Abstract
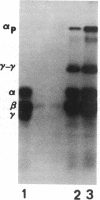
Factor XIII is a blood protransglutaminase that is distributed in plasma and platelets. The extracellular and intracellular zymogenic forms differ in that the plasma zymogen contains A and B subunits, while the platelet zymogen has A subunits only. Both zymogens form the same enzyme. Erythrocytes, in contrast, contain a tissue transglutaminase that is distinct from Factor XIII. In this study other bone marrow-derived cells were examined for transglutaminase activity. Criteria that were used to differentiate Factor XIII proteins from erythrocyte transglutaminase included: (a) immunochemical and immunohistochemical identification with monospecific polyclonal and monoclonal antibodies to Factor XIII proteins, (b) requirement for thrombin cleavage to express activity, (c) pattern of fibrin cross-linking catalyzed by the enzyme, and (d) different electrophoretic mobilities in nondenaturing gel systems. By these criteria human peripheral blood monocytes, peritoneal macrophages, and monocytes maintained in culture contain an intracellular protransglutaminase that is the same as platelet Factor XIII. The monocyte-macrophage protein is thrombin-sensitive, and under appropriate conditions there is no enzyme expression without activation of the zymogen. Both the monocyte-macrophage zymogen and enzyme have the same electrophoretic mobilities as platelet Factor XIII zymogen and enzyme. Antibody to A protein reacts with the monocyte-macrophage protein. B protein is not associated with this intracellular zymogen. By immunoperoxidase staining monocyte-macrophage protein seems to be localized in the cytoplasm, similar to the known cytoplasmic distribution of platelet and megakaryocyte Factor XIII. These procedures were also used to study populations of human granulocytes and lymphocytes, and protransglutaminase activity was not observed in these cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker S., Haskill S. Characterization of the presumptive sarcoma cells in primary MSV tumors. Int J Cancer. 1980 Apr 15;25(4):543–550. doi: 10.1002/ijc.2910250417. [DOI] [PubMed] [Google Scholar]
- Brenner S. C., Wold F. Human erythrocyte transglutaminase. Purification and properties. Biochim Biophys Acta. 1978 Jan 12;522(1):74–83. doi: 10.1016/0005-2744(78)90323-6. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chung S. I. Comparative studies on tissue transglutaminase and factor XIII. Ann N Y Acad Sci. 1972 Dec 8;202:240–255. doi: 10.1111/j.1749-6632.1972.tb16338.x. [DOI] [PubMed] [Google Scholar]
- Credo R. B., Curtis C. G., Lorand L. Ca2+-related regulatory function of fibrinogen. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4234–4237. doi: 10.1073/pnas.75.9.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis C. G., Brown K. L., Credo R. B., Domanik R. A., Gray A., Stenberg P., Lorand L. Calcium-dependent unmasking of active center cysteine during activation of fibrin stabilizing factor. Biochemistry. 1974 Aug 27;13(18):3774–3780. doi: 10.1021/bi00715a024. [DOI] [PubMed] [Google Scholar]
- Curtis C. G., Janus T. J., Credo R. B., Lorand L. Regulation of factor XIIIa generation by fibrinogen. Ann N Y Acad Sci. 1983 Jun 27;408:567–576. doi: 10.1111/j.1749-6632.1983.tb23273.x. [DOI] [PubMed] [Google Scholar]
- Davies P. J., Davies D. R., Levitzki A., Maxfield F. R., Milhaud P., Willingham M. C., Pastan I. H. Transglutaminase is essential in receptor-mediated endocytosis of alpha 2-macroglobulin and polypeptide hormones. Nature. 1980 Jan 10;283(5743):162–167. doi: 10.1038/283162a0. [DOI] [PubMed] [Google Scholar]
- Davies P. J., Murtaugh M. P. Transglutaminase and receptor-mediated endocytosis in macrophages and cultured fibroblasts. Mol Cell Biochem. 1984;58(1-2):69–77. doi: 10.1007/BF00240606. [DOI] [PubMed] [Google Scholar]
- Ewan V. A., Edwards R. L., Rickles F. R. Expression of procoagulant activity in a human monocyte-like cell line. J Lab Clin Med. 1983 Mar;101(3):401–410. [PubMed] [Google Scholar]
- Folk J. E. Structure and catalytic properties of hepatic transglutaminase. Ann N Y Acad Sci. 1972 Dec 8;202:59–76. doi: 10.1111/j.1749-6632.1972.tb16322.x. [DOI] [PubMed] [Google Scholar]
- Folk J. E. Transglutaminases. Annu Rev Biochem. 1980;49:517–531. doi: 10.1146/annurev.bi.49.070180.002505. [DOI] [PubMed] [Google Scholar]
- Ganrot P. O. Crossed immunoelectrophoresis. Scand J Clin Lab Invest Suppl. 1972;124:39–47. doi: 10.3109/00365517209102749. [DOI] [PubMed] [Google Scholar]
- Ikematsu S., McDonagh R. P., Reisner H. M., Skrzynia C., McDonagh J. Immunochemical studies of human factor XIII. J Lab Clin Med. 1981 May;97(5):662–671. [PubMed] [Google Scholar]
- Johnson D. A., Elder J. H. Antibody directed to determinants of a Moloney virus derived MCF GP70 recognizes a thymic differentiation antigen. J Exp Med. 1983 Nov 1;158(5):1751–1756. doi: 10.1084/jem.158.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski M., McDonagh J. Studies on the mechanism of thrombin. Interaction with fibrin. J Biol Chem. 1983 Sep 10;258(17):10530–10535. [PubMed] [Google Scholar]
- Kannagi R., Teshigawara K., Noro N., Masuda T. Transglutaminase activity during the differentiation of macrophages. Biochem Biophys Res Commun. 1982 Mar 15;105(1):164–171. doi: 10.1016/s0006-291x(82)80026-0. [DOI] [PubMed] [Google Scholar]
- Kaplan J., Nielsen M. L. Analysis of macrophage surface receptors. I. Binding of alpha-macroglobulin . protease complexes to rabbit alveolar macrophages. J Biol Chem. 1979 Aug 10;254(15):7323–7328. [PubMed] [Google Scholar]
- Kiesselbach T. H., Wagner R. H. Demonstration of factor XIII in human megakaryocytes by a fluorescent antibody technique. Ann N Y Acad Sci. 1972 Dec 8;202:318–328. doi: 10.1111/j.1749-6632.1972.tb16344.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lorand L., Campbell-Wilkes L. K., Cooperstein L. A filter paper assay for transamidating enzymes using radioactive amine substrates. Anal Biochem. 1972 Dec;50(2):623–631. doi: 10.1016/0003-2697(72)90074-7. [DOI] [PubMed] [Google Scholar]
- Lorand L., Conrad S. M. Transglutaminases. Mol Cell Biochem. 1984;58(1-2):9–35. doi: 10.1007/BF00240602. [DOI] [PubMed] [Google Scholar]
- Lorand L., Siefring G. E., Jr, Tong Y. S., Bruner-Lorand J., Gray A. J., Jr Dansylcadaverine specific staining for transamidating enzymes. Anal Biochem. 1979 Mar;93(2):453–458. doi: 10.1016/s0003-2697(79)80178-5. [DOI] [PubMed] [Google Scholar]
- Lorand L., Urayama T., De Kiewiet J. W., Nossel H. L. Diagnostic and genetic studies on fibrin-stabilizing factor with a new assay based on amine incorporation. J Clin Invest. 1969 Jun;48(6):1054–1064. doi: 10.1172/JCI106061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L., Weissmann L. B., Epel D. L., Bruner-Lorand J. Role of the intrinsic transglutaminase in the Ca2+-mediated crosslinking of erythrocyte proteins. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4479–4481. doi: 10.1073/pnas.73.12.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason T. E., Phifer R. F., Spicer S. S., Swallow R. A., Dreskin R. B. An immunoglobulin-enzyme bridge method for localizing tissue antigens. J Histochem Cytochem. 1969 Sep;17(9):563–569. doi: 10.1177/17.9.563. [DOI] [PubMed] [Google Scholar]
- McDonagh J., Messel H., McDonagh R. P., Jr, Murano G., Blombäck B. Molecular weight analysis of fibrinogen and fibrin chains by an improved sodium dodecyl sulfate gel electrophoresis method. Biochim Biophys Acta. 1972 Jan 26;257(1):135–142. doi: 10.1016/0005-2795(72)90262-0. [DOI] [PubMed] [Google Scholar]
- McDonagh R. P., McDonagh J., Blombäck B. Isolation and characterization of the S-carboxymethyl derivatives of crosslinked and noncrosslinked human fibrin. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3648–3652. doi: 10.1073/pnas.69.12.3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonagh J., Waggoner W. G., Hamilton E. G., Hindenbach B., Mcdonagh R. P. Affinity chromatography of human plasma and platelet factor XIII on organomercurial agarose. Biochim Biophys Acta. 1976 Oct 28;446(2):345–357. doi: 10.1016/0005-2795(76)90002-7. [DOI] [PubMed] [Google Scholar]
- Mosher D. F. Action of fibrin-stabilizing factor on cold-insoluble globulin and alpha2-macroglobulin in clotting plasma. J Biol Chem. 1976 Mar 25;251(6):1639–1645. [PubMed] [Google Scholar]
- Murtaugh M. P., Arend W. P., Davies P. J. Induction of tissue transglutaminase in human peripheral blood monocytes. J Exp Med. 1984 Jan 1;159(1):114–125. doi: 10.1084/jem.159.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh M. P., Mehta K., Johnson J., Myers M., Juliano R. L., Davies P. J. Induction of tissue transglutaminase in mouse peritoneal macrophages. J Biol Chem. 1983 Sep 25;258(18):11074–11081. [PubMed] [Google Scholar]
- Musson R. A., Henson P. M. Humoral and formed elements of blood modulate the response of peripheral blood monocytes. I. Plasma and serum inhibit and platelets enhance monocyte adherence. J Immunol. 1979 May;122(5):2026–2031. [PubMed] [Google Scholar]
- Novogrodsky A., Quittner S., Rubin A. L., Stenzel K. H. Transglutaminase activity in human lymphocytes: early activation by phytomitogens. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1157–1161. doi: 10.1073/pnas.75.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterud B., Lindahl U., Seljelid R. Macrophages produce blood coagulation factors. FEBS Lett. 1980 Oct 20;120(1):41–43. doi: 10.1016/0014-5793(80)81041-6. [DOI] [PubMed] [Google Scholar]
- Rider D. M., McDonagh J. Resistance of factor XIII to degradation or activation by plasmin. Biochim Biophys Acta. 1981 Jul;675(2):171–177. doi: 10.1016/0304-4165(81)90223-3. [DOI] [PubMed] [Google Scholar]
- SWIGERT S., KOPPEL J. L., OLWIN J. H. Selective inactivation of fibrin stabilizing factor contaminant in fibrinogen. Nature. 1963 May 25;198:797–798. doi: 10.1038/198797a0. [DOI] [PubMed] [Google Scholar]
- Schroff G., Neumann C., Sorg C. Transglutaminase as a marker for subsets of murine macrophages. Eur J Immunol. 1981 Aug;11(8):637–642. doi: 10.1002/eji.1830110809. [DOI] [PubMed] [Google Scholar]
- Schwartz B. S., Levy G. A., Fair D. S., Edgington T. S. Murine lymphoid procoagulant activity induced by bacterial lipopolysaccharide and immune complexes is a monocyte prothrombinase. J Exp Med. 1982 May 1;155(5):1464–1479. doi: 10.1084/jem.155.5.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. L., Pizzo S. V., Hill R. L., McKee P. A. Human Factor XIII from plasma and platelets. Molecular weights, subunit structures, proteolytic activation, and cross-linking of fibrinogen and fibrin. J Biol Chem. 1973 Feb 25;248(4):1395–1407. [PubMed] [Google Scholar]
- Sherman L. A., Lee J. L., Stewart C. C. Release of fibrinolytic enzymes by macrophages in response to soluble fibrin. J Reticuloendothel Soc. 1981 Nov;30(5):317–329. [PubMed] [Google Scholar]
- Skrzynia C., Reisner H. M., McDonagh J. Characterization of the catalytic subunit of factor XIII by radioimmunoassay. Blood. 1982 Nov;60(5):1089–1095. [PubMed] [Google Scholar]
- Sottrup-Jensen L., Stepanik T. M., Wierzbicki D. M., Jones C. M., Lønblad P. B., Kristensen T., Mortensen S. B., Petersen T. E., Magnusson S. The primary structure of alpha 2-macroglobulin and localization of a Factor XIIIa cross-linking site. Ann N Y Acad Sci. 1983;421:41–60. doi: 10.1111/j.1749-6632.1983.tb18091.x. [DOI] [PubMed] [Google Scholar]
- Stenberg P., Stenflo J. A rapid and specific fluorescent activity staining procedure for transamidating enzymes. Anal Biochem. 1979 Mar;93(2):445–452. doi: 10.1016/s0003-2697(79)80177-3. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy P. B., Rohrbach M. S., Mann K. G. Functional prothrombinase complex assembly on isolated monocytes and lymphocytes. J Biol Chem. 1983 Jun 25;258(12):7264–7267. [PubMed] [Google Scholar]
- Tycko B., DiPaola M., Yamashiro D. J., Fluss S., Maxfield F. R. Acidification of endocytic vesicles and the intracellular pathways of ligands and receptors. Ann N Y Acad Sci. 1983;421:424–433. doi: 10.1111/j.1749-6632.1983.tb18136.x. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]