Abstract
Background: Endovascular aortic repair was first performed nearly two decades ago and has become a well-established alternative therapy for many thoracoabdominal aortic diseases. Early survival results with the endovascular aortic repair were impressive, but it also brought many complications. Aortoesophageal fistula is little-known and may be underestimated because it is an unusual complication of thoracic endovascular aortic repair. Objective: To provide a review of the general features of aortoesophageal fistula as a little-known complication after thoracic endovascular aortic repair and to present a new insight regarding the hypothesized mechanisms of this complication based on clinical experience. Methods: The new insights regarding the hypothesized mechanisms built on the literature review and clinical experience. Literature Review from PubMed and Web of Knowledge for relevant studies with English paper. Searches were performed without year, and used the combinations of the following key words: “thoracic aortic aneurysm”, “endovascular”, “aortoesophageal fistula”, “complication”. Results: The authors’ hypothesized mechanisms of aortoesophageal fistula after thoracic aortic aneurysm endovascular repair include the relatively thin vessel wall on thoracic aortic aneurysm hard to prevent the relatively rigid stent graft projecting the aortic and direct erosion into the esophagus. Conclusion: Selecting flexibility and appropriate size stent graft, avoiding the thin aortic wall, and identifying the risk factors may reduce the morbidity of complications with aortoesophageal fistula after thoracic aortic aneurysm endovascular repair.
Keywords: Thoracic aortic aneurysm, endovascular, hypothesized mechanisms, complication, aortoesophageal fistula
The history and main complications of TEVAR
In 1991, endovascular techniques were first reported to be used in abdominal aortic aneurysms, 3 years later, it investigation was stimulated into the feasibility of EVAR on descending thoracic aortic aneurysms [1]. Since then, multiple authors have reported a potential for lower mortality and peri-procedural morbidity, TEVAR has become a well-established alternative therapy for many thoracic aortic diseases [2]. Although endovascular treatment has been proposed as a less-invasive and safe alternative to open surgery for repair of many thoracic aortic diseases, TEVAR would lead to long-term and serious complications in this setting [3]. Although long-term results are still being gathered, TEVAR became the new preferred therapeutic approach in most intervention centers or department of cardiothoracic surgery or department of vascular surgery. Moreover, technical approaches and overall strategies have evolved considerably in the recent past [4]. Intervention material technology, on the other hand, has not kept pace with these developments [5]. Most devices in use today are simple derivatives of grafts created for treatment of TAA. Their performance is less than optimal because they have not been designed to address the unique anatomic, hemodynamic, and pathologic features that characterize the aortic arch and DTA [6]. Such shortcomings brought many complications including endoleak, neurologic impairment, thrombosis, vessel injuries, graft collapse paraplegia and stroke [7].
The history and epidemiology of AEF
AEF is a rare and highly dangerous complication of foreign body ingestion. Nandi and his colleagues [8] followed up 2394 cases of impacted esophageal foreign bodies and two (0.08%) cases developed an AEF’s while Lai and his colleagues [9] looked at a consecutive series of 1338 foreign body ingestion and none had an AEF’s. Although AEF is a rare complication of foreign body ingestion, foreign bodies are the second leading cause of an AEF’s at 19% after thoracic aortic aneurysms at 51% [10]. In 1998, Norgren and his colleagues reported the aortoenteric fistula after TEVAR [11]. In recent years, few cases of AEF were reported with complication of TEVAR, about 50 cases have been reported in the last years (see Table 1) [12-42]. In these cases, Eggebrecht and his colleagues [13] report the largest and systematic study about occurrence of AEF as an unusual, catastrophic complication of TEVAR. It is rare, and is thus probably not widely known within the medical community. AEF complicated TEVAR will be reported increasingly over time due to growing numbers of interventional thoracic aortic procedures and increasing follow-up periods.
Table 1.
Published data of secondary AEF after TEVAR (except Chiesa R and his colleagues’ study data [40])
| Age (year) | AEF time after TEVAR (month) | AEF etiology | |
|---|---|---|---|
| Hance et al. | 24 | 15 | Type 1 endoleak |
| Eggebrecht et al. | 62 | 1 | Erosion by stent graft |
| 74 | 9 | Type 1 endoleak | |
| 77 | 2 | Erosion by stent graft and ischemic necrosis | |
| 67 | 6 | Type 1 endoleak | |
| 49 | 2 | NA | |
| 52 | 15 | NA | |
| Czerny et al. | 57 | 1 | Pressure erosion |
| Porcu et al. | 59 | 2 | Type 1 endoleak |
| Santo et al. | 31 | 8 | Pseudoaneurysm |
| Martens et al. | 64 | 3 | Infection |
| Riesenman et al. | 52 | 4.5 | Infection |
| Girdauskas et al. | NA | 49 | Type 1 endoleak |
| NA | 3.5 | Erosion by stent graft | |
| Christensen et al. | 79 | 4 | Infection |
| Isasti et al. | 74 | 24 | Infection |
| Kim et al. | 75 | 2 | NA |
| Sager et al. | 70 | 24 | Aortic vasa vasorum bleeding |
| Kasai et al. | 54 | 36 | Ischemic necrosis |
| Canaud et al. | 86 | 18 | NA |
| Albors et al. | 66 | 1 | NA |
| Gavens et al. | 80 | 3 | Erosion by stent graft and infection |
| Yavuz et al. | 60 | 48 | NA |
| Numan et al. | 68 | 4 | Infection |
| Abdulaziz et al. | 22 | 12 | Erosion by stent graft and surgery |
| Dołęga-Kozierowski et al. | 62 | 21 | NA |
| Muradi et al. | 65 | 4 | Infection |
| Canaud et al. | 86 | 18 | NA |
| Onodera et al. | 73 | 12 | NA |
| Munakata et al. | 38 | 3 | NA |
| Morisaki et al. | 76 | 1 | Erosion by stent graft and ischemic necrosis |
| Chiba et al. | 70 | 2 | Stent graft rigidity mechanical damage |
| Rispoli et al. | 66 | 26 | Infection and Type 3 endoleak |
Chiesa R and his colleagues’ study data have not mentioned any cause for the etiology of the AEF, so was not included this table.
Epidemiology and clinical presentation of AEF complicating TEVAR
Eggebrecht and his colleagues [13] followed up 268 patients undergoing retrograde or antegrade and TEVAR, AEF occurred in 5 (1.9%). In the other studies, secondary AEF is also known to develop late after thoracic aortic surgery in up to 1.7% of patients [43]. This survey revealed that the incidence of post-TEVAR AEF is comparable to the incidence after open repair surgery; therefore, in this respect, endovascular treatment of the thoracic aorta does not appear to provide any advantage over conventional repair [40]. Median age of Eggebrecht and his colleagues’ patients was 64.5 (range 49 to 77) years with one-half being men [13].
AEF occurred 1 month to 4 years, and median 11.6 months after TEVAR in our literature review. The primary clinical symptom is uncontrolled hematemesis, usually resulting in exsanguinations [13]. Other clinical symptom include: 1) intense backache [31]; 2) fever [31]; 3) chest pain [30]. The “classic” symptom of AEF involves Chiari’s triad of aortoesophageal syndrome-chest pain, episode of small haematemesis followed by massive haematemesis [44]. Eggebrecht and his colleagues [13] reported their patients died all due to fatal rebleeding (n=4) or mediastinitis (n=2). Often the interval between sentinel haemorrhage and exsanguination is about 24 hours with AEF of foreign body in the esophageal [45], perhaps the same is happening to AEF after TEVAR. This ‘window of opportunity’ should lead to early transfer of the patient to a center capable of treating AEF and save the valuable time.
The suggested mechanisms of AEF complicating TEVAR
The exact mechanisms of AEF formation complicating TEVAR are unknown. There were no anatomic or procedural similarities among the cases that have reported predisposed to AEF [13].
Stent graft infection should be considered as the main mechanism of fistula formation [23]. Other hypotheses include: 1) direct erosion of the relatively rigid stent graft through the aorta into the esophagus [13]; 2) pressure necrosis of the esophageal wall due to the continuing forces of the self-expanding endoprosthesis [13]; 3) ischemic esophageal necrosis due to stent-graft coverage of aortic side branches that feed the esophagus [13]; 4) optimal deployment of stent graft repair requires over-sizing the device by 10% to 20% [46] and in a few extreme cases needed the overlap of repeat stent-grafting or over-sizing stent graft led to the thoracic aortic protruded more, which increased diameter of aortic and led to esophageal lumenal turned smaller, it added injure of the esophageal when food through; 5) mycotic aortic aneurysms be treated by stent graft repair [30], bacterial was enclosed in aneurysm, and eventually extends to the esophagus eroding its wall; 6) large aneurism of descending aorta or endoleak into the residual aneurysm developed to large aneurism, with subsequent esophageal pressure [26]. However, Chiesa R and his colleagues’ survey found that the rate of residual type I endoleak in patients presenting an AEF was comparable to that of the global cohort; only one patient had an endoleak at the time of AEF detection [40]. This result could be due to the low incidence rate of endoleaks (type II error). 7) repeat stent-grafting or the length of stent grafts was too long, which increased the friction area between thoracic aortic and esophageal wall [15]; 8) TEVAR was taking in aortic lesions acute phase, the aortic wall was easily broken with tissue edema, the stent graft easily protruding, or even non-inflammatory reaction occurred at this phase, all these would increased the risk of this complication eventually [36].
The mechanisms of AEF complicating TEVAR on BTAI with a hypothesis
The most common anatomical site of BTAI is the medial aspect of the lumen, at the isthmus of the proximal aorta [47,48]. This site is the second physiological stenosis of esophageal. And it’s the tight place where the esophagus and aortic sticking (see Figure 1). The most common type of BTAI is false aneurysm [49]. Because of false aneurysm with no adventitia, the stent-graft would have no aortic wall protection into the aneurysm cavity at the site of aneurysm neck. This would make the stent-graft protrude at a sharp angle at this site. We treated 19 patients with BTAI in Wuhan General Hospital of Guangzhou Command in last 7 years, and 15 patients underwent single graft surgery. Some cases in these patients with a big pseudoaneurysm on the descending aorta were seen in digital substraction angiography (DSA). A stent-graft (Lifetech Scientific (Shenzhen) Co., LTD; Guangdong, China or COOK MEDICAL INC. Bloomington, Indiana, USA) was placed inside the descending aorta to isolate the pseudoaneurysm with via femoral artery puncture. Postoperative digital substraction angiography showed the stent-grafts were placed well, had normal morphology for the proximal aortic. But on the pseudoaneurysm bodies, the stent-grafts were over-spread into the aneurysm cavity and protruded a sharp angle (arrows in Figures 2, 3, 4 and 5) in the aneurysm cavity for during checking of DSA on the operation and for the subsequent checking of computed tomographic angiography about one week. The sharp angle would direct stab in the esophagus through the aorta. This phenomenon occurred in some others report also [38], Chiba D and his colleagues suggested mechanical damage caused by arterial pulsation, stent graft rigidity and interruption of the circulation of the esophageal wall by enlarged aneurysms are all expected to play roles in the development of AEFs also.
Figure 1.
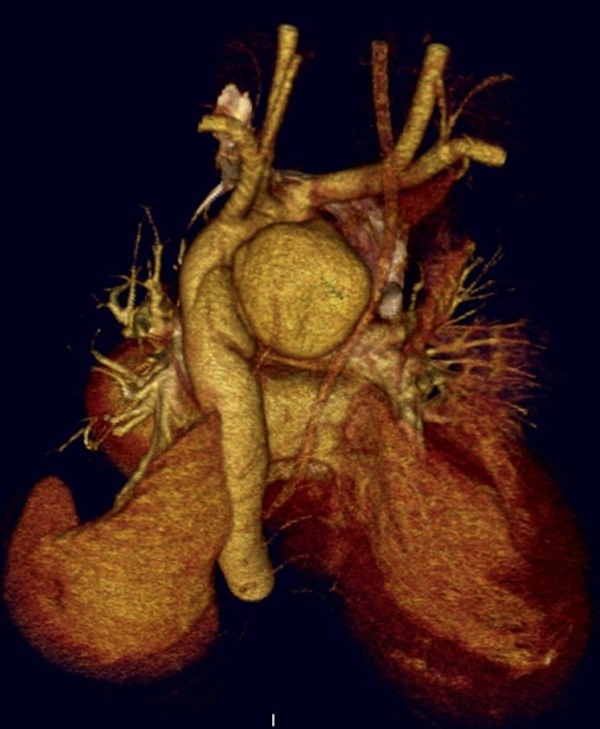
Three-dimensional 16-slice computed tomographic scan demonstrating a false aneurysm at the isthmus of the proximal aorta with the medial aspect of the lumen.
Figure 2.

Digital substraction angiography demonstrating the stent graft (Lifetech Scientific (Shenzhen) Co., LTD; Guangdong, China) over-spread into the aneurysm sac at the pseudoaneurysm with two sharp angles (arrow).
Figure 3.

Sagittal plane of a 16-slice computed tomographic demonstrating the stent graft (Lifetech Scientific (Shenzhen) Co., LTD; Guangdong, China) over-spread into the aneurysm sac at the pseudoaneurysm (arrow).
Figure 4.

Three-dimensional 16-slice computed tomographic demonstrating the stent graft (Lifetech Scientific (Shenzhen) Co., LTD; Guangdong, China) over-spread into the aneurysm sac at the pseudoaneurysm with two sharp angles (arrow).
Figure 5.

Three-dimensional 16-slice computed tomographic demonstrating the stent graft (COOK MEDICAL INC. Bloomington, Indiana, USA) over-spread into the aneurysm sac at the pseudoaneurysm with a sharp angle (arrow).
For example in our study, a case with a big pseudoaneurysm (with a diameter of about 14 mm) on the descending aorta (with a diameter of about 28 mm) was seen in computed tomographic angiography and DSA. A stent-graft (with a diameter of 30 mm at proximal and 24 mm at distal, and length were and 160 mm, Lifetech Scientific (Shenzhen) Co., LTD; Guangdong, China) was placed, and then the good completed angiogram was obtained. But at the subsequent checking of computed tomographic angiography on one week, the stent-graft at the pseudoaneurysm over-spread into the aneurysm cavity with a diameter of 43 mm. This phenomenon occurred some others case also but had not occurred at other types of aneurysms with these stent-grafts.
Through document retrieval and clinical experience analyzation, some other examples effectively proved this hypothesis, it had been reported as follow: 1) Taylor BJ and his colleagues suggested directly from pulsatile graft material in contact with the esophagus also [50]. Muradi A and his colleagues suggested direct erosion of the relatively rigid stent graft through the aorta into the esophagus as the most common cause of AEF after TEVAR at a similar view [13,41]. 2) Yamanaka K and his colleagues described an AEF caused by a piece of polytetrafluoroethylene (PTFE) thread after total aortic arch grafting in thoracotomy, this PTFE thread as a sharps direct stab in the esophagus [51]. 3) Li HP and his colleagues described an AEF, which was reasonable to suspect the aortic wall was gradually eroded by the vascular clip using in esophagectomy. They suggested it is possible that the vascular clip was applied in an angle against aorta, which induced aortic wall injury [52]. 4) Sansur CA and his colleagues described an esophageal fistula, which they suggested the perforation of the esophagus occur as a result of protruding screws from a cervical plate or fixation of the anterior cervical spine, these protruding screws as a sharps direct stab in the esophagus [56]. 5) Okita R and his colleagues described an esophageal fistula, which reported a 65-year-old man with complications of AEF after esophagectomy with a surgical stapler [54]. Chen YY and his colleagues described an esophageal fistula, which due to using a transoral circular stapler after thoracoscopic esophagogastrostomy [55]. These reports had a common characteristic: the staple line of the gastric tube at lesser curvature was not oversewn, the staple line of the gastric tube led to direct contact adjacent organs with the exposed metallic staples as a sharps [54,55].
Chiesa R and his colleague found pseudoaneurysm as an indication for TEVAR represents a significant risk factor for late fistulisation also [40]. But they consider that compression of the oesophagus or the airways by the pseudoaneurysm result in a local inflammatory response, formation of stable adhesions and tissue necrosis, leading to erosion and final fistulisation [40]. They also admit to the mechanism underlying this finding is unclear, but they speculate that aortic lesions that require emergent repair often entail extravasation of blood and periaortic haematoma, with increased local inflammatory response and compression of surrounding organs. These factors, which are not ameliorated by aortic stent grafting, may play a role in late fistulisation [40].
We do not oppose to this view, and limitations of the present phenomenon include its small sample size in our department. As with all the other studies exploring the suggested mechanisms of AEF complicating TEVAR on BTAI, our data were retrospectively collected and thus present increased potential for bias. Limited and variable duration of follow-up offers limited outcomes, but, at least with morphology of our date, it has a certain truth, which is sufficient to support a suggested mechanism of AEF complicating TEVAR on BTAI.
Screening and diagnosis of AEF complicating TEVAR
Once appear the symptoms with prior TEVAR, patients should be highly the clinical suspicion for AEF. Accurate diagnosis can use auxiliary examination. Endoscopy is the most sensitive and specific diagnostic test [56], it can provide a clear view onto the stent-graft in esophagus [23,51]. The supine chest x-ray has been the basic screening tool for AEF. High resolution CT provides clear view of blood vessels and can provide accurate diagnosis, and it also helps to assess a suspected aortic pseudoaneurysm, mediastinitis and the extent of soft tissue involvement. It is also helpful in planning for therapeutic drainage or aspiration [57]. CT scans rarely detect fistulous tracts, and suggestive signs are present in most patients, including air bubbles into the thrombus, periaortic fluid collection, oesophageal or bronchial wall thickening and lung consolidation [58].
The laboratory tests include such inflammatory changes as well as white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), blood cultures were obtained Positive found. A few have no symptoms, patients may appear negative result.
Treatment of AEF complicating TEVAR
Like other ordinary AEF, there was no special treatment of AEF complicating TEVAR. Although AEF is a rare and unusual complication of TEVAR, and once happened, it is a devastating complication resulting from a variety of thoracic conditions. Mortality after surgery for thoracic aortic fistulae has been shown to reach 61% in cases of primary etiology and 78% in cases of secondary fistulae [40]. The first was successfully treat AEF induced by a foreign body was in 1980 described by Ctercteko and Mok [44], who cross-clamped the descending thoracic aorta and primarily closed the esophageal and aorta with suture. Conventional treatment includes surgical aortic repair, which is most commonly performed via a left posterolateral thoracotomy. High-risk patients have been reported to survive with conservative treatment consisting of antimicrobial therapy and percutaneous drainage [59], almost all of them were fatal unless promptly treated. The infected stent graft should be removed if a patient’s condition permits in the most recent viewpoint of treatment of AEF complicating TEVAR [28], left thoracotomy with subsequent aortic graft replacement and esophageal fistula resection may be appropriate AEF therapy [46]. Temporary or permanent extra-anatomic bypass can be performed in the presence of no active bleeding [28]. Recently, TEVAR was proposed as an alternative strategy for the surgical management of AEF [19]. The operative mortality of post-TEVAR AEF repair was no significant differences in terms of either early or late mortality were observed [40]. In addition, we can’t ignore prevent grafts infection.
However, mediastinitis, sepsis, and bleeding are common complications after surgical treatment of AEF, and this treatment option has high morbidity and mortality rates. Those cases of successful treatment guided us that AEF after TEVAR was a lethal complication with very limited treatment options, avoidance and early diagnosis was essential.
Conclusions
Mid-term and long-term complications remain less well defined, and each emergence of new technology by using the good sides and brings the problems accompanying them, TEVAR bears more unusual complications. Previously unanticipated complication, AEF, is uniformly fatal but the mechanism is unclear. Pseudoaneurysm after BTAI as an indication for TEVAR represents a significant risk factor. The clinical characteristic, diagnosis and treatment of AEF complicating TEVAR had been accumulated some experience. Clinicians should be aware of AEF as one of the differential diagnoses in TEVAR patients. This would allow prompt diagnosis and/or immediate triage for treatment.
Last, we believe that significant technologic of TEVAR developments and the clearly mechanisms for AEF complicating TEVAR are sure to emerge in the very near future. And we hopefully would like to see our hypothesized mechanisms and management strategies could optimise the conditions for a favourable outcome in future cases.
Acknowledgements
This work was supported by grants from 2010 military clinical high-tech funded plan projects (No. 2010 gxjs036) and Wuhan General Hospital of Guangzhou Command funded plan (No. YZ201401).
Disclosure of conflict of interest
None.
Abbreviations
- TEVAR
Thoracic endovascular aortic repair
- TAA
Thoracic aortic aneurysm
- DTA
Descending thoracic aorta
- BTAI
blunt thoracic aortic injury
- AEF
Aortoesophageal fistula
- CT
Computed tomography
- EVAR
Endovascular aortic repair
References
- 1.Williams JB, Andersen ND, Bhattacharya SD, Scheer E, Piccini JP, McCann RL, Hughes GC. Retrograde ascending aortic dissection as an early complication of thoracic endovascular aortic repair. J Vasc Surg. 2012;55:1255–62. doi: 10.1016/j.jvs.2011.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Midulla M, Dehaene A, Godart F, Lions C, Decoene C, Serge W, Koussa M, Rey C, Prat A, Beregi JP. TEVAR in patients with late complications of aortic coarctation repair. J Endovasc Ther. 2008;15:552–7. doi: 10.1583/08-2436.1. [DOI] [PubMed] [Google Scholar]
- 3.Riesenman PJ, Farber MA, Mendes RR, Marston WA, Fulton JJ, Mauro M, Keagy BA. Endovascular repair of lesions involving the descending thoracic aorta. J Vasc Surg. 2005;42:1063–74. doi: 10.1016/j.jvs.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Neo WT, Pua U, Wong DE. Thoracic endovascular aortic repair: a local single institution experience. Ann Acad Med Singapore. 2011;40:414–7. [PubMed] [Google Scholar]
- 5.Xi EP, Zhu J, Zhu SB, Liu Y, Yin GL, Zhang Y, Zhang XM, Dong YQ. Surgical treatment of aortoesophageal fistula induced by a foreign body in the esophagus: 40 years of experience at a single hospital. Surg Endosc. 2013;27:3412–6. doi: 10.1007/s00464-013-2926-3. [DOI] [PubMed] [Google Scholar]
- 6.Criado FJ, Abul-Khoudoud OR, Domer GS, McKendrick C, Zuzga M, Clark NS, Monaghan K, Barnatan MF. Endovascular repair of the thoracic aorta: lessons learned. Ann Thorac Surg. 2005;80:857–63. doi: 10.1016/j.athoracsur.2005.03.110. discussion 863. [DOI] [PubMed] [Google Scholar]
- 7.White GH, Yu W, May J, Chaufour X, Stephen MS. Endoleak as a complication of endoluminal grafting of abdominal aortic aneurysms: Classification, incidence, diagnosis, and management. J Endovasc Surg. 1997;4:152–68. doi: 10.1177/152660289700400207. [DOI] [PubMed] [Google Scholar]
- 8.Nandi P, Ong GB. Foreign bodies in the oesophagus: review of 2394 cases. Br J Surg. 1978;65:5–9. doi: 10.1002/bjs.1800650103. [DOI] [PubMed] [Google Scholar]
- 9.Lai AT, Chow TL, Lee DT, Kwok SP. Risk factors predicting the development of complications after foreign body ingestion. Br J Surg. 2003;90:1531–5. doi: 10.1002/bjs.4356. [DOI] [PubMed] [Google Scholar]
- 10.Hollander JE, Quick G. Aortoesophageal Fistula: A comprehensive review of the literature. Am J Med. 1991;91:279–87. doi: 10.1016/0002-9343(91)90129-l. [DOI] [PubMed] [Google Scholar]
- 11.Norgren L, Jernby B, Engellau L. Aortoenteric fistula caused by a ruptured stent-graft: a case report. J Endovasc Surg. 1998;5:269–72. doi: 10.1583/1074-6218(1998)005<0269:AFCBAR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Canaud L, Ozdemir BA, Bee WW, Bahia S, Holt P, Thompson M. Thoracic endovascular aortic repair in management of aortoesophageal fistulas. J Vasc Surg. 2014;59:248–54. doi: 10.1016/j.jvs.2013.07.117. [DOI] [PubMed] [Google Scholar]
- 13.Eggebrecht H, Mehta RH, Dechene A, Tsagakis K, Kühl H, Huptas S, Gerken G, Jakob HG, Erbel R. Aortoesophageal fistula after thoracic aortic stent-graft placement: a rare but catastrophic complication of a novel emerging technique. JACC Cardiovasc Interv. 2009;2:570–6. doi: 10.1016/j.jcin.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Hance KA, Hsu J, Eskew T, Hermreck AS. Secondary aortoesophageal fistula after endoluminal exclusion because of thoracic aortic transection. J Vasc Surg. 2003;37:886–8. doi: 10.1067/mva.2003.159. [DOI] [PubMed] [Google Scholar]
- 15.Eggebrecht H, Baumgart D, Radecke K, von Birgelen C, Treichel U, Herold U, Hunold P, Gerken G, Jakob H, Erbel R. Aortoesophageal fistula secondary to stent-graft repair of the thoracic aorta. J Endovasc Ther. 2004;11:161–7. doi: 10.1583/03-1114.1. [DOI] [PubMed] [Google Scholar]
- 16.Czerny M, Zimpfer D, Fleck T, Gottardi R, Cejna M, Schoder M, Lammer J, Wolner E, Grabenwoger M, Mueller MR. Successful treatment of an aortoesophageal fistula after emergency endovascular thoracic aortic stent-graft placement. Ann Thorac Surg. 2005;80:1117–20. doi: 10.1016/j.athoracsur.2004.02.136. [DOI] [PubMed] [Google Scholar]
- 17.Porcu P, Chavanon O, Sessa C, Thony F, Aubert A, Blin D. Esophageal fistula after endovascular treatment in a type B aortic dissection of the descending thoracic aorta. J Vasc Surg. 2005;41:708–11. doi: 10.1016/j.jvs.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 18.Martens K, De Mey J, Everaert H, Delvaux G, Van Den Brande P. Aortoesophageal fistula following endovascular exclusion of a thoracic aneurysm. Int Angiol. 2007;26:292–6. [PubMed] [Google Scholar]
- 19.Santo KC, Guest P, McCafferty I, Bonser RS. Aortoesophageal fistula secondary to stent-graft repair of the thoracic aorta after previous surgical coarctation repair. J Thorac Cardiovasc Surg. 2007;134:1585–6. doi: 10.1016/j.jtcvs.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 20.Riesenman PJ, Farber MA, Mauro MA, Selzman CH, Feins RH. Aortoesophageal fistula after thoracic endovascular aortic repair and transthoracic embolization. J Vasc Surg. 2007;46:789–91. doi: 10.1016/j.jvs.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 21.Girdauskas E, Falk V, Kuntze T, Borger MA, Schmidt A, Scheinert D, Mohr FW. Secondary surgical procedures after endovascular stent grafting of the thoracic aorta: successful approaches to a challenging clinical problem. J Thorac Cardiovasc Surg. 2008;136:1289–94. doi: 10.1016/j.jtcvs.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 22.Christensen JD, Heyneman LE. Case of the season: aortoesophageal fistula complicating thoracic aortic aneurysm stent graft repair. Semin Roentgenol. 2009;44:4–7. doi: 10.1053/j.ro.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Isasti G, Gómez-Doblas JJ, Olalla E. Aortoesophageal fistula: an uncommon complication after stent-graft repair of an aortic thoracic aneurysm. Interact Cardiovasc Thorac Surg. 2009;9:683–4. doi: 10.1510/icvts.2009.207910. [DOI] [PubMed] [Google Scholar]
- 24.Kim HW, Suh JH, Jo KH, Yoon JS. Concomitant aortoesophageal and aortobronchial fistula after endovascular aortic repair. Ann Thorac Surg. 2010;90:2062. doi: 10.1016/j.athoracsur.2010.02.095. [DOI] [PubMed] [Google Scholar]
- 25.Sager HB, Wellhöner P, Wermelt JA, Schunkert H, Kurowski V. Lethal Hemorrhage Caused by Aortoesophageal Fistula Secondary to Stent-Graft Repair of the Thoracic Aorta. Cardiovasc Intervent Radiol. 2011;34:60–3. doi: 10.1007/s00270-010-9844-8. [DOI] [PubMed] [Google Scholar]
- 26.Albors J, Bahamonde JÁ, Sanchis JM, Boix R, Palmero J. Aortoesophageal fistula after thoracic stent grafting. Asian Cardiovasc Thorac Ann. 2011;19:352–6. doi: 10.1177/0218492311419230. [DOI] [PubMed] [Google Scholar]
- 27.Kasai K, Ushio A, Tamura Y, Sawara K, Kasai Y, Oikawa K, Endo M, Takikawa Y, Suzuki K. Conservative treatment of an aortoesophagial fistula after endovascular stent grafting for a thoracic aortic aneurysm. Med Sci Monit. 2011;17:CS39–42. doi: 10.12659/MSM.881702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Numan F, Gulsen F, Cantasdemir M, Solak S, Arbatli H. Percutaneous treatment of an infected aneurysmal sac secondary to aortoesophageal fistula with a history of stent-graft treatment for thoracic aortic aneurysm. Cardiovasc Intervent Radiol. 2012;35:690–4. doi: 10.1007/s00270-011-0256-1. [DOI] [PubMed] [Google Scholar]
- 29.Canaud L, Alric P, Gandet T, Albat B, Marty-Ané C, Berthet JP. Surgical conversion after thoracic endovascular aortic repair. J Thorac Cardiovasc Surg. 2011;142:1027–31. doi: 10.1016/j.jtcvs.2011.01.060. [DOI] [PubMed] [Google Scholar]
- 30.Gavens E, Zaidi Z, Al-Jundi W, Kumar P. Aortoesophageal fistula after endovascular aortic aneurysm repair of a mycotic thoracic aneurysm. Int J Vasc Med. 2011;2011:649592. doi: 10.1155/2011/649592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yavuz S, Kanko M, Ciftci E, Parlar H, Agirbas H, Berki T. Aortoesophageal fistula secondary to thoracic endovascular aortic repair of a descending aortic aneurysm rupture. Heart Surg Forum. 2011;14:E249–51. doi: 10.1532/HSF98.20101179. [DOI] [PubMed] [Google Scholar]
- 32.Iguchi A, Miyazaki S, Akimoto H, Ohmi M, Tabayashi K. Successful management of secondary aortoesophageal fistula with graft infection. Thorac Cardiovasc Surg. 2001;49:126–8. doi: 10.1055/s-2001-11707. [DOI] [PubMed] [Google Scholar]
- 33.Abdulaziz S, Abou-Shala N, Al-Sanouri I. A young patient with massive haematemesis. BMJ Case Rep. 2012:2012. doi: 10.1136/bcr-2012-007192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onodera M, Inoue Y, Fujino Y, Kikuchi S, Endo S. A case of secondary aortoesophageal fistula inserted a covered self-expanding esophageal stent to control gastrointestinal bleeding. Case Rep Gastrointest Med. 2013;2013:857135. doi: 10.1155/2013/857135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canaud L, Alric P, Gandet T, Ozdemir BA, Albat B, Marty-Ane C. Open surgical secondary procedures after thoracic endovascular aortic repair. Eur J Vasc Endovasc Surg. 2013;46:667–74. doi: 10.1016/j.ejvs.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 36.Munakata H, Yamanaka K, Okada K, Okita Y. Successful surgical treatment of aortoesophageal fistula after emergency thoracic endovascular aortic repair: Aggressive débridement including esophageal resection and extended aortic replacement. J Thorac Cardiovasc Surg. 2013;146:235–7. doi: 10.1016/j.jtcvs.2013.02.064. [DOI] [PubMed] [Google Scholar]
- 37.Morisaki A, Hirai H, Sasaki Y, Hige K, Bito Y, Suehiro S. Aortoesophageal Fistula after Endovascular Repair for Aberrant Right Subclavian Artery Aneurysm. Ann Thorac Cardiovasc Surg. 2013 doi: 10.5761/atcs.cr.12.02153. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Chiba D, Hanabata N, Araki Y, Sawaya M, Yoshimura T, Aoki M, Shimoyama T, Fukuda S. Aortoesophageal fistula after thoracic endovascular aortic repair diagnosed and followed with endoscopy. Intern Med. 2013;52:451–5. doi: 10.2169/internalmedicine.52.9139. [DOI] [PubMed] [Google Scholar]
- 39.Dołęga-Kozierowski B, Sokratous K, Dyś K, Lis M, Ferenc S, Drelichowski S, Witkiewicz W. Aortoesophageal fistula as a complication of thoracic aorta aneurism stent grafting - a case report and literature review. Pol J Radiol. 2012;77:77–80. doi: 10.12659/pjr.883635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiesa R, Melissano G, Marone EM, Marrocco-Trischitta MM, Kahlberg A. Aorto-oesophageal and aortobronchial fistulae following thoracic endovascular aortic repair: a national survey. Eur J Vasc Endovasc Surg. 2010;39:273–9. doi: 10.1016/j.ejvs.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Muradi A, Yamaguchi M, Kitagawa A, Nomura Y, Okada T, Okita Y, Sugimoto K. Secondary aortoesophageal fi stula after thoracic endovascular aortic repair for a huge aneurysm. Diagn Interv Radiol. 2013;19:81–4. doi: 10.4261/1305-3825.DIR.5912-12.1. [DOI] [PubMed] [Google Scholar]
- 42.Rispoli P, Bertoldo U, Oliaro A, Mossetti C, Varetto GF, Tallia C, Campanella A, Rinaldi M. Two-stage treatment of a secondary aortoesophageal fistula after thoracic endovascular aneurysm repair. J Cardiovasc Surg (Torino) 2012;53:531–5. [PubMed] [Google Scholar]
- 43.Grabenwöger M, Alfonso F, Bachet J, Bonser R, Czerny M, Eggebrecht H, Evangelista A, Fattori R, Jakob H, Lönn L, Nienaber CA, Rocchi G, Rousseau H, Thompson M, Weigang E, Erbel R. Thoracic Endovascular Aortic Repair (TEVAR) for the treatment of aortic diseases: a position statement from the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2012;33:1558–63. doi: 10.1093/eurheartj/ehs074. [DOI] [PubMed] [Google Scholar]
- 44.Aziz S, McWilliams R, Rashid A, Gosney JR, Harris PL, Stables RH. Late aortic rupture due to stent margin pseudoaneurysm formation complicating endovascular stent graft repair of a thoracic aortic mycotic aneurysm. EJVES Extra. 2006;12:30–4. [Google Scholar]
- 45.Ctercteko G, Mok CK. Aortoesophageal fistula induced by a foreign body: the first recorded survival. J Thorac Cardiovasc Surg. 1980;80:233–5. [PubMed] [Google Scholar]
- 46.Tehrani HY, Peterson BG, Katariya K, Morasch MD, Stevens R, DiLuozzo G, Salerno T, Maurici G, Eton D, Eskandari MK. Endovascular repair of thoracic aortic tears. Ann Thorac Surg. 2006;82:873–7. doi: 10.1016/j.athoracsur.2006.04.012. discussion 877-8. [DOI] [PubMed] [Google Scholar]
- 47.Siegel JH, Belwadi A, Smith JA, Shah C, Yang K. Analysis of the mechanism of lateral impact aortic isthmus disruption in real-life motor vehicle crashes using a computer-based finite element numeric model: with simulation of prevention strategies. J Trauma. 2010;68:1375–95. doi: 10.1097/TA.0b013e3181dcd42d. [DOI] [PubMed] [Google Scholar]
- 48.Demetriades D. Blunt thoracic aortic injuries: crossing the Rubicon. J Am Coll Surg. 2012;214:247–59. doi: 10.1016/j.jamcollsurg.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 49.Demetriades D, Velmahos GC, Scalea TM, Jurkovich GJ, Karmy-Jones R, Teixeira PG, Hemmila MR, O’Connor JV, McKenney MO, Moore FO, London J, Singh MJ, Lineen E, Spaniolas K, Keel M, Sugrue M, Wahl WL, Hill J, Wall MJ, Moore EE, Margulies D, Malka V, Chan LS American Association for the Surgery of Trauma Thoracic Aortic Injury Study Group. Operative repair or endovascular stent graft in blunt traumatic thoracic aortic injuries: results of an American Association for the Surgery of Trauma Multicenter Study. J Trauma. 2008;64:561–70. doi: 10.1097/TA.0b013e3181641bb3. discussion 570-1. [DOI] [PubMed] [Google Scholar]
- 50.Taylor BJ, Stewart D, West P, Dunn JT, Cisek P. Endovascular repair of a secondary aortoesophageal fistula: a case report and review of the literature. Ann Vasc Surg. 2007;21:167–71. doi: 10.1016/j.avsg.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Yamanaka K, Nonaka M, Iwakura A, Asao Y. Repair of aortoesophageal fistula after total aortic arch grafting. Interact Cardiovasc Thorac Surg. 2011;12:655–6. doi: 10.1510/icvts.2010.257501. [DOI] [PubMed] [Google Scholar]
- 52.Li HP, Hsieh CC, Chiang HH, Wang TH, Lee JY, Huang MF, Chou SH. Aortobronchial fistula after esophagectomy for esophageal cancer -- a very rare complication. Kaohsiung J Med Sci. 2011;27:247–50. doi: 10.1016/j.kjms.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sansur CA, Early S, Reibel J, Arlet V. Pharyngocutaneous fistula after anterior cervical spine surgery. Eur Spine J. 2009;18:586–91. doi: 10.1007/s00586-009-0951-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okita R, Mukaida H, Takiyama W, Oheda M, Uchida N, Ishihara H. Successful surgical treatment of aortoesophageal fistula after esophagectomy. Ann Thorac Surg. 2005;79:1059–61. doi: 10.1016/j.athoracsur.2003.09.116. [DOI] [PubMed] [Google Scholar]
- 55.Chen YY, Chang JM, Lai WW. Tracheo-neo-esophageal fistula caused by exposed metallic staples erosion. Ann Thorac Surg. 2012;94:1375. doi: 10.1016/j.athoracsur.2012.03.089. [DOI] [PubMed] [Google Scholar]
- 56.Chughtai TS, Sheiner NM. Successful repair of aortoesophageal fistula secondary to traumatic pseudoaneurysm. Ann Thorac Surg. 1998;66:936–8. doi: 10.1016/s0003-4975(98)00647-x. [DOI] [PubMed] [Google Scholar]
- 57.Giménez A, Franquet T, Erasmus JJ, Martínez S, Estrada P. Thoracic complications of esophageal disorders. Radiographics. 2002;22:S247–58. doi: 10.1148/radiographics.22.suppl_1.g02oc18s247. [DOI] [PubMed] [Google Scholar]
- 58.Bergqvist D, Björck M. Secondary arterioenteric fistulation--a systematic literature analysis. Eur J Vasc Endovasc Surg. 2009;37:31–42. doi: 10.1016/j.ejvs.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 59.Saleem BR, Berger P, Zeebregts CJ, Slart RH, Verhoeven EL, van den Dungen JJ. Periaortic endograft infection due to Listeria monocytogenes treated with graft preservation. J Vasc Surg. 2008;47:635–7. doi: 10.1016/j.jvs.2007.09.029. [DOI] [PubMed] [Google Scholar]


