Abstract
The purpose of this study is to characterize brain gray matter volumetric changes in HIV seropositive without neurocognitive impairment and seronegative men in Asia. We investigate 36 males with HIV seropositive (mean age 34.5±9.1 years) and 33 age- and gender-matched seronegative controls (mean age 31.4±7.6 years) in Asia. The cognitive competence of 36 males with HIV seropositive has no impaired based on performance in the international HIV dementia scale. High-resolution T1-weighted magnetic resonance imaging is performed on a 3.0 T MR system using a standard 32-channel birdcage head coil. Voxel-based morphometry is used to derive volumetric measurements at the level of the individual voxel (p < 0.001, none corrected for multiple comparisons). Compared to the control group, HIV seropositive male lower gray matter volumes are found in left inferior frontal gyrus triangular part and orbital part, left superior temporal gyrus, right middle frontal gyrus and ant cingulum; significant increases gray matter volumes can be seen in Periaqueductal gray and gray around lateral ventricle. HIV infection can change the gray matter volume early without cognitive competence impaired and MR can recognize that changes.
Keywords: Morphometry, MRI, gray matter, HIV
Introduction
Despite the widening use of highly active anti-retroviral therapy (HAART), neurocognitive impairment remains common among HIV-infected (HIV+) individuals. It has been established that viral invasion of the brain occurs early in infection [1-3], and leads to cognitive impairment [4]. Brain atrophy, and injury to subcortical regions, have been shown later in the course of infection [5-7].
Although various neuroimaging studies investigated structural brain changes in HIV, resent MR-based studies reported volumetric changes of the whole brain, basal ganglia in HIV patients [8-10]. Various correlations of MR parameters to cognitive decline were shown in these studies. However, it remains unclear if HIV positive patients without cognitive decline would also suffer from brain tissue atrophy.
VBM is a method to identify regional brain volume differences of gray matter, white matter and cerebrospinal fluid (CSF) using voxel-wise statistics in the context of Gaussian random fields [11]. The VBM procedure allows the detection of highly localized differences, consistently observed across the samples, over the entire brain, even in areas where the region of interest analysis would be difficult. The procedure has proved to be a powerful method in detecting regional tissue differences, even in clinical conditions where routine imaging does not show any visible abnormality [12,13], and provides the opportunity for an unbiased general search of abnormalities in the whole-brain volume [11]. Thus VBM may be useful to examine regional gray matter changes in HIV-infected male.
In this study, we have compared a group of HIV-infected males with gender- and age-matched healthy control group with VBM, using high-resolution T1-weighted images and statistical parametric mapping method.
Materials and methods
In this study we enrolled 36 males patients with HIV seropositive (mean age 34.5±9.1 years, range: 18-50 years; mean current CD4 cell count 206±187 cells/ml, range: 2-673 cells/ml; mean nadir CD4 cell count 199±189 cells/ml, range: 1-673 cells/ml); 33 age- and gender-matched HIV negative healthy controls(mean age 31.4±7.6 years, range 22-58 years) without any neurological limitation. All patients were recruited from You’an Hospital (Beijing) between June 2011 and May 2013. Inclusion criteria were HIV seropositive, informed consent and age older than 18 years. Exclusion criteria were opportunistic systemic or CNS infection, CNS neoplasm, active alcohol or drug abuse, concomitant neurological disease caused by factors other than HIV, major depression or other severe psychiatric disease. All patients and controls underwent a careful neurological examination by experienced neurologists. All patients and healthy subjects gave their written informed consent according to the Declaration of Helsinki prior to study inclusion. The study was approved by the local ethics committee.
Neurocognitive testing was performed with the international HIV dementia scale (IHDS) [14]. The IHDS comprises four tasks that evaluate the domains of memory (recall of four items at 5 min), attention (anti-saccadic errors), psychomotor speed (timed written alphabet), and construction. The maximum score achievable in the IHDS is 16 and a score < 10 predict HIV dementia. At this stage, we enrolled patients with no cognitive impairment (IHDS score ≥ 15).
History of HAART was received by 11 patients mean duration 3.3±3.8 years, range: 0.25-11.5 years.
Imaging data were performed on a single magnetic resonance scanner, at a 3.0 T MAGNETOM Tim Trio (Siemens, Erlangen, Germany) using a standard 32-channel birdcage head coil. T1-weighted sagittal whole-brain magnetic resonance imaging 3D datasets were obtained using magnetization prepared rapid acquisition gradient echo (MP-Rage) sequence (TR/TE/TI=1900 ms/2.52 ms/900 ms, flip angle=9°, field of view=246×256 mm, slice thickness=1 mm; resolution=1×1 mm; slices=176). The same scanner and the same scanning protocol were used for all patients and healthy controls.
We used statistical parametric mapping (SPM8) software (Wellcome (or Welcome?) Department of Cognitive Neurology, University College of London, London, England; http://www.fil.ion.ucl.ac.uk/spm) running under MATLAB version 8.0 (MathWorks Inc., Natick, Massachusetts) to data processing and analysis.
The standard VBM method can be summarized by the following sequence of steps: spatial normalization, segmentation, modulation, and smoothing. Pre-processing involved all voxel signal intensities in the final GM segmented images which multiplied by the Jacobian determinants (Jacobian modulation) derived from the spatial normalization [15]; segmentation into gray matter, white matter and CSF, and spatial smoothing with an isotropic Gaussian kernel of 8 mm full-width at half maximum (FWHM) [15]. After smoothing, each voxel of the image was a locally weighted average of GM or WM density from a region of surrounding voxels, and statistical analyses can be performed [16,17]. VBM analysis was performed in a cross sectional setting regional gray matter volumes of HIV positive patients without cognitive deficits and healthy controls, which were compared in Two-sample t-test analysis. Level of significance was set to a P value of < 0.001 with a cluster threshold of 100 voxels. To avoid edge effects at the border of gray matter, we excluded all voxels with a gray matter value below 0.2 (maximum value 0.8). Results were superimposed on a Montreal Neurologic Institute (MNI) template (Colin Brain).
We compared patient and control groups with age using independent t-tests and chi-square tests; when appropriate, nonparametric analogues (Mann-Whitney tests and Fisher’s exact tests) were conducted. All tests were two-tailed with alpha level=0.05.
Result
Initially, 131 HIV-positive patients were enrolled in the study. Before processing and analyzing the images with VBM, in accordance with T1WI and T2WI we excluded a total of 76 cases because of subclinical CNS infection and neoplasm, lacunar infarct, image 160 slices and the images have motion artifact or other various artifacts which did not pass our quality control checks; excluded 8 female, and 11 cognitive impairment patients. All these subjects were excluded from further analysis. And then remaining 36 male patients showed no abnormalities in T1WI and T2WI.
Table 1 gave an overview of gray matter of brain reduces detectable when comparing controls with HIV patients without cognitive decline. Most severe gray matter reduction was present the bilateral anterior cingulate cortex especially the right side, as well as the left inferior frontal gyrus triangular part and orbital part (Figure 1). Furthermore, atrophy was present within left superior temporal gyrus, Right middle frontal gyrus marked volume reduction comprising three separate clusters (Table 1). In the crura cerebelli gray matter reduction was detected on the right side.
Table 1.
Regions of reduced gray matter volumes in HIV positive patients without cognitive impairment compared with age- and gender-matched healthy controls
| Cluster kE | T value | Coordinates (x, y, z) | Anatomic location | ||
|---|---|---|---|---|---|
| 1608 | 4.58 | 5 | 35 | 24 | Right anterior cingulate gyrus |
| 1515 | 4.51 | -45 | 47 | 3 | Left inferiorfrontalgyrus triangularpart |
| 139 | 4.08 | -32 | 38 | -18 | Left inferiorfrontalgyrus orbitalpart |
| 196 | 4.04 | -51 | -36 | 21 | Left superior temporal gyrus |
| 197 | 4.03 | 32 | 36 | 33 | Right middle frontalgyrus |
| 317 | 4.03 | 39 | 20 | 45 | Right middle frontalgyrus |
| 214 | 3.73 | 47 | 44 | 6 | Right middle frontalgyrus |
| 126 | 3.96 | 51 | -51 | -32 | Right crura cerebelli |
Coordinates (x, y, z) refer to the location within the MNI space and denote the cluster peak location (defined as voxel with the highest T value); kE Cluster size expressed in mm3.
Figure 1.
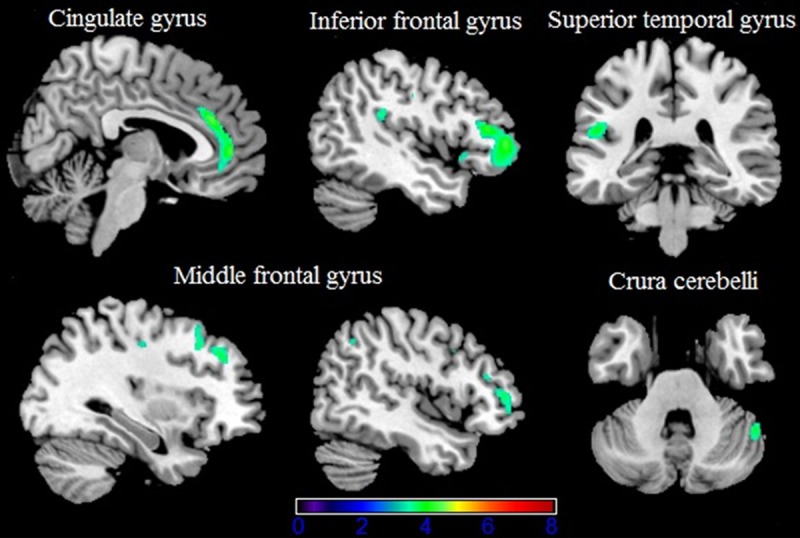
Gray matter (green) decreases in patients with HIV without cognitive impairment compared to controls (p < 0.001 none corrected for multiple comparisons) are superimposed on an MNI template (Colin brain). Right side of the image is right side of the brain.
Table 2 showed an overview of structural brain gray matter increased when comparing controls with HIV patients without cognitive decline. Most severe gray matter increase was present around the lateral ventricles (Figure 2). Furthermore, Periaqueductal gray matter was increased (Figure 2).
Table 2.
Regions of increased gray matter volumes in HIV positive patients without cognitive impairment compared with age- and gender-matched healthy controls
| Cluster kE | T value | Coordinates (x, y, z) | Anatomic location | ||
|---|---|---|---|---|---|
| 158 | 3.84 | -5 | -16 | -14 | Periaqueductal gray matter |
| 134 | 4.17 | -21 | -78 | 18 | Periventricular gray matter |
| 286 | 4.36 | -23 | -12 | 15 | Periventricular gray matter |
| 341 | 5.06 | -3 | 3 | 19 | Periventricular gray matter |
| 277 | 5.53 | -14 | -45 | 19 | Periventricular gray matter |
Coordinates (x, y, z) refer to the location within the MNI space and denote the cluster peak location (defined as voxel with the highest T value); kE Cluster size expressed in mm3.
Figure 2.
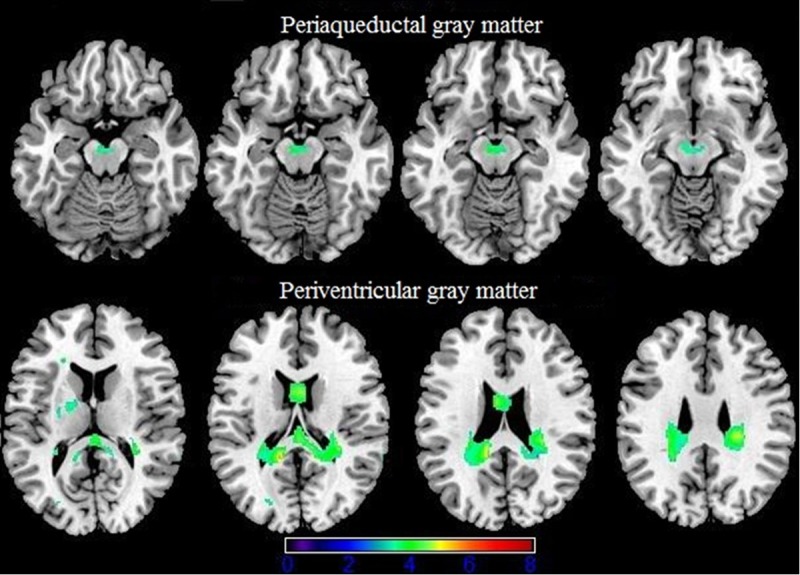
Gray matter (green) increases in patients with HIV without cognitive impairment compared to controls (p < 0.001 none corrected for multiple comparisons) are superimposed on an MNI template (Colin brain). Right side of the image is right side of the brain.
At the beginning of the study, we did a pretest study, and discovered that gender had a significant effect on gray matter volume change (Figure 3). That was the reason why we excluded the female patients from further analysis.
Figure 3.
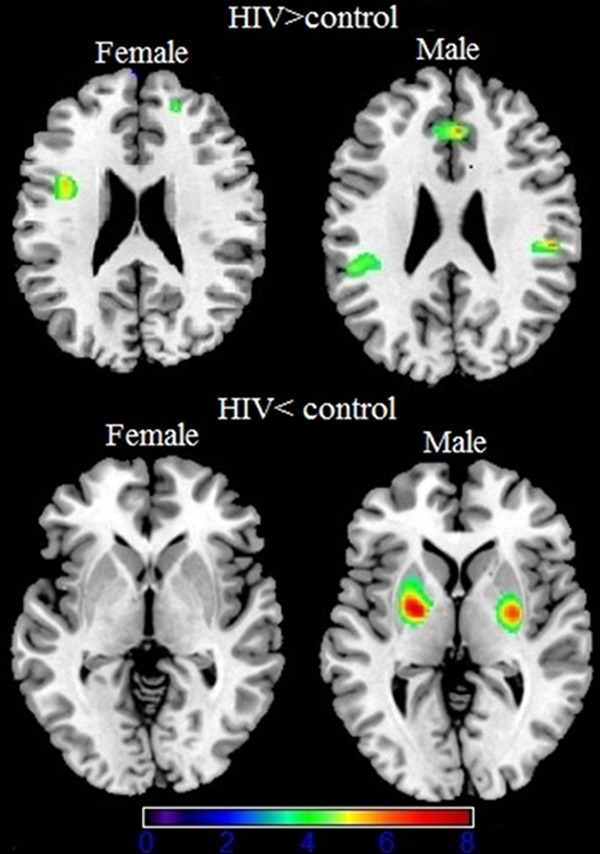
The first figure is gray matter (green) decreases in female patients with HIV compared to controls. The second is gray matter (green) decreases male patients with HIV compared to controls. The third is gray matter makes no difference in female patients with HIV compared to controls. The forth column is gray matter (green) increases in male patients with HIV compared to controls. (p < 0.001 none corrected for multiple comparisons) are superimposed on an MNI template (Colin brain). Right side of the image is right side of the brain.
Discussion
It’s well known that clinical symptoms of central nervous system (CNS) disease are based on neuronal dysfunction or death, rather than HIV directly infected neurons. However, the mechanism of HIV-related brain damage is not fully clear. The current theory is that the CNS infection is continuous and self-supporting, and does not depend on subsequent cycle of the spread of the virus.
Before HAART, HIV Associated Dementia (HAD) mainly detected in advanced HIV infectors. With the widespread use of HAART, prevalence of HAD decreased significantly, the health status of patients also improved. However, HIV-associated neurocognitive disorder (HAND) prevalence close to 40% in HIV-infected persons [18-20], is still a serious impact on the health of patients [21]. Therefore, detecting HAND early has a very important clinical significance.
Although HAART effectively inhibit viral replication and rebuild the immune system, mild HAND can still occur in each period of HIV infections [22,23]. A study [24] found significant psychological behavior abnormalities in these patients even more than the movement abnormalities. Cognitive impairment is still the main symptom of HAND patients, and the symptom was ingravescent. As we conclude from our IHDS test to these HIV patients, we found that with the disease developing, cognitive impairment become more obvious.
Conventional MR examination can find exact atrophy of cortex, but when it does not find a confirm brain structural abnormalities early, VBM can discover the change of brain volume. Through analysis of the data, we found that gray matter reduction of HIV patients without cognitive impairment was in bilateral anterior cingulate gray matter, the left inferior frontal gyrus triangular part and orbital part, right middle frontal gyrus, a smaller volume of the left superior temporal gyrus, and the cerebellar crus compared to the control group.
With a review of the literatures, we found that, as studies have shown that cognitive impairment of HAND is associated with early all subcortical and frontal lobe damage [25], in our study, the left frontal lobe (including inferior frontal gyrus triangular part and orbital part) brain tissue decreased significantly, these two areas mainly involves memory, cognitive and emotional decision function. We also found that the volume of the left superior temporal lobe cortex tissue is reduced, which is the same as the recent several studies. One of the research on visual attention task by fMRI-BOLD showed that HIV positive patients compared with control group the left temporal lobe signals continue increasing in 1 year [26], proved that the temporal lobe cortex sustained damage in HIV infection; Another study showed that temporal lobe cortex tissue decreased significantly with impaired cognitive function [27], also prompt the function of temporal lobe cortex associated with cognitive function. But the function of the posterior superior temporal lobe is mainly the Wernicke area associated with impaired auditory language comprehension [28], can further induce the symptom of sensory aphasia for HAND. At the same time, we found several volumes decrease in the right middle frontal gyrus. These regions were positively correlated with increased activity in the episodic memory network [29], activity in specific nodes of this network was associated with better item and context retrieval, have been associated with age-related declines in episodic memory retrieval [30-32], but our patient is young, the mean age is 34.5±9.1 years. So it can infer that HIV infect can accelerate aging. While in other studies it also correlated with the ability to resolve interference efficiently [33,34]; it reduced will infect selection and inhibition in Chinese semantic judgement.
Meanwhile, we found that, comparing with the control group, bilateral cingulate gyrus volume of HIV patients was the most significantly induced, which is the common changed part in gray matter caused by HIV infection, the result was same with some similar morphologic analysis studies by VBM [27,35]; the related fMRI studies [36] found that the function of anterior cingulate associated with cognitive was selective attention and conflict management. Then, we found that patient’s gray matter volume decreases lateralization to the left brain, confirms the function of the left brain is closely related to the conscious. Some MRS and MRI results showed HIV-related gray matter atrophy is most common in the basal ganglia (especially the caudate nucleus) [37,38]. In our study, we did not find the volume of gray matter in the basal ganglia nuclei decreases.
In our study, we also found the right cerebellar crus local volume decreased, as a study of stroke patients showed the cerebellar crus was located in the affected cerebral cortex, which further supports these decreased may represent the impaired cognitive function [39]. So we can infer cognitive impaired from the cerebellar crus local volume decreased.
We found patients’ gray matter volumes around the lateral ventricles and the periaqueductal were increased than the control group. Periaqueductal gray matter (PAG) is constituted with nerve cells gathered around cerebral aqueduct. It is known that PAG functions include the descending modulation of pain, defensive behavior, reproductive behavior and vocal function [40]. Currently there is no literature reported the volume of PAG increase in HIV patients, while there is no literature involved the reason too. We conjectured that it may be the upper neurons damaged leading to the function weakened, and then the lower neurons enhance their function causing gray matter volume increased. The relevant documents can prove periventricular gray matter (PVG) is associated to pain [41] and hypertension [42], the evidence maybe for a non-opioid mechanism [41]. But no evidence support that related to HIV. It is necessary to collect a large number of data to analysis, and proof by clinical pathological anatomy.
As the pretest study showed that female and male patients involved different brain regions of HIV CNS infection. In the further, we will enlarge the sample of female patients to confirm the effect of gender in HIV infection of the CNS.
Conclusion
In this study we observed: Bilateral anterior cingulate cortex, left inferior frontal gyrus triangular part and orbital part, left superior temporal gyrus, right middle frontal gyrus and right cerebellar crus are key brain regions to HIV infection of the central nervous system. In neuroanatomical, the brain structure change can explain some clinical symptoms in patients directing to cognitive impairment. The results of this study provide some evidence of the functional MR imaging for prevention, treatment and assessment of HAND early.
Acknowledgements
This author would like to thank Dr. Panli Zuo at Siemens Limited China for supporting for data processing. This work was supported by Beijing Nature Science Foundation (7132108) and Capital Health Research and Development Special Fund (2011-2018-01).
Disclosure of conflict of interest
None.
References
- 1.Chiodi F, Fenyo EM. Neurotropism of human immunodeficiency virus. Brain Pathol. 1991;1:185–191. doi: 10.1111/j.1750-3639.1991.tb00658.x. [DOI] [PubMed] [Google Scholar]
- 2.Chiodi F, Sonnerborg A, Albert J, Gaines H, Norkrans G, Hagberg L, Asjö B, Strannegård O, Fenyö EM. Human immunodeficiency virus infection of the brain. I. Virus isolation and detection of HIV specific antibodies in the cerebrospinal fluid of patients with varying clinical conditions. J Neurol Sci. 1988;85:245–257. doi: 10.1016/0022-510x(88)90184-0. [DOI] [PubMed] [Google Scholar]
- 3.Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Young SA, Mills RG, Wachsman W, Wiley CA. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- 4.Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- 5.Dal Pan GJ, McArthur JH, Aylward E, Selnes OA, Nance-Sproson TE, Kumar AJ, Mellits ED, McArthur JC. Patterns of cerebral atrophy in HIV-1-infected individuals: results of a quantitative MRI analysis. Neurology. 1992;42:2125–2130. doi: 10.1212/wnl.42.11.2125. [DOI] [PubMed] [Google Scholar]
- 6.Gelman BB, Guinto FC Jr. Morphometry, histopathology, and tomography of cerebral atrophy in the acquired immunodeficiency syndrome. Ann Neurol. 1992;32:31–40. doi: 10.1002/ana.410320107. [DOI] [PubMed] [Google Scholar]
- 7.Jernigan T, Archibald S, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, Notestine RJ, Wolfson T, Letendre SL, Ellis RJ, Heaton RK, Gamst AC, Franklin DR Jr, Clifford DB, Collier AC, Gelman BB, Marra C, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Grant I CHARTER Group. Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol. 2011;17:248–257. doi: 10.1007/s13365-011-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stout JC, Ellis RJ, Jernigan TL, Archibald SL, Abramson I, Wolfson T, McCutchan JA, Wallace MR, Atkinson JH, Grant I. Progressive cerebral volume loss in human immunodeficiency virus infection: a longitudinal volumetric magnetic resonance imaging study. HIV Neurobehavioral Research Center Group. Arch Neurol. 1998;55:161–168. doi: 10.1001/archneur.55.2.161. [DOI] [PubMed] [Google Scholar]
- 9.Chiang M, Dutton RA, Hayashi KM, Lopez OL, Aizenstein HJ, Toga AW, Becker JT, Thompson PM. 3D pattern of brain atrophy in HIV/AIDS visualized using tensor-based morphometry. Neuroimage. 2007;34:44–60. doi: 10.1016/j.neuroimage.2006.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardenas V, Meyerhoff D, Studholme C, Kornak J, Rothlind J, Lampiris H, Neuhaus J, Grant RM, Chao LL, Truran D, Weiner MW. Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. J Neurovirol. 2009;15:1–10. doi: 10.1080/13550280902973960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashburner J, Friston KJ. Voxel-based morphometry-the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 12.Padovani A, Borroni B, Brambati SM, Agosti C, Broli M, Alonso R, Scifo P, Bellelli G, Alberici A, Gasparotti R, Perani D. Diffusion tensor imaging and voxel based morphometry study in early progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2006;77:457–463. doi: 10.1136/jnnp.2005.075713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin YW, Kwon JS, Ha TH, Park HJ, Kim DJ, Hong SB, Moon WJ, Lee JM, Kim IY, Kim SI, Chung EC. Increased water diffusivity in the frontal and temporal cortices of schizophrenic patients. Neuroimage. 2006;30:1285–1291. doi: 10.1016/j.neuroimage.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, Robertson K, McArthur JC, Ronald A, Katabira E. The International HIV Dementia Scale: a new rapid screening test for HIV dementia. AIDS. 2005;19:1367–1374. [PubMed] [Google Scholar]
- 15.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 16.Yasuda CL, Valise C, Saúde AV, Pereira AR, Pereira FR, Ferreira Costa AL, Morita ME, Betting LE, Castellano G, Mantovani Guerreiro CA, Tedeschi H, de Oliveira E, Cendes F. Dynamic changes in white and gray matter volume are associated with outcome of surgical treatment in temporal lobe epilepsy. Neuroimage. 2010;49:71–79. doi: 10.1016/j.neuroimage.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Yasuda CL, Betting LE, Cendes F. Voxel-based morphometry and epilepsy. Expert Rev Neurother. 2010;10:975–984. doi: 10.1586/ern.10.63. [DOI] [PubMed] [Google Scholar]
- 18.Antinori A, Trotta MP, Lorenzini P, Torti C, Gianotti N, Maggiolo F, Ceccherini-Silberstein F, Nasto P, Castagna A, De Luca A, Mussini C, Andreoni M, Perno CF GNOMO Study Group. Virological response to salvage therapy in HIV-infected persons carrying the reverse transcriptase K65R mutation. Antivir Ther. 2007;12:1175–1183. [PubMed] [Google Scholar]
- 19.Boisse L, Gill MJ, Power C. HIV infection of the central nervots system: clinical features and neuropathogeneshs. Neurol Clin. 2008;26:799–819. doi: 10.1016/j.ncl.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Minagar A, Commins D, Alexander JS, Hoque R, Chiappelli F, Singer EJ, Nikbin B, Shapshak P. NeuroAIDS: characteristics and diagnosis of the neurological complications of AIDS. Mol Diagn Ther. 2008;12:25–43. doi: 10.1007/BF03256266. [DOI] [PubMed] [Google Scholar]
- 21.Robinson-Papp J, Elliott KJ, Simpson DM. HIV-related neurocognitive impairment in the HAART era. Curr HIV/AIDS Rep. 2009;6:146–152. doi: 10.1007/s11904-009-0020-1. [DOI] [PubMed] [Google Scholar]
- 22.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I CHARTER Group; HNRC Group. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pascual-Sedano B, Iranzo A, Marti-Fabregas J, Domingo P, Escartin A, Fuster M, Barrio JL, Sambeat MA. Prospective study of new-onset seizures in patients with human immunodeficiency virus infection: etiologic and clinical aspects. Arch Neurol. 1999;56:609–612. doi: 10.1001/archneur.56.5.609. [DOI] [PubMed] [Google Scholar]
- 24.Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, Calmy A, Chave JP, Giacobini E, Hirschel B, Du Pasquier RA. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- 25.Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, McCutchan JA, Taylor MJ, Kelly MD, Ellis RJ. The HNRC 500-neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- 26.Ernst T, Yakupov R, Nakama H, Crocket G, Cole M, Watters M, Ricardo-Dukelow ML, Chang L. Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Ann Neurol. 2009;65:316–325. doi: 10.1002/ana.21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Küper M, Rabe K, Esser S, Gizewski ER, Husstedt IW, Maschke M, Obermann M. Structural gray and white matter changes in patients with HIV. J Neurol. 2011;258:1066–1075. doi: 10.1007/s00415-010-5883-y. [DOI] [PubMed] [Google Scholar]
- 28.Naeser MA, Nancy Helm-Estabrooks N, Haas G, Auerbach S, Srinivasan M. Relationship between lesion extent in ‘Wernicke’s area’on computed tomographic scan and predicting recovery of comprehension in Wernicke’s aphasia. Arch Neurol. 1987;44:73–82. doi: 10.1001/archneur.1987.00520130057018. [DOI] [PubMed] [Google Scholar]
- 29.Rajah MN, Languay R, Grady CL. Age-related changes in right middle frontal gyrus volume correlate with altered episodic retrieval activity. J Neurosci. 2011;31:17941–17954. doi: 10.1523/JNEUROSCI.1690-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckner RL, Snyder AZ, Sanders AL, Raichle ME, Morris JC. Functional brain imaging of young, nondemented, and demented older adults. J Cogn Neurosci. 2000;12(Suppl 2):24–34. doi: 10.1162/089892900564046. [DOI] [PubMed] [Google Scholar]
- 31.West R. In defense of the frontal lobe hypothesis of cognitive aging. J Int Neuropsychol. 2000;6:727–729. doi: 10.1017/s1355617700666109. [DOI] [PubMed] [Google Scholar]
- 32.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 33.Garavan H, Stein E. Right hemispheric dominance for inhibitory control: an event-related functional MRI study. Proc Natl Sci U S A. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122:981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- 35.Chiang M, Dutton RA, Hayashi KM, Lopez OL, Aizenstein HJ, Toga AW, Becker JT, Thompson PM. 3D pattern of brain atrophy in HIV/AIDS visualized using tensor-based morphometry. Neuroimage. 2007;34:44–60. doi: 10.1016/j.neuroimage.2006.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell RL. Linear increases in BOLD response associated with increasing proportion of incongruent trials across time in a colour Stroop task. Exp Brain Res. 2010;203:193–204. doi: 10.1007/s00221-010-2225-3. [DOI] [PubMed] [Google Scholar]
- 37.Schouten J, Cinque P, Gisslen M, Reiss P, Portegies P. HIV-1 infection and cognitive impairment in the cART-ear: a review. AIDS. 2011;25:561–575. doi: 10.1097/QAD.0b013e3283437f9a. [DOI] [PubMed] [Google Scholar]
- 38.Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ CHARTER Group. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li W, Han T, Qin W, Zhang J, Liu H, Li Y, Meng L, Ji X, Yu C. Altered functional connectivity of cognitive-related cerebellar subregions in well-recovered stroke patients. Neural Plast. 2013;2013:452439. doi: 10.1155/2013/452439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schenberg LC, Bittencourt AS, Sudré EC, Vargas LC. Modeling panic attacks. Neurosci Biobehav Rev. 2001;25:647–659. doi: 10.1016/s0149-7634(01)00060-4. [DOI] [PubMed] [Google Scholar]
- 41.Young RF, Chambi VI. Pain relief by electrical stimulation of the periaqueductal and periventricular gray matter. Evidence for a non-opioid mechanism. J Neurosurg. 1987;66:364–371. doi: 10.3171/jns.1987.66.3.0364. [DOI] [PubMed] [Google Scholar]
- 42.Patel NK, Javed S, Khan S, Papouchado M, Malizia AL, Pickering AE, Paton JF. Deep brain stimulation relieves refractory hypertension. Neurology. 2011;76:405–407. doi: 10.1212/WNL.0b013e3182088108. [DOI] [PMC free article] [PubMed] [Google Scholar]


