Abstract
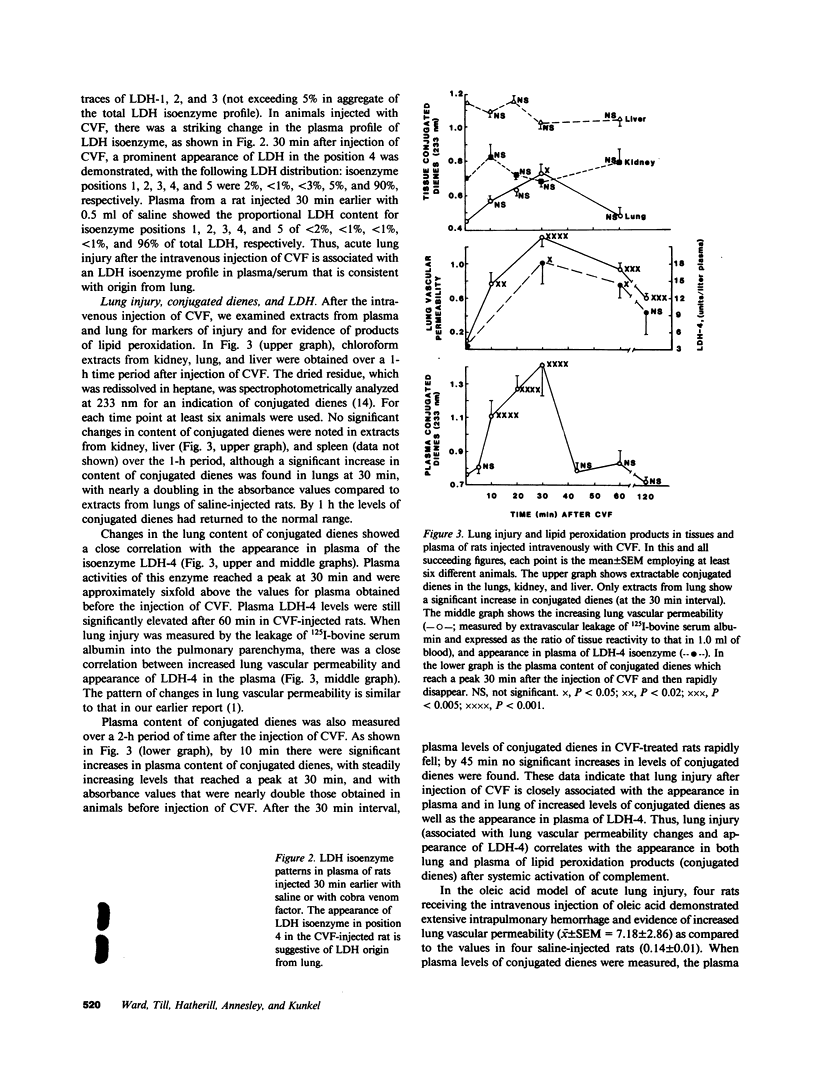
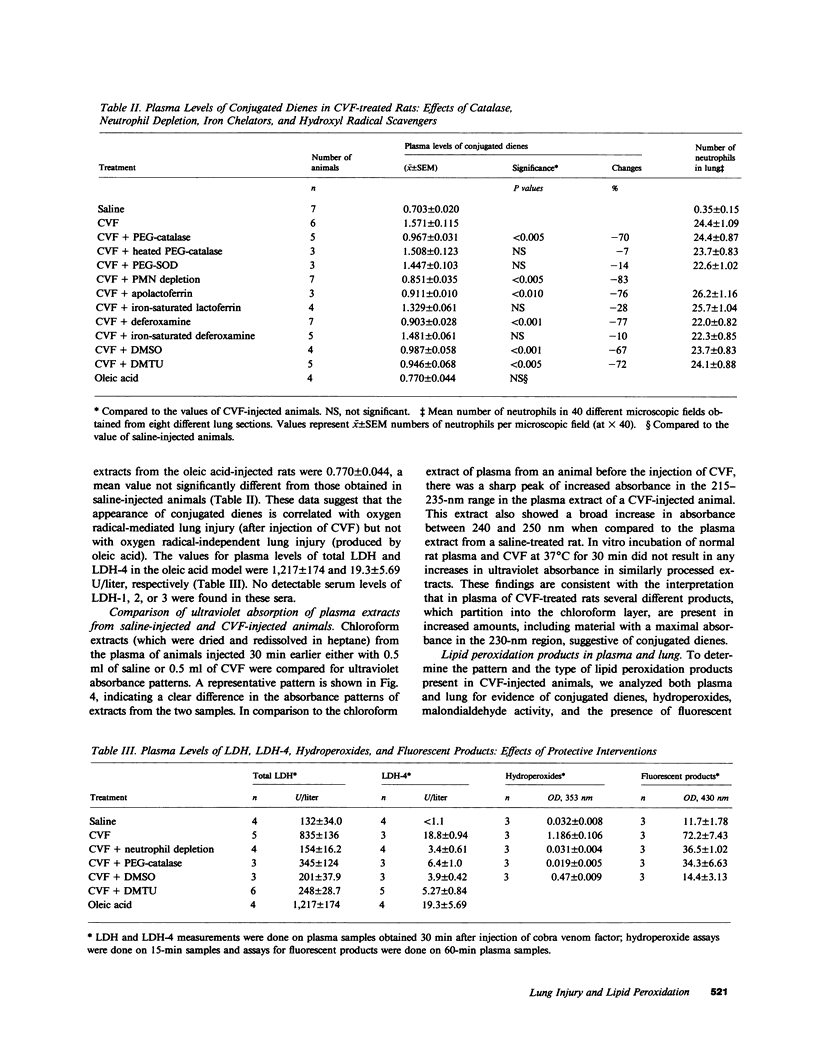
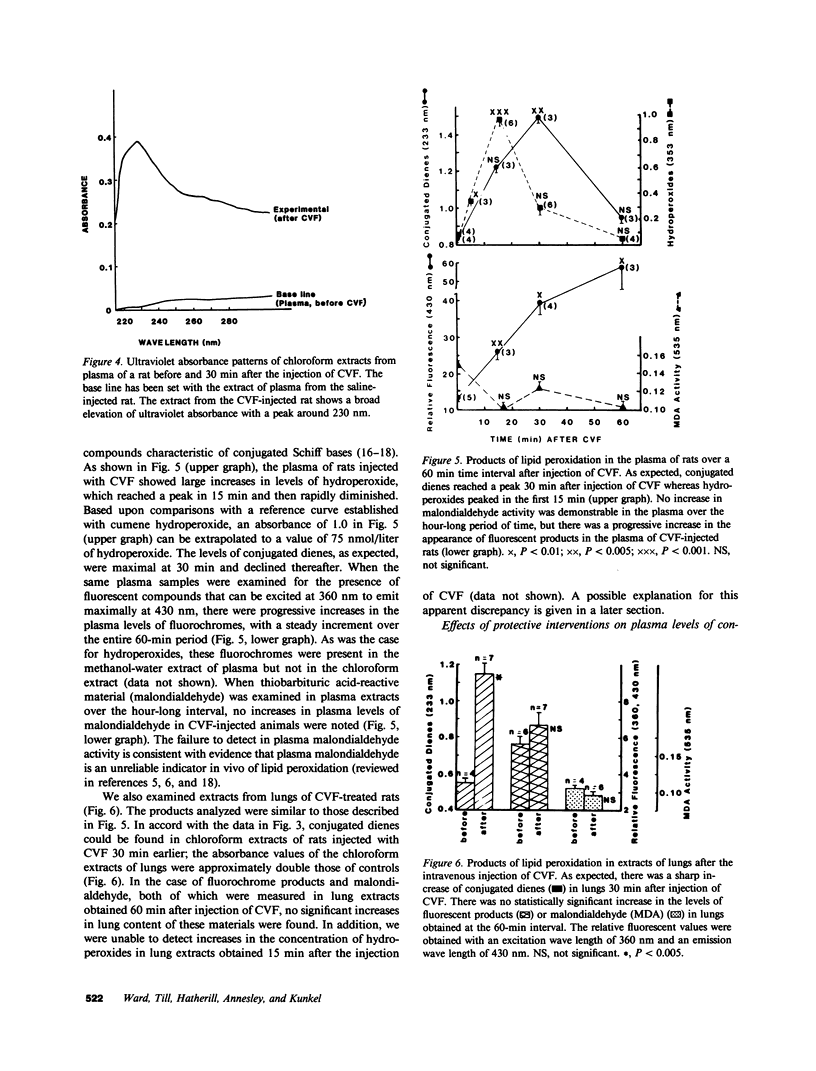
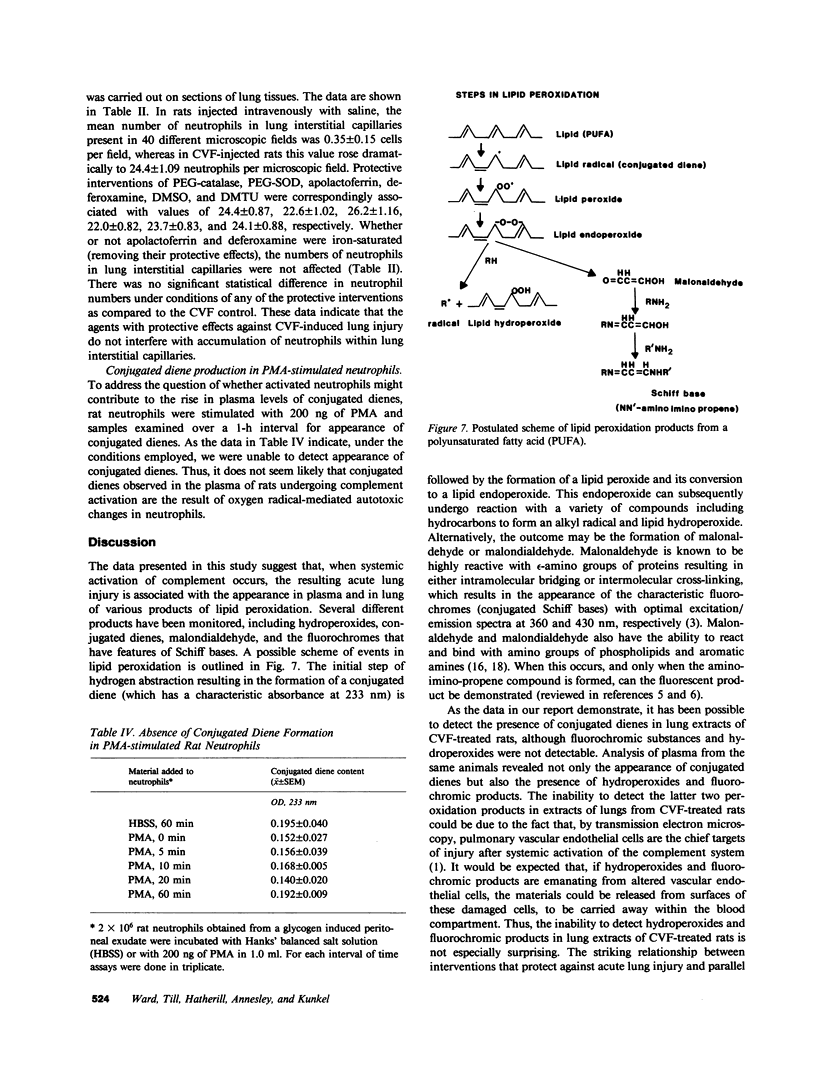
Previously we have demonstrated that systemic activation of the complement system after intravenous injection of cobra venom factor (CVF) results in acute lung injury as reflected by increases in the vascular permeability of the lung as well as by morphologic evidence of damage to lung vascular endothelial cells. In using the vascular permeability of the lung as the reference, the current studies show a quantitative correlation between lung injury and the appearance in plasma of lipid peroxidation products (conjugated dienes) as well as increased concentrations of lactic dehydrogenase (LDH) and one of its isoenzymes (LDH-4). After injection of CVF, extracts of lungs also showed elevated levels of conjugated dienes, whereas no elevations were found in extracts of liver, kidney, and spleen. There was no evidence in CVF-injected rats of renal or hepatic injury as reflected by the lack of development of proteinuria and the failure to detect increased serum levels of liver-related enzymes. Other peroxidation products identified in plasma of CVF-injected rats involved hydroperoxides and fluorescent compounds with features of Schiff bases. Not surprisingly, malondialdehyde was not found to be a reliable plasma indicator of lipid peroxidation associated with oxygen radical-mediated lung vascular injury. In using a model of oxygen radical-independent lung injury induced by oleic acid, although large amounts of LDH and LDH-4 were found in the plasma, no increases in plasma levels of conjugated dienes were detected. In CVF-injected animals treated with interventions protective against lung injury (neutrophil depletion, catalase, hydroxyl radical scavengers, or iron chelators), there were striking reductions in the plasma levels of conjugated dienes, hydroperoxides, and fluorochromic products. Morphometric analysis of lung sections revealed that the protective interventions did not interfere with the accumulation of neutrophils in lung interstitial capillaries after systemic activation of complement. In vitro studies with phorbol-stimulated neutrophils failed to demonstrate appearance of conjugated dienes, suggesting that the dienes appearing in plasma of CVF-injected animals are not the result of autotoxic changes in neutrophils. The data presented in this paper suggest that acute lung injury mediated by oxygen radicals derived from phagocytic cells can be monitored by the appearance in plasma of products of lipid peroxidation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amador E., Massod M. F., Franey R. J. Reliability of glutamic-oxalacetic transaminase methods. Am J Clin Pathol. 1967 Apr;47(4):419–428. doi: 10.1093/ajcp/47.4.419. [DOI] [PubMed] [Google Scholar]
- Bidlack W. R., Tappel A. L. Fluorescent products of phospholipids during lipid peroxidation. Lipids. 1973 Apr;8(4):203–207. doi: 10.1007/BF02544636. [DOI] [PubMed] [Google Scholar]
- Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Chio K. S., Tappel A. L. Inactivation of ribonuclease and other enzymes by peroxidizing lipids and by malonaldehyde. Biochemistry. 1969 Jul;8(7):2827–2832. doi: 10.1021/bi00835a020. [DOI] [PubMed] [Google Scholar]
- Chio K. S., Tappel A. L. Synthesis and characterization of the fluorescent products derived from malonaldehyde and amino acids. Biochemistry. 1969 Jul;8(7):2821–2826. doi: 10.1021/bi00835a019. [DOI] [PubMed] [Google Scholar]
- Claster S., Chiu D. T., Quintanilha A., Lubin B. Neutrophils mediate lipid peroxidation in human red cells. Blood. 1984 Nov;64(5):1079–1084. [PubMed] [Google Scholar]
- Cochrane C. G., Spragg R., Revak S. D. Pathogenesis of the adult respiratory distress syndrome. Evidence of oxidant activity in bronchoalveolar lavage fluid. J Clin Invest. 1983 Mar;71(3):754–761. doi: 10.1172/JCI110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey B. F., Thrall R. S., McCormick J. R., Ward P. A. Oleic-acid-induced lung injury in the rat. Failure of indomethacin treatment or complement depletion to ablate lung injury. Am J Pathol. 1981 Jun;103(3):376–383. [PMC free article] [PubMed] [Google Scholar]
- Dillard C. J., Dumelin E. E., Tappel A. L. Effect of dietary vitamin E on expiration of pentane and ethane by the rat. Lipids. 1977 Jan;12(1):109–114. doi: 10.1007/BF02532981. [DOI] [PubMed] [Google Scholar]
- Dillard C. J., Tappel A. L. Fluorescent products from reaction of peroxidizing polyunsaturated fatty acids with phosphatidyl ethanolamine and phenylalanine. Lipids. 1973 Apr;8(4):183–189. doi: 10.1007/BF02544632. [DOI] [PubMed] [Google Scholar]
- Eiermann G. J., Dickey B. F., Thrall R. S. Polymorphonuclear leukocyte participation in acute oleic-acid-induced lung injury. Am Rev Respir Dis. 1983 Nov;128(5):845–850. doi: 10.1164/arrd.1983.128.5.845. [DOI] [PubMed] [Google Scholar]
- Fox R. B. Prevention of granulocyte-mediated oxidant lung injury in rats by a hydroxyl radical scavenger, dimethylthiourea. J Clin Invest. 1984 Oct;74(4):1456–1464. doi: 10.1172/JCI111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay R. J., McComb R. B., Bowers G. N., Jr Optimum reaction conditions for human lactate dehydrogenase isoenzymes as they affect total lactate dehydrogenase activity. Clin Chem. 1968 Aug;14(8):740–753. [PubMed] [Google Scholar]
- Glader B. E., Conrad M. E. Hemolysis by diphenylsulfones: comparative effects of DDS and hydroxylamine-DDS. J Lab Clin Med. 1973 Feb;81(2):267–272. [PubMed] [Google Scholar]
- Goldstein B. D., Rozen M. G., Amoruso M. A. Relation of fluorescence in lipid-containing red cell membrane extracts to in vivo lipid peroxidation. J Lab Clin Med. 1979 Apr;93(4):687–694. [PubMed] [Google Scholar]
- Gutteridge J. M., Kerry P. J. Detection by fluorescence of peroxides and carbonyls in samples of arachidonic acid. Br J Pharmacol. 1982 Jul;76(3):459–461. doi: 10.1111/j.1476-5381.1982.tb09240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENRY R. J., CHIAMORI N., GOLUB O. J., BERKMAN S. Revised spectrophotometric methods for the determination of glutamic-oxalacetic transaminase, glutamic-pyruvic transaminase, and lactic acid dehydrogenase. Am J Clin Pathol. 1960 Oct;34:381–398. doi: 10.1093/ajcp/34.4_ts.381. [DOI] [PubMed] [Google Scholar]
- Jacob H. S., Lux S. E., 4th Degradation of membrane phospholipids and thiols in peroxide hemolysis: studies in vitamin E deficiency. Blood. 1968 Oct;32(4):549–568. [PubMed] [Google Scholar]
- McCord J. M., Roy R. S. The pathophysiology of superoxide: roles in inflammation and ischemia. Can J Physiol Pharmacol. 1982 Nov;60(11):1346–1352. doi: 10.1139/y82-201. [DOI] [PubMed] [Google Scholar]
- NAGEL W., WILLIG F., SCHMIDT F. H. UBER DIE AMINOSAEUREARYLAMIDASE-(SOG. LEUCINAMINOPEPTIDASE-) AKTIVITAET IM MENSCHLICHEN SERUM. Klin Wochenschr. 1964 May 1;42:447–449. doi: 10.1007/BF01486025. [DOI] [PubMed] [Google Scholar]
- Reiss U., Tappel A. L., Chio K. S. DNA-malonaldehyde reaction: formation of fluorescent products. Biochem Biophys Res Commun. 1972 Aug 21;48(4):921–926. doi: 10.1016/0006-291x(72)90696-1. [DOI] [PubMed] [Google Scholar]
- Romson J. L., Hook B. G., Kunkel S. L., Abrams G. D., Schork M. A., Lucchesi B. R. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983 May;67(5):1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- Tappel A., Fletcher B., Deamer D. Effect of antioxidants and nutrients on lipid peroxidation fluorescent products and aging parameters in the mouse. J Gerontol. 1973 Oct;28(4):415–424. doi: 10.1093/geronj/28.4.415. [DOI] [PubMed] [Google Scholar]
- Till G. O., Johnson K. J., Kunkel R., Ward P. A. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel C. W., Müller-Eberhard H. J. Cobra venom factor: improved method for purification and biochemical characterization. J Immunol Methods. 1984 Oct 12;73(1):203–220. doi: 10.1016/0022-1759(84)90045-0. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Duque R. E., Sulavik M. C., Johnson K. J. In vitro and in vivo stimulation of rat neutrophils and alveolar macrophages by immune complexes. Production of O-2 and H2O2. Am J Pathol. 1983 Mar;110(3):297–309. [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Till G. O., Kunkel R., Beauchamp C. Evidence for role of hydroxyl radical in complement and neutrophil-dependent tissue injury. J Clin Invest. 1983 Sep;72(3):789–801. doi: 10.1172/JCI111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J. The role of superoxide in the destruction of erythrocyte targets by human neutrophils. J Biol Chem. 1980 Oct 25;255(20):9912–9917. [PubMed] [Google Scholar]
- Wilkinson J. H., Boutwell J. H., Winsten S. Evaluation of a new system for the kinetic measurement of serum alkaline phosphatase. Clin Chem. 1969 Jun;15(6):487–495. [PubMed] [Google Scholar]




