Abstract
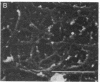
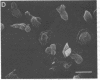
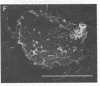
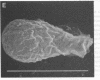
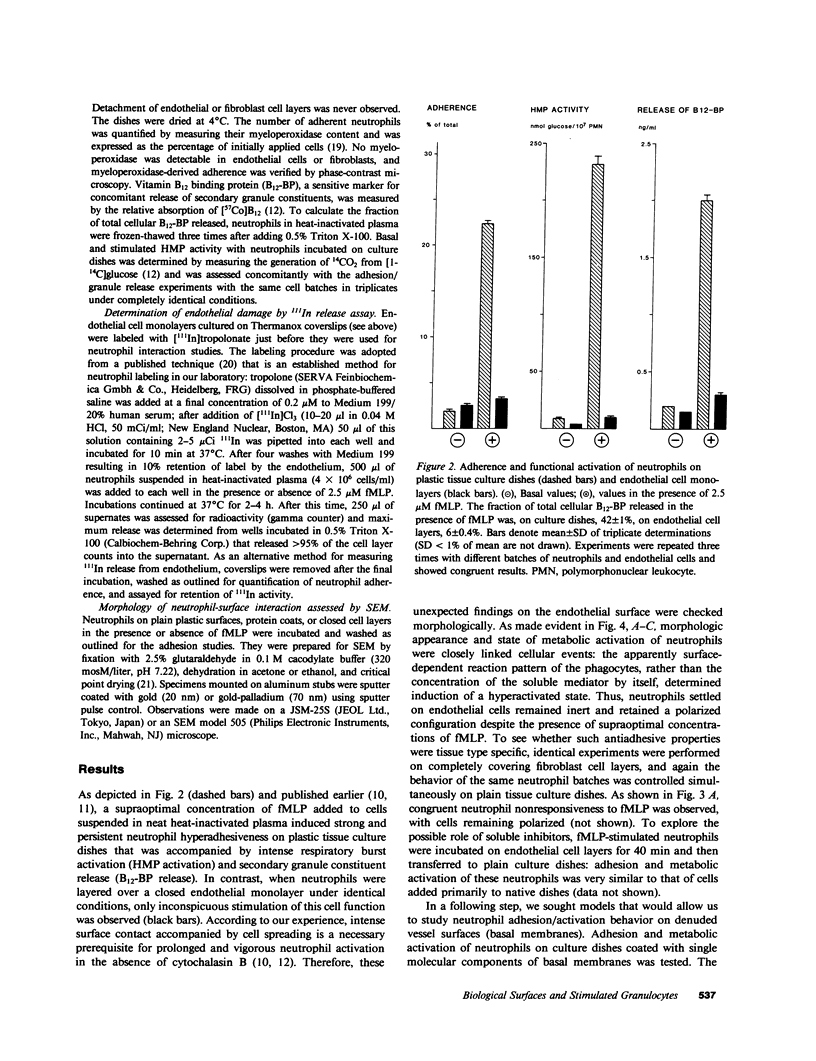
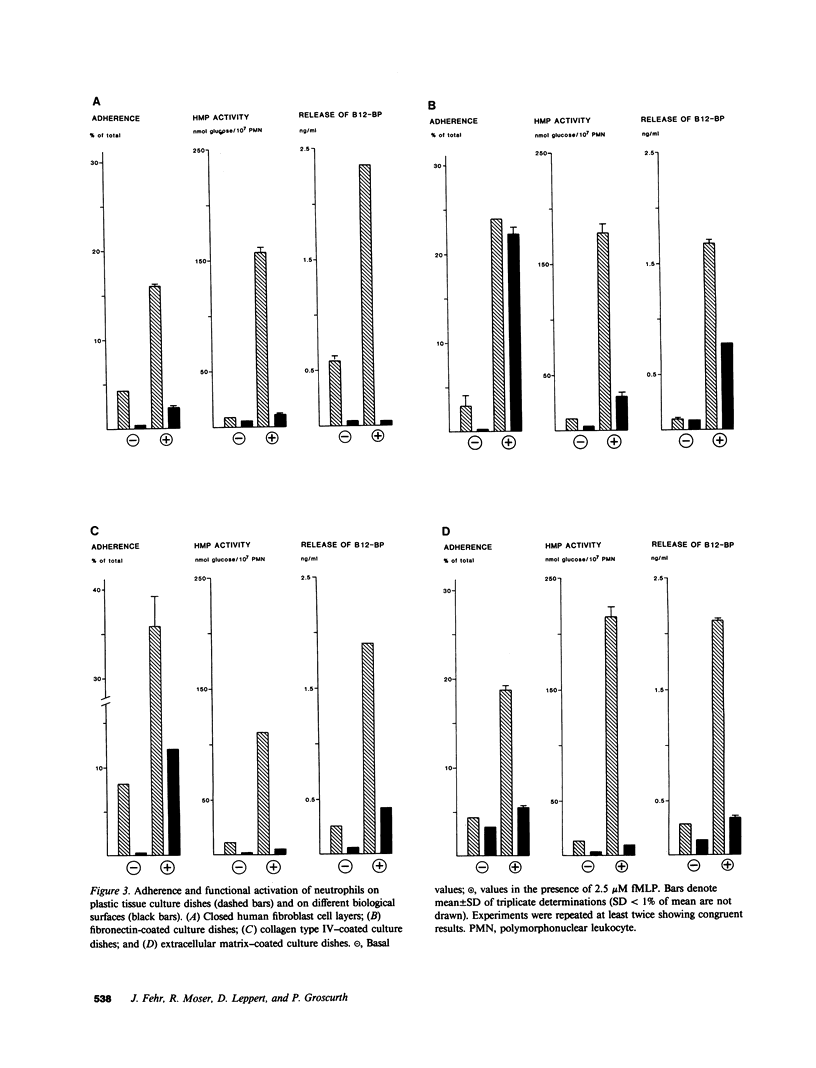
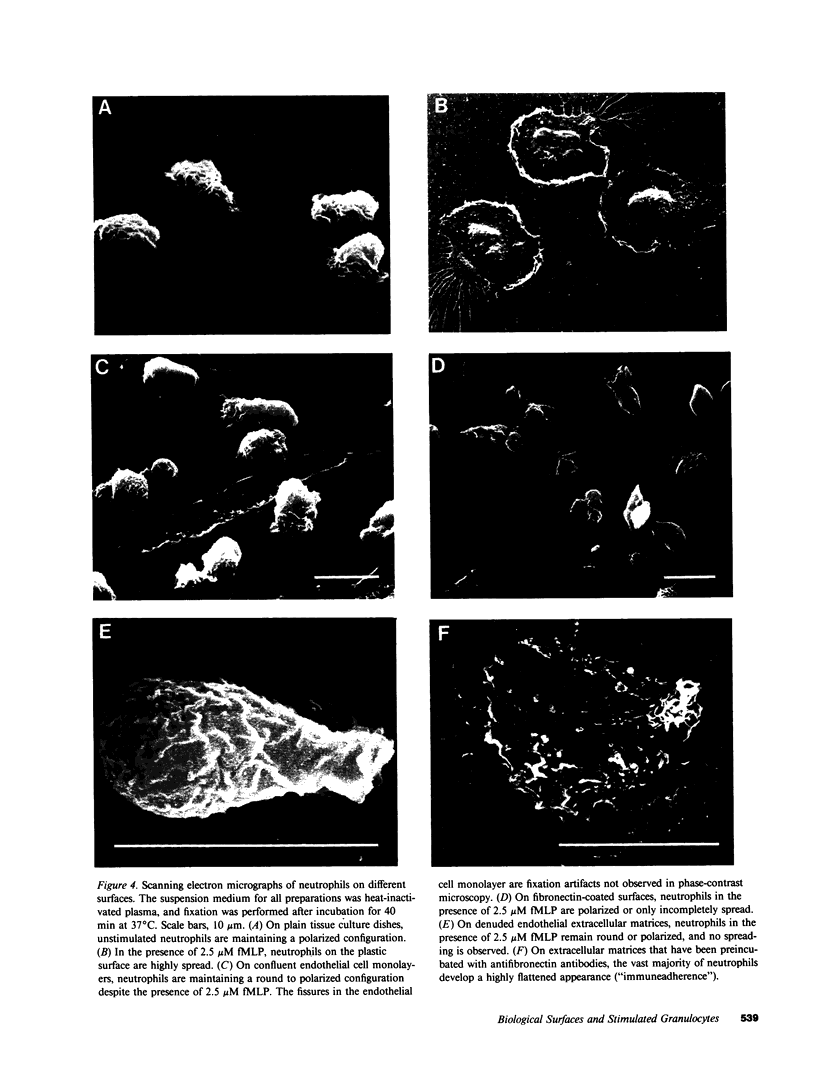
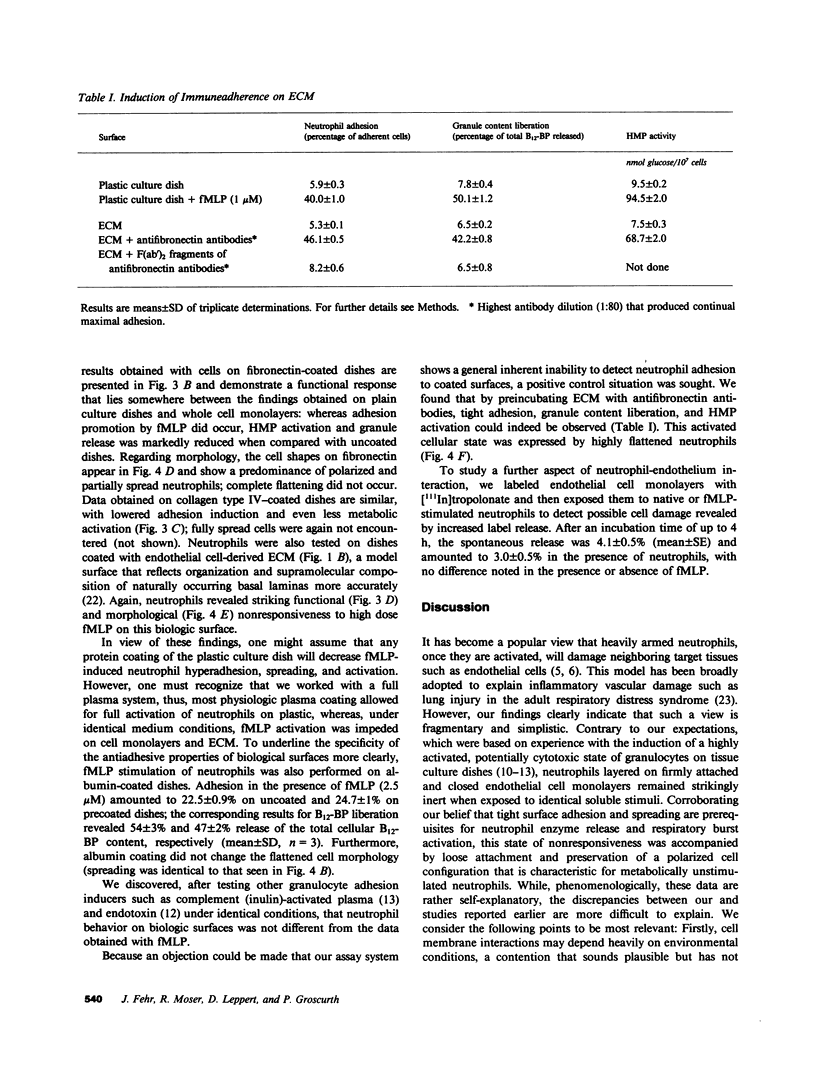
Despite the fact that a series of endogenous and exogenous inflammatory mediators are potent activators of circulating granulocytes, damage of vascular endothelium, a primary target tissue, is a rather unusual event in systemic inflammatory states. Since mediator-induced neutrophil hyperadhesiveness on plastic tissue culture dishes is invariably accompanied by intense release of lysosomal granule constituents and respiratory burst activation, thus representing a powerful model to investigate neutrophil cytotoxic states, comparative studies with neutrophils suspended in autologous plasma in the presence or absence of N-formyl-Met-Leu-Phe (2.5 microM), the most potent adhesion inducer, were performed on different biologic surfaces. On optimally adherent closed monolayers of cultured endothelial cells or fibroblasts we observed poor stimulation of adhesion as well as minimal granule release and hexose monophosphate pathway activation. Functional behavior of neutrophils on single molecular components of basal laminas such as fibronectin and collagen (type IV) coats was intermediate, with positive adhesion promotion but markedly reduced metabolic activation. When tested on endothelial cell-derived extracellular matrices, neutrophils again showed functional nonresponsiveness to N-formyl-Met-Leu-Phe. Scanning electron microscopy revealed an impressive congruency between the degree of cellular spreading and metabolic activation in the presence of N-formyl-Met-Leu-Phe, with maximally flattened neutrophils on plastic vs. nonspread, polarized cells on monolayers. Identical results were obtained by using other adhesion inducers such as complement-activated plasma or endotoxin. Lack of cell injury by N-formyl-Met-Leu-Phe-exposed neutrophils was corroborated by the absence of tracer release from [111In]tropolonate-labeled endothelium. These results indicate that biologic surfaces possess antiadhesive properties that protect them from cytotoxic damage by stimulated angry phagocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Schwartz J. H., Tauber A. I. Proton secretion by stimulated neutrophils. Significance of hexose monophosphate shunt activity as source of electrons and protons for the respiratory burst. J Clin Invest. 1984 Aug;74(2):455–459. doi: 10.1172/JCI111442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahinden C., Fehr J. Receptor-directed inhibition of chemotactic factor-induced neutrophil hyperactivity by pyrazolon derivatives. Definition of a chemotactic peptide antagonist. J Clin Invest. 1980 Nov;66(5):884–891. doi: 10.1172/JCI109955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahinden C., Galanos C., Fehr J. Granulocyte activation by endotoxin. I. Correlation between adherence and other granulocyte functions, and role of endotoxin structure on biologic activity. J Immunol. 1983 Feb;130(2):857–862. [PubMed] [Google Scholar]
- Danpure H. J., Osman S., Brady F. The labelling of blood cells in plasma with 111In-tropolonate. Br J Radiol. 1982 Mar;55(651):247–249. doi: 10.1259/0007-1285-55-651-247. [DOI] [PubMed] [Google Scholar]
- Fehr J., Dahinden C. Modulating influence of chemotactic factor-induced cell adhesiveness on granulocyte function. J Clin Invest. 1979 Jul;64(1):8–16. doi: 10.1172/JCI109466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr J., Huber A. Complement-induced granulocyte adhesion and aggregation are mediated by different factors: evidence for non-equivalence of the two cell functions. Immunology. 1984 Nov;53(3):583–593. [PMC free article] [PubMed] [Google Scholar]
- Fehr J., Jacob H. S. In vitro granulocyte adherence and in vivo margination: two associated complement-dependent functions. Studies based on the acute neutropenia of filtration leukophoresis. J Exp Med. 1977 Sep 1;146(3):641–652. doi: 10.1084/jem.146.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr Culture of vascular endothelium. Prog Hemost Thromb. 1976;3:1–28. [PubMed] [Google Scholar]
- Goldstein I. M., Kaplan H. B., Edelson H. S., Weissmann G. Ceruloplasmin. A scavenger of superoxide anion radicals. J Biol Chem. 1979 May 25;254(10):4040–4045. [PubMed] [Google Scholar]
- Gospodarowicz D., Lui G. M. Effect of substrata and fibroblast growth factor on the proliferation in vitro of bovine aortic endothelial cells. J Cell Physiol. 1981 Oct;109(1):69–81. doi: 10.1002/jcp.1041090109. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Harker L. A., Striker G. E., Wright D. G. Neutrophil-mediated endothelial injury in vitro mechanisms of cell detachment. J Clin Invest. 1981 Dec;68(6):1394–1403. doi: 10.1172/JCI110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Levine J. D., Callahan K. S., Schwartz B. R., Harker L. A. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J Clin Invest. 1984 Mar;73(3):706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. Mechanisms of exocytosis in phagocytic inflammatory cells. Parke-Davis Award Lecture. Am J Pathol. 1980 Dec;101(3):494–511. [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppel P., Peterhans E., Bertoni G., Keist R., Groscurth P., Wyler R., Keller R. Induction of chemiluminescence during interaction of tumoricidal effector cell populations and tumor cells is dependent on the presence of mycoplasma. J Immunol. 1984 Apr;132(4):2021–2029. [PubMed] [Google Scholar]
- MacGregor R. R., Friedman H. M., Macarak E. J., Kefalides N. A. Virus infection of endothelial cells increases granulocyte adherence. J Clin Invest. 1980 Jun;65(6):1469–1477. doi: 10.1172/JCI109811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag T., Hoover G. A., Stemerman M. B., Weinstein R. Serial propagation of human endothelial cells in vitro. J Cell Biol. 1981 Nov;91(2 Pt 1):420–426. doi: 10.1083/jcb.91.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri J. A. Endothelial cell-matrix interactions in hemostasis. Prog Hemost Thromb. 1982;6:1–24. [PubMed] [Google Scholar]
- Rinaldo J. E., Rogers R. M. Adult respiratory-distress syndrome: changing concepts of lung injury and repair. N Engl J Med. 1982 Apr 15;306(15):900–909. doi: 10.1056/NEJM198204153061504. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C., Patrick R. A., Hassett C. Motility and adhesiveness in human neutrophils. Effects of chemotactic factors. J Clin Invest. 1979 Feb;63(2):221–229. doi: 10.1172/JCI109293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Vercellotti G. M., McCarthy J., Furcht L. T., Jacob H. S., Moldow C. F. Inflamed fibronectin: an altered fibronectin enhances neutrophil adhesion. Blood. 1983 Nov;62(5):1063–1069. [PubMed] [Google Scholar]
- Weiss S. J., LoBuglio A. F. Phagocyte-generated oxygen metabolites and cellular injury. Lab Invest. 1982 Jul;47(1):5–18. [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Smolen J. E., Hoffstein S. Polymorphonuclear leukocytes as secretory organs of inflammation. J Invest Dermatol. 1978 Jul;71(1):95–99. doi: 10.1111/1523-1747.ep12544444. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Smolen J. E., Korchak H. M. Release of inflammatory mediators from stimulated neutrophils. N Engl J Med. 1980 Jul 3;303(1):27–34. doi: 10.1056/NEJM198007033030109. [DOI] [PubMed] [Google Scholar]