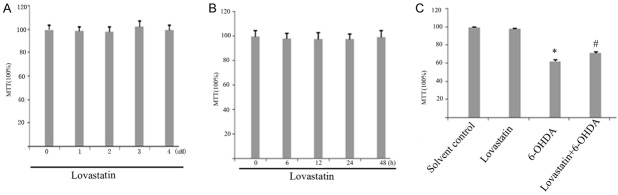
Viability in lovastatin-treated cells was evaluated using the MTT assay. Cells were incubated with lovastatin (1 to 4 μM) (A) and with 2 μM lovastatin for 6 to 48 h (B) and 6-OHDA (100 μM) for 24 h (C). Lovastatin protected PC12 cells against 6-OHDA neurotoxicity. MTT value in 6-OHDA treated group significantly reduced compared with controls (*p < 0.001, 6-OHDA vs solvent controls), but lovastatin upregulated this reduction (#p < 0.001, 6-OHDA vs 6-OHDA + lovastatin), the results are displayed as the percentages of the control samples. The data are presented as the means ± S.E.M. (n = 9) for three independent experiments.
To investigate the effect of 6-OHDA and lovastatin on PC12 cells, we exposed the cells to 6-OHDA (100 uM) and lovastatin (2 μM) for 24 h period of time, and examined a variety of markers of cell death. MTT value in 6-OHDA treated group significantly reduced compared with controls, but lovastatin upregulated this reduction (Figure 1C).
Effects of 6-OHDA and simvastatin on LDH and glutamate
LDH is released from the cells following membrane collapse, and the released LDH is usually considered as a sign of late cell death [10]. Our result showed that LDH increased in 6-OHDA-incubated PC12 cells, when compared with that in controls (Figure 2A), whereas lovastatin incubation prevented this elevation (Figure 2A). Glutamate is the most abundant excitatory neurotransmitter and recognized as another important sign of cell death. Our result indicated that glutamate increased in 6-OHDA-incubated PC12 cells, when compared with that in controls (Figure 2B), whereas lovastatin incubation prevented this elevation (Figure 2B), demonstrating a significant neuroprotective effect of lovastatin in vitro PD model.
Figure 2.
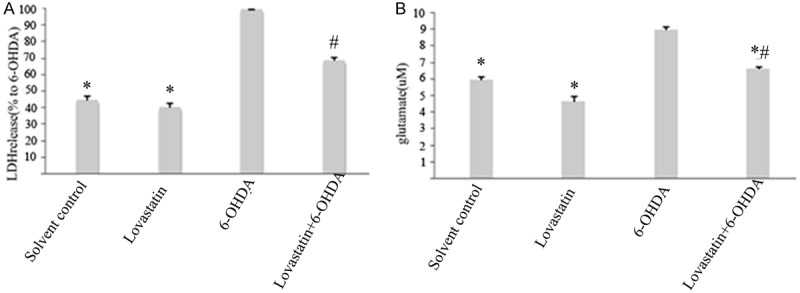
The effects of lovastatin on 6-OHDA-induced LDH and glutamate. Lovastatin reduced 6-OHDA-induced LDH and glutamate (*p < 0.001, 6-OHDA vs solvent controls, n = 9; A), while lovastatin incubation abolished this elevation (#p < 0.001, 6-OHDA vs 6-OHDA + lovastatin, n = 9; A). In 6-OHDA incubated PC12, glutamate was increased compared with controls (6-OHDA vs controls, *p < 0.001; B), while lovastatin treatment abolished this elevation (6-OHDA vs 6-OHDA + Lovastatin, #p < 0.001, B). All the results are expressed as mean ± S.E.M. (n = 9).
Analysis of NMDAR1 and TNF-α mRNA by real-time RT-PCR
Using quantitative RT-PCR, we were able to detect that the expression of NMDAR1 (Figure 3A) and TNF-α (Figure 3B) mRNA was significantly elevated in 6-OHDA mediated PC12 compared with the solvent control, while lovastatin incubation abolished this elevation.
Figure 3.
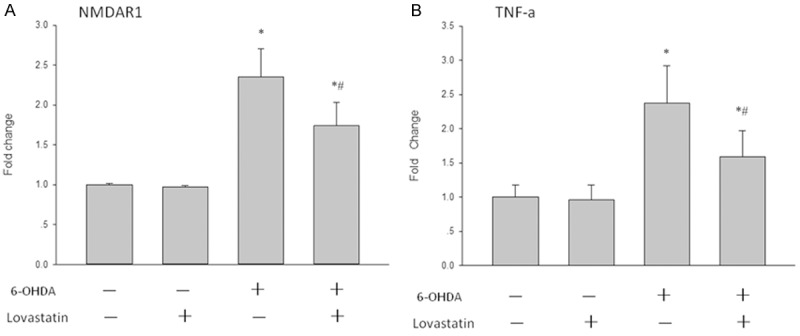
The effects of lovastatin on 6-OHDA-induced TNF-α and NMDAR1 mRNA expression in PC12 cells. The cells were treated with the indicated doses of lovastatin and 6-OHDA. After incubation for 24 h, the mRNA levels of TNF-α and NMDAR1 were determined by RT-PCR. The data are presented as the mean ± S.E.M. (n = 5 wells for each condition) for three independent experiments (*p < 0.001 vs. solvent controls; #p < 0.001 vs. 6-OHDA).
Lovastatin regulates the levels of NMDANR1 receptors and TNF-α in 6-OHDA-treated PC12 cells using western blot analysis
To confirm that the neuroprotection of lovastatin in vitro PD model is associated with NMDA receptors, NMDANR1 receptors were measured by western blot. After 24 h, 6-OHDA incubation pronouncedly increased the NR1 receptors compared with controls (Figure 4A); while this elevation was significantly abolished following lovastatin treatment. In order to explore if the modulation of NR1 receptor following lovastatin treatment is correlated to anti-inflammatory responses, the levels of inflammatory mediators TNF-α were also determined by western blot. Compared with controls, 6-OHDA produced significant increases in the total amount of TNF-α (Figure 4B); however, this elevation was significantly prevented following lovastatin treatment (Figure 4B).
Figure 4.
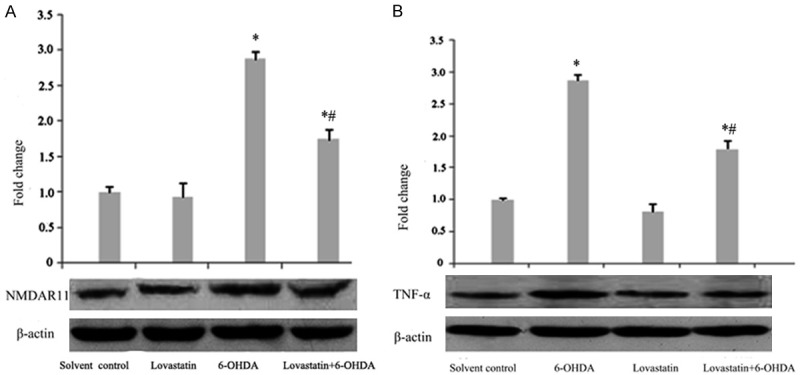
The effects of lovastatin on 6-OHDA-induced TNF-α and NMDAR1 protein expression in PC12 cells. The cells were treated with the indicated doses of lovastatin and 6-OHDA. After incubation for 24 h, the protein levels of TNF-α (B) and NMDAR1 (A) were analyzed by western blot. The data are presented as the mean ± S.E.M. (n = 5 wells for each condition) for three independent experiments (*p < 0.001 vs. solvent controls; #p < 0.001 vs. 6-OHDA).
Discussion
The study aimed to investigate the neuroprotective effect of Lovastatin and explain the mechanisms in experimental Parkinsonian cell models. The cell viability was obviously decreased in 6-OHDA-treated PC12 cells (Figure 1). However, pre-incubation with lovastatin displayed a restoration of reduction of cell viability. In addition, our results showed that LDH and glutamate were significantly increased in 6-OHDA-induced PC12 cells and the elevations were obviously prevented after lovastatin incubation, demonstrating that lovastatin displayed pronouncedly neuroprotective effects. PC12 cells mainly express the functional NR1 receptor, therefore NR1 was chosen to detect the effects of 6-OHDA neurotoxicity in this study. It has been shown that the elevation of NMDA receptors is closely correlated to inflammation responses and induced neuronal death [11-13]. In the current study, the increased NR1 expression and excitatory glutamate concentration following 6-OHDA incubation were observed (Figure 2). This 6-OHDA induced elevation of glutamate excessively activated the increased NMDANR1, subsequently further aggravating PC12 damage [14] and may be involved in the susceptibility to excitotoxicity in PC12 cells. However, the addition of lovastatin significantly abolished this elevation of NR1 and glutamate, accompanying the reduction in PC12 cell death. Considering the elevation of NR1 and glutamate will lead to excitotoxicity and neuronal cell death [15], we reasonably speculated that in the current study lovastatin prevented PC12 cell death, at least partially, via exerting neuroprotective against NR1-induced excitotoxicity. This result showing that the upregulation of NR1 was correlated to neuronal cell death and the abolishment of this NR1 elevation prevented the neuronal loss [16]. The present study is consistent with our previous observation in which simvastatin upregulated NMDA receptors in the naïve rat brain, and further validates our proposal that statins may exhibit NMDA antagonist-like effects [17].
In order to explore if inflammatory mediators in PC12 cells changed following 6-OHDA and lovastatin treatment, we measured the expression of TNF-α. Our study showed increased expression of TNF-α in 6-OHDA-induced PC12 cells, implying these inflammatory mediators involved in our study. The elevations of NR1 and TNF-α were significantly abolished following lovastatin treatment, strongly suggesting a direct anti-inflammatory property of lovastatin through NMDA receptor modulation. TNF-α in pathological brain processes including the mediation of neuronal death [18,19]. In order to further verify that the alteration of NMDA receptors is associated with inflammatory cytokine TNF-α in this study, we focused specifically on 6-OHDA -treated PC12 expressing NR1 and TNF-α protein. We detected a significant increased NR1 protein after 6-OHDA exposure and this increase was abolished following lovastatin treatment; while TNF-α proteins displayed a similar pattern after 6-OHDA neurotoxicity and lovastatin treatment (Figure 4), which demonstrated that NR1 proteins was closely associated with inflammatory cytokine TNF-α following 6-OHDA and lovastatin treatment. This result is consistent with other studies showing that pro-inflammatory mediator TNF-α was involved lovastatin mediated neuroprotection and associated with NMDA receptors [19,20]. To our best of knowledge, this is the first time to describe the correlation of TNF-α and NR1 in PC12.
In summary, our study presents the first evidence demonstrating the effects of lovastatin on NMDA receptors in 6-OHDA lesioned PC12 cells and reveals NMDA modulation effect, which provides an exciting and potential paradigm to ameliorate PD. Our results strongly demonstrated that lovastatin provided robust neuroprotection on dopaminergic neurodegeneration partially via NMDA receptor mediated anti-inflammatory mechanisms.
Acknowledgements
This work was supported by Grant-In-Aid of The First Affiliated Hospital of Henan University of Science and Technology and National Natural Science Foundations. (U1304809) of China from Jun-Qiang Yan.
Disclosure of conflict of interest
None.
References
- 1.Undela K, Gudala K, Malla S, Bansal D. Statin use and risk of Parkinson’s disease: a meta-analysis of observational studies. J Neurol. 2013;260:158–165. doi: 10.1007/s00415-012-6606-3. [DOI] [PubMed] [Google Scholar]
- 2.Lee YC, Lin CH, Wu RM, Lin MS, Lin JW, Chang CH, Lai MS. Discontinuation of statin therapy associates with Parkinson disease: a population-based study. Neurology. 2013;81:410–416. doi: 10.1212/WNL.0b013e31829d873c. [DOI] [PubMed] [Google Scholar]
- 3.Janssen WG, Vissavajjhala P, Andrews G, Moran T, Hof PR, Morrison JH. Cellular and synaptic distribution of NR2A and NR2B in macaque monkey and rat hippocampus as visualized with subunit-specific monoclonal antibodies. Exp Neurol. 2005;191(Suppl 1):S28–44. doi: 10.1016/j.expneurol.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson A, Eriksson M, Muly EC, Akesson E, Samuelsson EB, Bogdanovic N, Benedikz E, Sundstrom E. Analysis of NR3A receptor subunits in human native NMDA receptors. Brain Res. 2007;1186:102–112. doi: 10.1016/j.brainres.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Mishizen-Eberz AJ, Rissman RA, Carter TL, Ikonomovic MD, Wolfe BB, Armstrong DM. Biochemical and molecular studies of NMDA receptor subunits NR1/2A/2B in hippocampal subregions throughout progression of Alzheimer's disease pathology. Neurobiol Dis. 2004;15:80–92. doi: 10.1016/j.nbd.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Hallett PJ, Spoelgen R, Hyman BT, Standaert DG, Dunah AW. Dopamine D1 activation potentiates striatal NMDA receptors by tyrosine phosphorylation-dependent subunit trafficking. J Neurosci. 2006;26:4690–4700. doi: 10.1523/JNEUROSCI.0792-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallett PJ, Standaert DG. Rationale for and use of NMDA receptor antagonists in Parkinson’s disease. Pharmacol Ther. 2004;102:155–174. doi: 10.1016/j.pharmthera.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Fiorentini C, Rizzetti MC, Busi C, Bontempi S, Collo G, Spano P, Missale C. Loss of synaptic D1 dopamine/N-methyl-D-aspartate glutamate receptor complexes in L-DOPA-induced dyskinesia in the rat. Mol Pharmacol. 2006;69:805–812. doi: 10.1124/mol.105.016667. [DOI] [PubMed] [Google Scholar]
- 9.Bibbiani F, Oh JD, Kielaite A, Collins MA, Smith C, Chase TN. Combined blockade of AMPA and NMDA glutamate receptors reduces levodopa-induced motor complications in animal models of PD. Exp Neurol. 2005;196:422–429. doi: 10.1016/j.expneurol.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Blanco J, Martín V, Herrera F, García-Santos G, Antolín I, Rodriguez C. Intracellular signaling pathways involved in post-mitotic dopaminergic PC12 cell death induced by 6-hydroxydopamine. J Neurochem. 2008;107:127–140. doi: 10.1111/j.1471-4159.2008.05588.x. [DOI] [PubMed] [Google Scholar]
- 11.Yeh SH, Hung JJ, Gean PW, Chang WC. Hypoxia-inducible factor-1alpha protects cultured cortical neurons from lipopolysaccharide-induced cell death via regulation of NR1 expression. J Neurosci. 2008;28:14259–14270. doi: 10.1523/JNEUROSCI.4258-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galic MA, Riazi K, Henderson AK, Tsutsui S, Pittman QJ. Viral-like brain inflammation during development causes increased seizure susceptibility in adult rats. Neurobiol Dis. 2009;36:343–351. doi: 10.1016/j.nbd.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki J, Kofuji S, Itoh R, Momiyama T, Takayama K, Murakami H, Chida S, Tsuya Y, Takasuga S, Eguchi S, Asanuma K, Horie Y, Miura K, Davies EM, Mitchell C, Yamazaki M, Hirai H, Takenawa T, Suzuki A, Sasaki T. The PtdIns(3,4)P(2) phosphatase INPP4A is a suppressor of excitotoxic neuronal death. Nature. 2010;465:497–501. doi: 10.1038/nature09023. [DOI] [PubMed] [Google Scholar]
- 14.Xia P, Chen HS, Zhang D, Lipton SA. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J Neurosci. 2010;30:11246–11250. doi: 10.1523/JNEUROSCI.2488-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Betzen C, White R, Zehendner CM, Pietrowski E, Bender B, Luhmann HJ, Kuhlmann CR. Oxidative stress upregulates the NMDA receptor on cerebrovascular endothelium. Free Radic Biol Med. 2009;47:1212–1220. doi: 10.1016/j.freeradbiomed.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Sadovova N, Hotchkiss C, Fu X, Scallet AC, Patterson TA, Hanig J, Paule MG, Slikker W Jr. Blockade of N-methyl-D-aspartate receptors by ketamine produces loss of postnatal day 3 monkey frontal cortical neurons in culture. Toxicol Sci. 2006;91:192–201. doi: 10.1093/toxsci/kfj144. [DOI] [PubMed] [Google Scholar]
- 17.Yan J, Xu Y, Zhu C, Zhang L, Wu A, Yang Y, Xiong Z, Deng C, Huang XF, Yenari MA, Yang YG, Ying W, Wang Q. Simvastatin prevents dopaminergic neurodegeneration in experimental parkinsonian models: the association with anti-inflammatory responses. PLoS One. 2011;6:e20945. doi: 10.1371/journal.pone.0020945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michaluk P, Mikasova L, Groc L, Frischknecht R, Choquet D, Kaczmarek L. Matrix metalloproteinase-9 controls NMDA receptor surface diffusion through integrin beta1 signaling. J Neurosci. 2009;29:6007–6012. doi: 10.1523/JNEUROSCI.5346-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lebrun-Julien F, Duplan L, Pernet V, Osswald I, Sapieha P, Bourgeois P, Dickson K, Bowie D, Barker PA, Di Polo A. Excitotoxic death of retinal neurons in vivo occurs via a non-cell-autonomous mechanism. J Neurosci. 2009;29:5536–5545. doi: 10.1523/JNEUROSCI.0831-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheeler D, Knapp E, Bandaru VV, Wang Y, Knorr D, Poirier C, Mattson MP, Geiger JD, Haughey NJ. Tumor necrosis factor-alpha-induced neutral sphingomyelinase-2 modulates synaptic plasticity by controlling the membrane insertion of NMDA receptors. J Neurochem. 2009;109:1237–1249. doi: 10.1111/j.1471-4159.2009.06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


