Abstract
Exposure to house dust is a significant source of exposure to flame retardant chemicals (FRs), particularly in the US. Given the high exposure there is a need to understand the bioaccessibility of FRs from dust. In this study, Tenax beads (TA) encapsulated within a stainless steel insert were used as an adsorption sink to estimate the dynamic absorption of a suite of FRs commonly detected in indoor dust samples (n = 17), and from a few polyurethane foam samples for comparison. Organophosphate flame retardants (OPFRs) had the highest estimated bioaccessibility (∼80%) compared to brominated compounds (e.g., PBDEs), and values generally decreased with increasing Log Kow, with <30% bioaccessibility measured for BDE209. These measurements were in very close agreement with reported PBDE bioavailability measures from an in vivo rat exposure study using indoor dust. The bioaccessibility of very hydrophobic FRs (Log Kow > 6) in foam was much less than that in house dust, and increasing bioaccessibility was observed with decreasing particle size. In addition, we examined the stability of more labile FRs containing ester groups (e.g., OPFRs and 2-ethylhexyl-tetrabromo-benzoate (EH-TBB)) in a mock-digestive fluid matrix. No significant changes in the OPFR concentrations were observed in this fluid; however, EH-TBB was found to readily hydrolyze to tetrabromobenzoic acid (TBBA) in the intestinal fluid in the presence of lipases. In conclusion, our study demonstrates that the bioaccessibility and stability of FRs following ingestion varies by chemical and sample matrix and thus should be considered in exposure assessments.
Introduction
Flame retardants (FRs) are common additives applied to consumer products and construction materials. As additives they are not chemically bound to these components and over time they migrate out. Due to high octanol-air partitioning coefficients (Log KOA), many FRs are ubiquitous and abundant in house dust.1,2 Several studies have now found that house dust ingestion is one of the most important exposure pathways for FRs, especially for infants and toddlers.2,3 In current risk assessments, 100% bioaccessibility is often assumed when evaluating human exposure to FRs in house dust. However, previous studies have shown that hydrophobic organic compounds sorbed to organic matter (e.g., soil and sediment), cannot be completely released from these matrices and subsequently absorbed into the gastrointestinal tract.4,5 Therefore, understanding the bioaccessibility of FRs in dust is of great significance for adequate risk evaluations.
Though some studies have examined the bioaccessibility (i.e., the fraction which can desorb from the ingested matrix) of polybrominated diphenyl ethers (PBDEs) in house dust,6,7 no information is available for several new alternate FRs such as organophosphate FRs (OPFRs) and Firemaster550 (FM550), which are the major replacements for the pentaBDE commercial formulations following their phase-out.8,9 Furthermore, FRs in dust may have heterogeneous sources. FRs may be sorbed to organic material in the dust following partitioning from air, or be associated with debris in dust that results from product weathering (e.g., foam or plastic weathering). Using microscopic forensic methods, Webster et al.10 found that a strong bromine signal in a dust sample was associated with particles/debris suggestive of weathered commercial products. In another study using dust collected from a gymnasium, an abundance of polyurethane foam (PUF) debris was observed using scanning electron microscopy,9 again suggesting the FR signatures in dust may be associated with weathered materials/polymers. Infants or toddlers also tend to mouth toys or furniture made of PUF impregnated with FRs and phthalate additives. Therefore, it is important to evaluate the bioaccessibility of FRs from both dust particles and PUF material.
In vitro physiologically based extraction methods are predominately used in bioaccessibility studies due to the advantages of reduced cost/time and animal use. Various models have been proposed and most of them use simulated digestive fluid to sequentially, or continuously extract contaminants within a matrix during relevant physiological residence times.11 However, absorption in the gastro-intestinal tract is a dynamic process and traditional in vitro methods might underestimate the bioaccessibility due to a failure to maintain and consider the concentration gradient, especially for very hydrophobic compounds. Recent studies found that sorption-assisted bioaccessible extractions using a silicon rod, an activated carbon impregnated silicon rod, or a C18 membrane as an “infinite sink” could increase the bioaccessibility of polycyclic aromatic hydrocarbons (PAHs) in sediment, and were more comparable with in vivo studies.12−14 However, activated carbon impregnated silicon rods may may not be amenable to back-extraction, and silicon rods need large surface areas (∼2 m) to ensure a high sorption capacity. Tenax beads (TA), a porous polymer with desirable adsorption/desorption characteristics, have been validated as an effective material to evaluate bioaccessibilty due to their strong sorption capacity, easy back extraction and ability to be recycled. A 6 h TA extraction was widely used to predict the bioavailability of PAHs and pesticides in soils and sediments.15−17 Therefore, it seems feasible to predict that TA may also predict bioaccessibility of FRs in dust; however, to the authors’ knowledge, no studies have investigated this potential application.
Another knowledge gap is the stability of FRs in digestive fluids. Due to stricter environmental health regulations and intense public awareness, the FR market has moved from persistent FRs like PBDEs to less persistent FRs such as OPFRs and FM550.1 These latter chemicals have more labile functional groups, such as phosphate and carboxylic esters, which could be vulnerable to nucleophilic reactions. The half-times of these esters in water (pH 7) vary significantly between chemicals, which could range from several minutes to years.18 Furthermore, hydrolysis reactions might also be different across the dynamic pH conditions along the human digestive system. Thus, it is important to examine the stability of these less stable FRs in digestive fluids. Given these issues, the primary objectives of this study were to (1) develop an effective TA-sorption assisted in vitro physiologically based bioaccessible extraction method; (2) examine the bioaccessibility of OPFRs, FM550, and PBDEs in house dust samples; (3) test the bioaccessibility of several FRs in PUF and its dependence on particle size; and (4) investigate the stability of several less persistent FRs, for example, OPFRs and components of FM550, in simulated digestive fluids.
Materials and Methods
Design of the TA-Assisted Bioaccessible Extraction Method
In our preliminary experiment, we found that the bile salts could precipitate the TA, which was probably due to the decreased tension force caused by the biodetergent. Therefore, TA could not be used in the bioaccessible experiment until good separation from dust after incubation was achieved. In this study, TA beads (60–80 mesh, Supelco) were first cleaned by sonication using acetone:hexane (1:1, v/v) and sieved through 100 mesh (152 μm, USA standard testing sieve) to minimize the lost of small beads during the experiment. An insert was designed for use in this study (see Supporting Information (SI) Figure S1) that would contain the TA. A 100 mesh stainless steel material (Small Parts, Logansport, IN) was cut into ∼11 × 7 cm (length × width) dimensions. The mesh was rolled and fixed at the ends with 0.4 mm copper wire. A half-cut 4 mL glass vial was inserted to one end as a cap. After loading TA (0.5 g), another precleaned 4 mL vial was used as a cap on the other side. After incubation, the TA insert was rinsed thoroughly with deionized water to remove any dust residue attached to the TA, which were then collected in an aluminum weight boat. The rinsing water was combined with the colon fluid. Most of the dust remained in the colon fluid due to its smaller size (<60 μm) and rinsing step could further separate TA from dust matrix. After collecting the beads, the insert was extracted together with the dust to guarantee that dust sticking to the stainless steel mesh insert could be recovered. Diffusion of methylene blue was used to confirm circulation of the digestive fluid across the stainless mesh and the result showed that the circulation was very efficient without any blockage. In this study we used a house dust Standard Reference Material (SRM) 2585 (National Institute of Standards and Technology (NIST), Gaithersburg, MD) to validate the method. One advantage was that a previous in vivo study used SRM2585 to examine PBDE bioavailability in rats,19 making it possible to compare the in vitro and in vivo data.
Dust and PUF Preparation
Indoor dust samples (n = 17) collected during our previous studies20−22 were used here to examine bioaccessibility in actual dust samples. Since most ingestible dust particles adhering to hands have a diameter less than 60 μm,23 all the dust used in this study was first sieved to <53 μm. To investigate the factors affecting the bioaccessibility in the house dust, total organic carbon (TOC), nitrogen, and hydrogen content were analyzed by Elemental Analyzer Vario MICRO Cube (Elementar). Log Kow values of PBDEs were taken from a previous study24 and values for 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB), bis(2-ethylhexyl) tetrabromophthalate (BEH-TEBP), and all the OPFRs were calculated using EPI suite (EPIWEB 4.1). PUFs treated either with TCDPP, FM550, or penta-BDEs were selected to study FR bioaccessibility in commercial products due to their frequent detection.25,26 To determine if particle size affected bioaccessibility, PUF samples were first freeze-dried in liquid nitrogen and then scraped across a stainless mesh sieve with apertures of 500, 250, and 106 μm in series. An attempt to further fragment the foam to <53 μm failed due to very limited yield rate.
Physiologically Based Extraction of FRs in Dust and PUFs
The procedure used here was modified from a recently developed colon-extended physiological based extraction method27 (See flowchart diagram in SI Figure S2). The composition of each type of simulated digestive fluid followed a previous published study13 with the addition of porcine lipase (Type II, 100–400 units/mg protein using olive oil, Sigma-Aldrich, St Louis, MO) at a final concentration of 1.6 mg/mL in the intestinal fluid. 50 mL glass centrifuge tubes were used for the incubation and fixed onto a rotatory device (RKVS, Appropriate Technical Resources, Inc., Laurel, MD), which was used to agitate the mixture with a speed of ∼40 resolution per minute. The incubation was maintained at 37 °C in an oven (Precision, Thermo). Briefly, ∼0.4 g house dust was incubated with 0.5 g TA as well as 45 mL prewarmed simulated gastric fluid for 1.5 h. Then sodium carbonate (NaHCO3) was added to adjust pH to ∼6.5 and bile salts (bovine and ovine, Sigma-Aldrich), lipases, and pancreatin (porcine, 8 USP, Sigma-Aldrich) were added to prepare the intestinal fluid. After incubating for ∼4 h, TA insert was taken out and dust was separated from intestinal fluid by centrifugation at 1000g for 10 min. Then TA insert was reinstalled and the colon fluid was added, followed by incubation for ∼16 h. Bead collection is described above and dust was separated by centrifugation. The experiments with the PUF (∼20 mg for each incubation) were conducted in a similar manner as the dust samples except that a different method was used to separate foam particles from the digestive fluid. Centrifugation did not work well due to the resuspension of foam particles. Instead, the digestive fluid was filtered through glass wool packed in a 15 mL serological borosilicate glass pipet. The glass wool containing the foam particles was recovered by pumping air into the pipet from the bottom.
Hydrolysis Experiments for OPFRs and FM550
The FM550 commercial mixture (Great Lakes Chemical, West Lafayette, IN) and OPFRs including tris(2-chloroethyl) phosphate (TCEP), TDCIPP, and tris (1-chloro-2-propyl) phosphate (TCIPP) dissolved in methanol were spiked into 100 mL gastric fluid, intestinal fluid, and colon fluid stored separately in amber glass bottles to obtain a final concentration ∼200 ng/mL for each compound. An additional 1.5 mL of methanol was added to increase the solubility of hydrophobic compounds like EH-TBB and BEH-TEBP in the mixtures. Glass coated stir bars were used to minimize the sorption onto the coating material. One mL aliquots (in duplicate) were transferred to 6 mL precleaned glass tubes at selected sampling times (from 0 to 20 h) and the reaction was immediately quenched by adding 100 μL 6 M HCl. One aliquot was spiked with a monofluorinated tetrabrominated diphenyl ether (F-BDE69), deuterated TDCIPP (d-TDCIPP), and d-TPHP as surrogates to quantify EH-TBB/BEH-TEBP, and other OPFRs, respectively. The other aliquot was spiked with 2,3,5 triiodo-benzoic acid (TIBA; 98%, Sigma-Aldrich, St. Louis, MI) to quantify the metabolite TBBA from EH-TBB. Both aliquots were warmed at 50 °C for 15 min to further denature the protein. Parent compounds were extracted using liquid–liquid extraction with hexane: ethyl acetate (1:1) three times. Extraction and analysis of TBBA is based on a method in a previous study.28 Briefly, the sample was extracted with acetone:water (1:1, v/v), concentrated, and cleaned using an Agilent-OPT SPE column. One control sample (without lipase and pancreatin, but with the FR spike) was run alongside to observe the effect of lipases on the hydrolysis of OPFRs and FM550. After confirming the formation of TBBA from EH-TBB in the intestinal fluid, a further measurement of the bioaccessibility of TBBA was assessed to determine if this metabolite could be absorbed during the residence time in the intestinal tract. TBBA was spiked into simulated intestinal fluids with two different pH values (5.7 and 8.0) and 0.6 g TA was added. Aliquots of 1 mL fluid were transferred to 6 mL borosilicate tubes at different sampling time and the concentration of TBBA was analyzed in each sample.
Chemical Analysis
A detailed chemical analysis of FRs in dust/foam, TA, and digestive fluids are described in the SI.
Data Analysis and Quality Control
Bioaccessibility in this study was calculated using the following equation:
Bioaccessibility = 1 – (FRs remaining in dust (or foam) after incubation/the sum of FRs measured in the dust (or foam), TA and digestive fluid). SRM2585 was used as the reference material to observe the intraday (n = 3) and interday variability (n = 3). The relative standard deviation of both was less than 15% for most compounds. In the foam extraction, duplicate samples were prepared for each sample. The recoveries of F-BDE69, 13C-BDE209, and OPFR surrogate standard ranged from 65 to 120%, 50–110%, and 71–115%; respectively, during sample solvent extraction and cleanup. All statistical analyses were conducted using SigmaPlot 12.0 software, testing hypotheses at α = 0.05, and all tests were two-tailed. When comparing the aging effect in dust samples, a two factor ANOVA analysis was used.
Result and Discussion
Performance of the TA-Assisted Method
To validate the sorption efficiency of PBDEs and less hydrophobic OPFRs by TA, sorption kinetics of the FRs were first investigated in the three digestive fluids using pure chemicals. Solutions containing either low or high levels of OPFRs and PBDEs (in methanol) were spiked into each digestive fluid with a final concentration of either ∼10 ng/mL (low dose) or ∼2 μg/mL (high dose) for each FR, which span typical concentrations of FRs measured in house dust extracts. Additional methanol was added to make a final concentration of 1% methanol in the digestive fluid to increase the solubility at the initiation of the experiment. Duplicate samples of 0.5 mL each were collected at various incubation time points, chemically analyzed and averaged. As shown in SI Figure S4, the majority of the FRs partitioned from the digestive fluid into the TA within 2 h of incubation, especially in the gastric and intestinal fluid for both dose levels. The sorption kinetics of OPFRs and BDE209 in the low level spike (∼10 ng/mL) are not shown and were below detection limits. It did appear that the sorption of higher molecular weight PBDEs, such as BDE209, was slower than for the lower molecular weight congeners, and sorption of FRs in the intestinal fluid appeared to occur more quickly than in the gastric and colon fluid. This might be due to the presence of high levels of bile salts, which could increase the solubility and diffusion of hydrophobic FRs. Even for the less hydrophobic TCEP (logKow ∼ 1.78), more than 90% of the spiked TCEP partitioned to the TA after a 6 h incubation. Therefore, considering the average residence time in the human digestive tract, sorption of the FRs to the TA will not be a rate-limiting step for the FRs studied here.
To test the efficacy of separation between TA and dust using this method, the recovered mass of SRM2585 (n = 3) and TA (n = 3) following the incubation was recorded. Triplicate incubations of SRM2585 without TA was also run alongside the samples for comparison. As shown in SI Figure S3, more than 94% of the added TA (by mass) could be recovered using the designed TA trap. The recovery of the dust incubated with TA was no different than the one without TA; however, the mass of dust recovered was low overall (∼60%). The low recovery may be due to the loss of either inorganic carbon in the acidic gastric fluid or dissolved organic matter in the fluid.
Comparing In Vitro and In Vivo Methods Using SRM2585
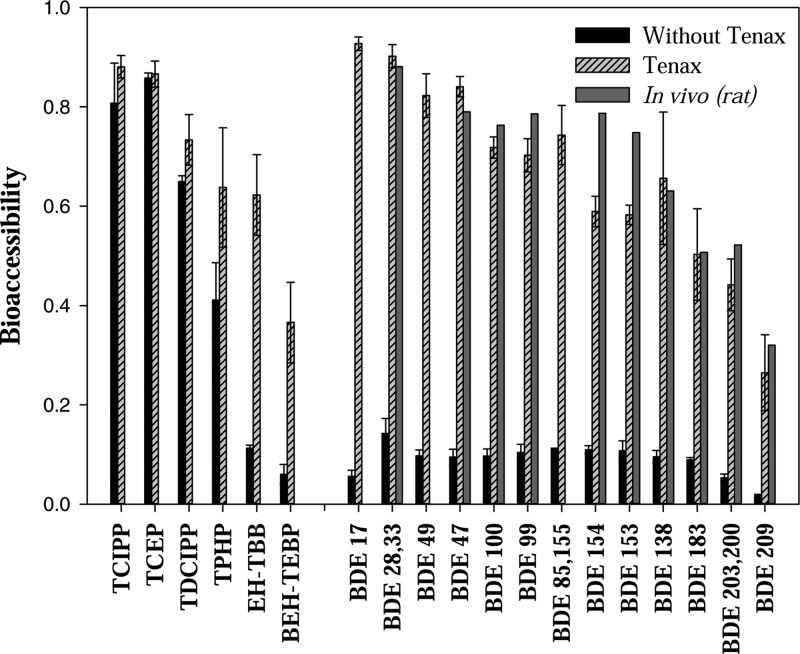
The bioaccessibility of FRs in SRM2585 was compared using digestive fluid with and without TA employed as an infinite sink (see Figure 1). No significant difference between the two methods was observed for several less hydrophobic OPFRs such as TCEP, TCIPP, and TDCIPP. However, large differences were observed for hydrophobic compounds such as EH-TBB, BEH-TEBP, and PBDEs. For example, the bioaccessibility of BDE47 in SRM 2585 was ∼80% using the TA method, but was only ∼10% using only digestive fluid. In general, the bioaccessibility of FRs in SRM2585 using the TA method was several folds higher than the method without the infinite sink, especially for the fairly hydrophobic compounds. Lepom et al.29 also measured bioaccessibility of PBDEs in SRM2585 using an in vitro incubation method without including an infinite sink and the average bioaccessibility for the tri- to hepta-BDEs ranged from 27 to 42%, and BDE209 was about 10% (range 7–14%), which were slightly higher than the values measured in this study without TA, but lower than that measured with TA. To further validate the method, an in vivo data set on the net absorption efficiency of several PBDEs (calculated as the fraction of the ingested chemical that was not excreted via the feces) in rats exposed to SRM2585, was also included for comparison.6 As seen in Figure 1, the in vivo net absorption data are quite comparable with the bioaccessibility measure using the TA method. These findings confirm several recent studies12−14 that inclusion of an infinite sink could maintain the concentration gradient between matrices and fluid, and is essential in evaluating the bioaccessibility of fairly hydrophobic organic compounds (Log Kow > 5).
Figure 1.
Measured bioaccessibility of OPFRs, EH-TBB, BEH-TEBP, and PBDEs in SRM2585 sieved to <53 μm (n = 3) with and without TA-assisted extractions. The net absorption rate of several PBDEs in a previous in vivo study using Sprague–Dawley rats dosed with SRM2585 was also included as comparison.6 Error bar represents standard deviation of triplicates.
The distribution of several FRs among the four different compartments (i.e., GI fluid, colon fluid, dust, and TA) was also investigated (SI Figure S5). For BDE47 and 99, most of the mass either sorbed to the TA or remained in the dust matrix, which could be explained by the higher hydrophobicity of those chemicals. However, for less hydrophobic compounds such as TCEP and TCIPP, ∼ 20% of the total mass was partitioned into the intestinal fluid, which was slightly higher than the measured value in the sorption efficiency experiment using the spiking method (SI Figure S4). Therefore, the fraction of these chemicals (Log Kow < 4) in the fluid should also be considered even with the presence of an infinite sink.
Bioaccessibility of FRs in House Dust (n = 17)
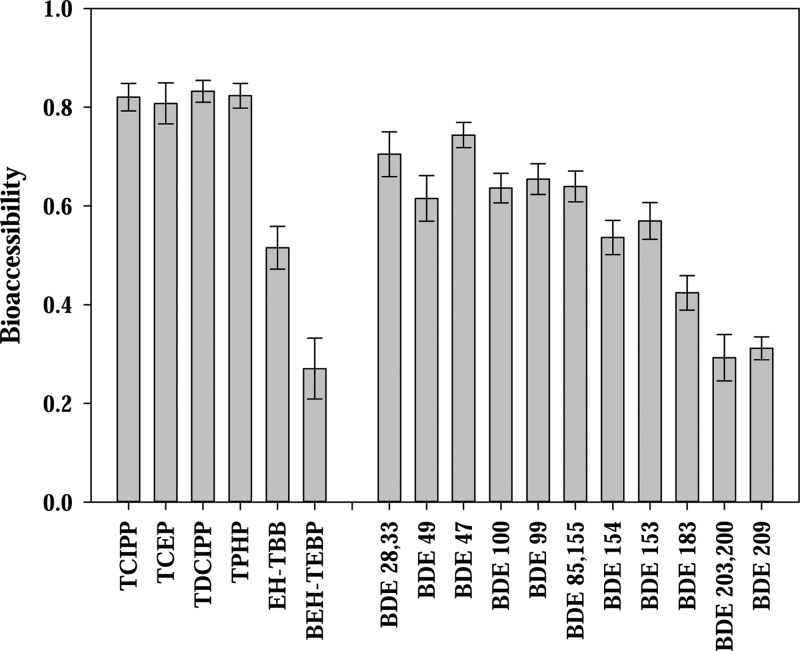
After validating the TA method, the bioaccessibility of FRs in 17 different dust samples was investigated. FRs were frequently detected in these dust samples. Concentrations as follows: ∑10BDEs (BDE 17, 28/33, 49, 47, 100, 99, 85/155, 154, 153, and 138, range: 150–76 540 ng/g dust, GM: 1700 ng/g dust); BDE209 (range: 370–86 000 ng/g dust, GM: 1720 ng/g dust); TCEP (range: <MDL–6935 ng/g dust, GM: 360 ng/g dust); TDCIPP (range: <MDL–54 010 ng/g dust, GM: 1620 ng/g dust); TCIPP (range: <MDL–65 820 ng/g dust, GM: 3510 ng/g dust); TPHP (range: <MDL–1 532 000 ng/g dust, GM: 6520 ng/g dust); EH-TBB (range: <MDL–3500 ng/g dust, GM: 1020 ng/g dust), and BEH-TEBP (range: <MDL–17 600 ng/g dust, GM: 530 ng/g dust). The calculated bioaccessibility of the FRs in individual dust samples can be found in SI Table S1; average values are presented in Figure 2. OPFRs, including TCEP, TCIPP, TDCIPP, and TPHP, are highly bioaccessible and 80% of the measured OPFR compounds in the house dust can be readily desorbed into the digestive fluid. In contrast, the bioaccessibility of PBDEs varied among congeners. The bioaccessibile fraction was over ∼60% for the lower molecular weight PBDEs, such as tri- to penta-BDE congeners, but it decreased to ∼25% for the higher molecular weight compounds, particularly BDE209. The bioaccessibility of EH-TBB was ∼50% and BEH-TEBP was similar to BDE209.
Figure 2.
Average estimated bioaccessibility (%) of FRs in 17 dust samples. Error bar represents the standard error (n = 17).
Factors Affecting the Bioaccessibility of FRs in Dust
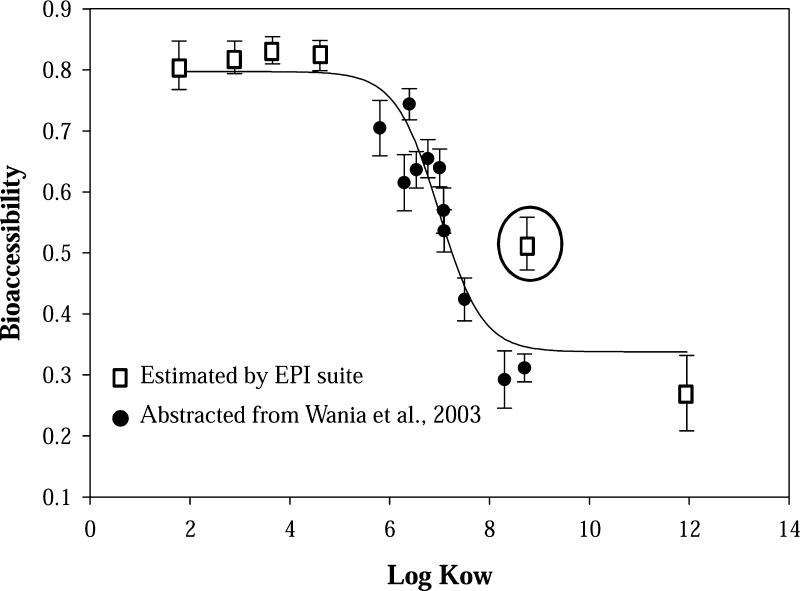
The FRs investigated here have a wide range in molecular weight, solubilities and partitioning properties, which likely influence their bioaccessibility. As shown in Figure 3, a general decreasing trend in bioaccessibility was observed with increasing Log Kow. It should be noted that Log Kow for some chemicals (e.g, OPFRs and EH-TBB/BEH-TEBP) were estimated using EPI suite (EPIWEB 4.1) while some (e.g., PBDEs) were based on experimental measures.24 The different sources for the Kow values may explain why EH-TBB is a relative outlier in this relationship (Figure 3). However, overall the Log Kow value was a good predictor of the bioaccessibility of the FR chemicals. No difference in the bioaccessibility was observed for FRs with Log Kow values <5, but a reverse relationship was observed for FRs with Log Kow values >5. A two resistance model in absorption has been proposed in a previous study.30 One resistance is the organic barrier (e.g, TA) with lipid like properties while the second is an aqueous barrier. At low Log Kow values the lipid barrier provides the dominant resistance for absorption, which is independent of Log Kow. However, as Log Kow increases, diffusive transport through the aqueous barrier becomes increasingly limited and the absorption decreases. The relationship between Kow and bioaccessibility observed in this study was very similar to a previous in vivo study in cows assessing the PBDE absorption potential.31 However, Lepom et al.29 reported that the bioaccessibility of individual PBDE congeners did not appear to be correlated with degree of bromination for tri- to hepta-BDEs using an in vitro method. And Ruby et al.32 did not find any correlation between bioaccessibility of polychlorinated dibenzodioxins/furan (PCDD/Fs) in soil and the degree of chlorination using a different in vitro method. In this study, the bioaccessibility of PBDEs in SRM2585 using the traditional incubation method (without TA) did not show a decreasing trend for tri- to hepta-BDEs either. These findings suggest that the measured bioaccessibility is very method-dependent.
Figure 3.
Relationship between bioaccessibility and hydrophobicity (Log Kow) of the FRs in 17 house dust samples. The circled symbol represents EH-TBB. Error bar represents the standard error (n = 17).
Dust samples from different sampling years were also analyzed to examine the potential effect of aging on the bioaccessibility of FRs, as previous studies have shown that aging could reduce the bioaccessibility of hydrophobic compounds in soils and sediments.33,34 In these previous studies, the mobility of hydrophobic compounds sorbed to organic matter was reduced with aging of the soils/sediments. As shown in SI Figure S6, the bioaccessibility of dust samples collected in 2006 (n = 7) was significantly different from those collected in 2010 (n = 10; p < 0.001, two-way ANOVA). Significantly higher bioaccessibility of TCIPP, EH-TBB, BEH-TEBP, BDE100, BDE183, and BDE200/203 was observed in the dust samples collected in 2010. However, BDE209 was not significantly different between the two groups, which might be explained by the fact that most of BDE209 in the dust may be associated with weathered polymers from commercial products, and not from sorption onto dust particles directly.10 These results suggest for the first time that aging could decrease the bioaccessibility of some FRs in dust.
Since organic carbon content and composition of dust may also affect bioaccessibility, we investigated the relationship between these variables. No significant relationships were observed with TOC (SI Figure S7), or ratios of C/N, and C/H in the dust. In a previous study in soils, no particular relationship between TOC, black carbon content (BC), and bioaccessibility of PAHs was observed either.12 Due to the complexity and heterogeneity of the source for dust, it might be difficult to establish a model to effectively predict the bioaccessibility of one compound in the dust based on its composition.
Bioaccessibility of FRs in PUFs and Size Effect
Because dust samples may also contain small pieces of PUF from furniture, we also investigated the bioaccessibility of FRs directly from FR-treated PUF. Measured concentrations of FRs in three PUF samples were 1.8, 2.4, and 4.3 mg/g PUF for TDCIPP, ∑10BDEs, and FM550 (the sum of EH-TBB and BEH-TEBP); respectively. As the FRs are more highly concentrated in the PUF relative to the dust, the mass of TA used in the incubations with PUF (0.6 g TA) was increased to reduce the likelihood of saturation. Also, no difference was observed using either 0.6 or 1.0 g TA in a bioaccessibility test for FM550 impregnated foam (data not shown), suggesting 0.6 g TA can serve as an infinite sink of FRs from the foam. Microscopic imaging of the three size fractions showed that PUF could be effectively fragmented into microfoam particles (SI Figure S8), which also showed a similar shape with those identified in dust collected from a gymnasium.9
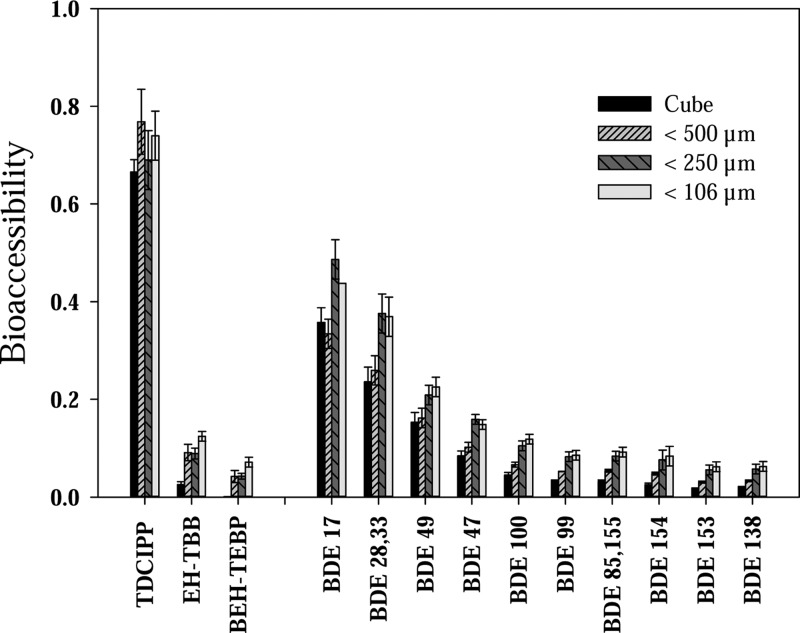
The measured bioaccessibility of TDCIPP, pentaBDEs, and EH-TBB/BEH-TEBP in PUFs, as well as their fragmented foam particles, are shown in Figure 4. TDCIPP was quite bioaccessible (∼70–80%) in the PUF, similar to the dust samples, and no particle size effect was observed. The bioaccessibility of PBDEs in the PUF was also related to hydrophobicity (i.e., Log Kow), similar to the dust. Bioaccessibilty ranged from 20 to 40% for the tri- and tetra-BDEs and less than 10% for the penta- and hexa-BDEs. The bioaccessibility of EH-TBB and BEH-TEBP was less than 10% and no bioaccessible BEH-TEBP was observed in the nonfragmented foam (i.e., the PUF cube). An effect with particle size was observed with the higher molecular weight FRs, in which higher bioaccessibility was observed in the smaller particle size fractions (<250 μm and <100 μm) for both PBDEs and FM500 impregnated foam. The smaller particles have a larger total surface area and likely can facilitate the transport of FRs from the PUF to the digestive fluid. Here, the bioaccessibility of the more hydrophobic FRs in the foam was much less than what was observed in the dust samples, which might be due to lower fugacities of these chemicals in the PUF. PUF has a stronger retention capacity for semivolatile organic chemicals and has been widely used as adsorbent in air monitoring for these types of compounds. A similar result was observed in an in vivo study with earth worms, where the bioaccumulation factor in worms fed PUF was several times lower than that of worms fed PBDEs in spiked soil.35 Therefore, our results suggest that the bioaccessibility of more hydrophobic FRs in PUF is lower than in dust.
Figure 4.
Estimated bioaccessibility of OPFRs, FM550, and PBDEs in three respective PUF samples with different particle sizes. Error bar represents the standard deviation of duplicate foam samples. Cube represents the whole piece of foam with a weight of ∼20 mg.
Hydrolysis of OPFRs, EH-TBB, and BEH-TEBP
We also investigated potential degradation of the more labile FRs during the incubation process. After incubating the FRs directly with three digestive fluids over a physiological residence time, no significant drop in the OPFR concentration was observed (SI Figure S9), suggesting negligible hydrolysis or degradation of OPFRs occurred in the digestive fluid. In contrast, ∼70% of the initial EH-TBB concentration disappeared after incubating with the intestinal fluid for 20 h (Figure 5), which was not observed in either the gastric fluid or colon fluid. BEH-TEBP, another component in FM550, did not show any hydrolysis during the incubation (Figure 5). This observation was consistent with our previous study investigating the in vitro metabolism of EH-TBB and BEH-TEBP in human and rat subcellular hepatic fractions.28 Due to the similar pH range in the colon fluid and intestinal fluid, it was hypothesized that an enzyme in the intestinal fluid could hydrolyze EH-TBB in the intestinal fluid. Two enzymes (lipases and pancreatins) were added to the intestinal fluid in this study. The lipase, which is a type of esterase, can perform essential roles in the digestion, transport and processing of dietary lipids such as triglycerides (a carboxylic ester) to small fatty acids.36 The pancreatin purchased in this study is a mixture of several digestive enzymes composed of amylase, lipase, and protease. EH-TBB is a carboxylic ester and we therefore hypothesized that lipases in the intestinal fluid could degrade EH-TBB. To test this hypothesis an intestinal fluid mixture without lipases and pancreatins was prepared and incubated with EH-TBB. No decrease in the EH-TBB concentration was observed (SI Figure S10), supporting our hypothesis that the enzymes were mediating the transformation of EH-TBB. Subsequent experiments tested the degradation of EH-TBB with two different lipase concentrations, 1.6 and 10 mg/mL, since the efficacy of porcine lipase used in this study may be much weaker than human lipase.37 No difference was observed with the two different lipase concentrations (data not show). In previous studies, diethylhexyl phthalate (DEHP) was found to be hydrolyzed by rat lipases in several tissues, and the quantitative data on rates of phthalate ester hydrolysis by intestinal enzymes suggested that low amounts of orally ingested DEHP would have little opportunity to be absorbed as the parent compound.22,36
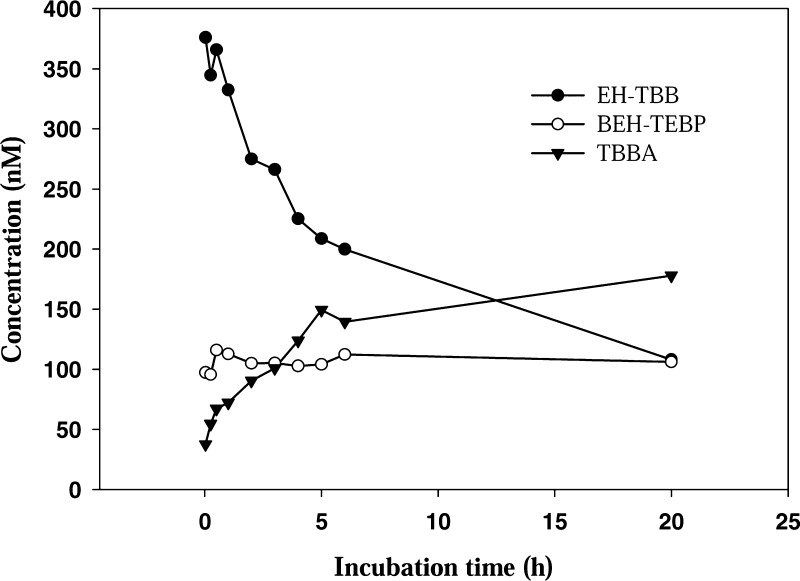
Figure 5.
Concentration (nM) of spiked EH-TBB and BEH-TEBP and the formation of TBBA incubated with 1.6 mg procine lipases/mL intestinal fluid at 37 °C and pH ∼ 7 measured at different sampling times. Each value was the average of duplicate samples at each sampling time.
The EH-TBB degraded in these experiments was transformed to tetrabromobenzoic acid (TBBA), which was confirmed using liquid chromatography tandem mass spectrometry (LC-MS/MS). As shown in Figure 5, increasing amounts of TBBA were observed with time. A mass balance analysis showed that TBBA was the major metabolite of EH-TBB in the intestinal fluid (∼73% of EH-TBB degraded). TBBA was found as the major metabolite of EH-TBB in our in vitro study28 and has been identified as a potential urinary biomarker for FM550 exposure.38 Though little toxicity has been reported for this compound, TBBA has found to be a possible moderate peroxisome proliferator activated receptor (PPARγ) ligand.39 Since TBBA was rapidly formed, we also investigated the bioaccessibility of TBBA in the intestine or colon after hydrolysis. Due to the induction effect of the bromine atoms on the molecule, the estimated acid dissociation constant (pKa) of TBBA is 2.3 (using the Hammett Equation18) and thus pH may influence the fate of TBBA. In our study, we tested the bioaccessibility of TBBA at two extreme pH values in the intestinal fluid (pH: 5.3 and 8).40 TBBA was spiked into the digestive solution with a final concentration of ∼1 μg/mL. At both pH values, the concentration of the TBBA did not change with time, suggesting that there is no absorption by TA (SI Figure S11). This result was not unexpected, since nearly all of the TBBA will be deprotonated at pH > 5. In vivo absorption might not occur in the intestine if no active transportation was involved. Overall, the hydrolysis experiment in this study revealed that more labile FRs can undergo transformation in the digestive fluid prior to absorption and should be considered in further exposure/risk assessment. However, it should be noted that this in vitro method only included a limited set of enzymes and also did not include other factors such as microfloral activity, which could also affect absorption and fate in the gastrointestinal tract.
Environmental Implications
In this study, an in vitro bioaccessibility test using TA as an adsorption sink was developed and our results are very comparable to an in vivo study, confirming several recent findings that the use of an infinite sink is necessary in evaluating in vitro bioaccessibility. The bioaccessibility of FRs varied greatly between compounds/matrices and it should be considered in future exposure and risk assessments, particularly for highly hydrophobic compounds (Log Kow > 5). The results of this study also showed that less hydrophobic FRs such as OPFRs are quite bioaccessible in both dust and in PUF, suggesting a higher risk of exposure for those compounds, despite the fact that they are generally less bioaccumulative. To date, the stability of the ingested organic contaminants in the gastro-intestinal tract was not well studied, although metabolism after absorption has been a focus of several studies. In this study, EH-TBB was readily transformed into TBBA in the presence of intestinal enzymes. Due to the abundance and variety of enzymes present in the digestive fluid, more labile organic contaminants with low ingestion rates might not be absorbed into the body as parent compounds. This may be an important consideration as chemical industries shift from producing persistent/bioaccumulative chemicals to less persistent forms. However, it should be noted that the in vitro artificial digestive fluid in the present study does not completely resemble human or rodent digestive fluids. Furthermore, the role of microflora on the metabolism and absorption should also be considered in future studies.
Acknowledgments
This study was funded by a grant from the National Institute of Environmental Health Sciences (1R01ES016099).
Supporting Information Available
Additional information as noted in text. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Dodson R. E.; Perovich L. J.; Covaci A.; Van den Eede N.; Ionas A. C.; Dirtu A. C.; Brody J. G.; Rudel R. A. After the PBDE Phase-out: A broad suite of flame retardants in repeat house dust samples from California. Environ. Sci. Technol. 2012, 462413056–13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M.; Eagle S.; Sjodin A.; Webster T. F. Serum PBDEs in a North Carolina toddler cohort: Associations with handwipes, house dust, and socioeconomic variables. Environ. Health Perspect. 2012, 12071049–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. I.; Stapleton H. M.; Slodin A.; Meeker J. D. Relationships between polybrominated diphenyl ether concentrations in house dust and serum. Environ. Sci. Technol. 2010, 44145627–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costera A.; Feidt C.; Dziurla M. A.; Monteau F.; Le Bizec B.; Rychen G. Bioavailability of polycyclic aromatic hydrocarbons (PAHs) from soil and hay matrices in lactating goats. J. Agric. Food Chem. 2009, 57125352–5357. [DOI] [PubMed] [Google Scholar]
- Lei L.; Suidan M. T.; Khodadoust A. P.; Tabak H. H. Assessing the bioavailability of PAHs in field-contaminated sediment using XAD-2 assisted desorption. Environ. Sci. Technol. 2004, 3861786–1793. [DOI] [PubMed] [Google Scholar]
- Huwe J. K.; Hakk H.; Smith D. J.; Diliberto J. J.; Richardson V.; Stapleton H. M.; Birnbaum L. S. Comparative absorption and bioaccumulation of polybrominated diphenyl ethers following ingestion via dust and oil in male rats. Environ. Sci. Technol. 2008, 4272694–700. [DOI] [PubMed] [Google Scholar]
- Yu Y. X.; Pang Y. P.; Li C.; Li J. L.; Zhang X. Y.; Yu Z. Q.; Feng J. L.; Wu M. H.; Sheng G. Y.; Fu J. M. Concentrations and seasonal variations of polybrominated diphenyl ethers (PBDEs) in in- and out-house dust and human daily intake via dust ingestion corrected with bioaccessibility of PBDEs. Environ. Int. 2012, 42, 124–131. [DOI] [PubMed] [Google Scholar]
- Stapleton H. M.; Klosterhaus S.; Keller A.; Ferguson P. L.; van Bergen S.; Cooper E.; Webster T. F.; Blum A. Identification of flame retardants in polyurethane foam collected from baby products. Environ. Sci. Technol. 2011, 45125323–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M.; Sharma S.; Getzinger G.; Ferguson P. L.; Gabriel M.; Webster T. F.; Blum A. Novel and high volume use flame retardants in U.S. couches reflective of the 2005 pentaBDE phase out. Environ. Sci. Technol. 2012, 462413432–13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster T. F.; Harrad S.; Millette J. R.; Holbrook R. D.; Davis J. M.; Stapleton H. M.; Allen J. G.; McClean M. D.; Ibarra C.; Abdallah M. A.; Covaci A. Identifying transfer mechanisms and sources of decabromodiphenyl ether (BDE 209) in indoor environments using environmental forensic microscopy. Environ. Sci. Technol. 2009, 4393067–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen A. G.; Hack A.; Minekus M.; Zeijdner E.; Cornelis C.; Schoeters G.; Verstraete W.; Van de Wiele T.; Wragg J.; Rompelberg C. J. M.; Sips A. J. A. M.; Van Wijnen J. H. Comparison of five in vitro digestion models to study the bioaccessibility of soil contaminants. Environ. Sci. Technol. 2002, 36153326–3334. [DOI] [PubMed] [Google Scholar]
- Collins C. D.; Mosquera-Vazquez M.; Gomez-Eyles J. L.; Mayer P.; Gouliarmou V.; Blum E. Is there sufficient ’sink’ in current bioaccessibility determinations of organic pollutants in soils?. Environ. Pollut. 2013, 181, 128–132. [DOI] [PubMed] [Google Scholar]
- Gouliarmou V.; Collins C. D.; Christiansen E.; Mayer P. Sorptive physiologically based extraction of contaminated solid matrices: Incorporating silicone rod as absorption sink for hydrophobic organic contaminants. Environ. Sci. Technol. 2013, 472941–8. [DOI] [PubMed] [Google Scholar]
- Gouliarmou V.; Mayer P. Sorptive bioaccessibility extraction (SBE) of soils: Combining a mobilization medium with an absorption sink. Environ. Sci. Technol. 2012, 461910682–10689. [DOI] [PubMed] [Google Scholar]
- Harwood A. D.; Landrum P. F.; Weston D. P.; Lydy M. J. Using SPME fibers and Tenax to predict the bioavailability of pyrethroids and chlorpyrifos in field sediments. Environ. Pollut. 2013, 173, 47–51. [DOI] [PubMed] [Google Scholar]
- van der Heijden S. A.; Jonker M. T. O. PAH bioavailability in field sediments: Comparing different methods for predicting in situ bioaccumulation. Environ. Sci. Technol. 2009, 43103757–3763. [DOI] [PubMed] [Google Scholar]
- You J.; Pehkonen S.; Landrum P. F.; Lydy M. J. Desorption of hydrophobic compounds from laboratory-spiked sediments measured by Tenax absorbent and matrix solid-phase microextraction. Environ. Sci. Technol. 2007, 41165672–8. [DOI] [PubMed] [Google Scholar]
- Schwarzenbach R. P.; Gschwend M. P.; Imboden M. D.. Environmental Organic Chemistry; Willey Interscience, 2005. [Google Scholar]
- Huwe J. K.; Hakk H.; Smith D. J.; Diliberto J. J.; Richardson V.; Stapleton H. M.; Birnbaum L. S. Comparative absorption and bioaccumulation of polybrominated diphenyl ethers following ingestion via dust and oil in male rats. Environ. Sci. Technol. 2008, 4272694–2700. [DOI] [PubMed] [Google Scholar]
- Stapleton H. M.; Eagle S.; Sjodin A.; Webster T. F. Serum PBDEs in a north carolina toddler cohort: Associations with handwipes, house dust, and socioeconomic variables. Environ. Health Perspect. 2012, 12071049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M.; Misenheimer J.; Hoffman K.; Webster T. F.. Flame retardant associations between children’s handwipes and house dust. Chemosphere 2014, in press [DOI] [PMC free article] [PubMed]
- Watkins D. J.; McClean M. D.; Fraser A. J.; Weinberg J.; Stapleton H. M.; Webster T. F. Associations between PBDEs in office air, dust, and surface wipes. Environ. Int. 2013, 59, 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choate L. M.; Ranville J. F.; Bunge A. L.; Macalady D. L. Dermally adhered soil: 1. Amount and particle-size distribution. Integr. Environ. Assess. Manage. 2006, 24375–84. [PubMed] [Google Scholar]
- Wania F.; Dugani C. B. Assessing the long-range transport potential of polybrominated diphenyl ethers: A comparison of four multimedia models. Environ. Toxicol. Chem. 2003, 2261252–1261. [PubMed] [Google Scholar]
- Dodson R. E.; Perovich L. J.; Covaci A.; Van den Eede N.; Ionas A. C.; Dirtu A. C.; Brody J. G.; Rudel R. A. After the PBDE phase-out: A broad suite of flame retardants in repeat house dust samples from California. Environ. Sci. Technol. 2012, 462413056–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M.; Klosterhaus S.; Eagle S.; Fuh J.; Meeker J. D.; Blum A.; Webster T. F. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ. Sci. Technol. 2009, 43197490–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilston E. L.; Gibson G. R.; Collins C. D. Colon extended physiologically based extraction test (CE-PBET) increases bioaccessibility of soil-bound PAH. Environ. Sci. Technol. 2011, 45125301–8. [DOI] [PubMed] [Google Scholar]
- Roberts S. C.; Macaulay L. J.; Stapleton H. M. In vitro metabolism of the brominated flame retardants 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (TBB) and bis(2-ethylhexyl) 2,3,4,5-tetrabromophthalate (TBPH) in human and rat tissues. Chem. Res. Toxicol. 2012, 2571435–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepom P.; Berndt M.; Duffek A.; Warmbrunn-Suckrow E.. Oral bioaccessibility of PBDEs in dust using an in vitro gastrointestinal model. IN Brominated Flame Retardant Symposium, 2013.
- McLachlan M. S. Model of the fate of hydrophobic contaminants in cows. Environ. Sci. Technol. 1994, 28132407–14. [DOI] [PubMed] [Google Scholar]
- Kierkegaard A.; De Wit C. A.; Asplund L.; McLachlan M. S.; Thomas G. O.; Sweetman A. J.; Jones K. C. A mass balance of tri-hexabrominated diphenyl ethers in lactating cows. Environ. Sci. Technol. 2009, 4372602–7. [DOI] [PubMed] [Google Scholar]
- Ruby M. V.; Fehling K. A.; Paustenbach D. J.; Landenberger B. D.; Holsapple M. P. Oral bioaccessibility of dioxins/furans at low concentrations (50–350 ppt toxicity equivalent) in soil. Environ. Sci. Technol. 2002, 36224905–4911. [DOI] [PubMed] [Google Scholar]
- Wong F.; Bidleman T. F. Aging of organochlorine pesticides and polychlorinated biphenyls in muck soil: Volatilization, bioaccessibility, and degradation. Environ. Sci. Technol. 2011, 453958–963. [DOI] [PubMed] [Google Scholar]
- Luo L.; Lin S.; Huang H. L.; Zhang S. Z. Relationships between aging of PAHs and soil properties. Environ. Pollut. 2012, 170, 177–182. [DOI] [PubMed] [Google Scholar]
- Gaylor M. O.; Harvey E.; Hale R. C. Polybrominated diphenyl ether (PBDE) accumulation by earthworms (Eisenia fetida) exposed to biosolids-, polyurethane foam microparticle-, and penta-BDE-amended soils. Environ. Sci. Technol. 2013, 472313831–9. [DOI] [PubMed] [Google Scholar]
- Svendsen A. Lipase protein engineering. Biochim. Biophys. Acta 2000, 15432223–238. [DOI] [PubMed] [Google Scholar]
- Carrière F.; Grandval P.; Gregory P. C.; Renou C.; Henniges F.; Sander-Struckmeier S.; Laugier R. Does the pancreas really produce much more lipase than required for fat digestion?. JOP 2005, 63206–15. [PubMed] [Google Scholar]
- Hoffman K.; Fang M.; Horman B.; Patisaul H. B.; Garantziotis S.; Birnbaum L. S.; Stapleton H. M. Urinary tetrabromobenzoic acid (TBBA) as a biomarker of exposure to the flame retardant mixture Firemaster 550. Environ. Health Perspect. 2014, 122, 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M.; Webster T. F.; Ferguson L. P.; Stapleton H. M.. Characterizing the peroxisome proliferator activated receptor (PPARγ) ligand binding potential of several major flame retardants, their metabolites, and chemical mixtures in house dust, 2014, in press. [DOI] [PMC free article] [PubMed]
- Fallingborg J. Intraluminal pH of the human gastrointestinal tract. Dan. Med. Bull. 1999, 463183–196. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.