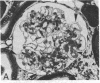
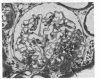
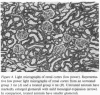
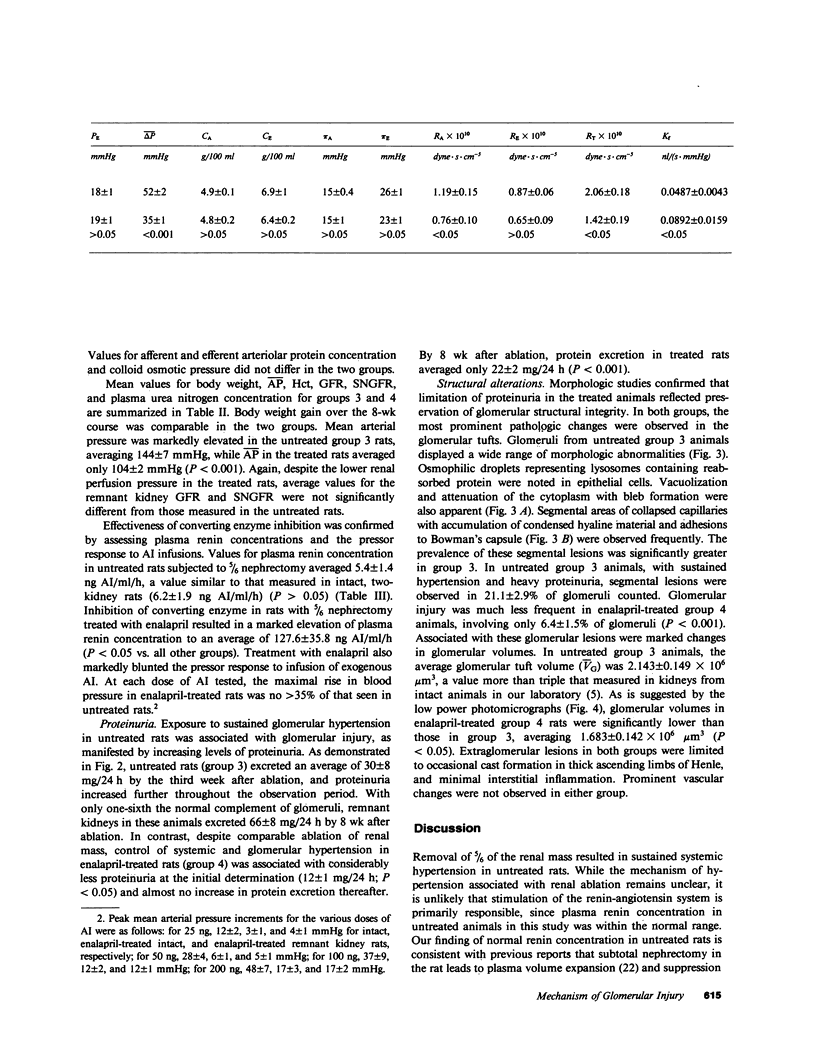
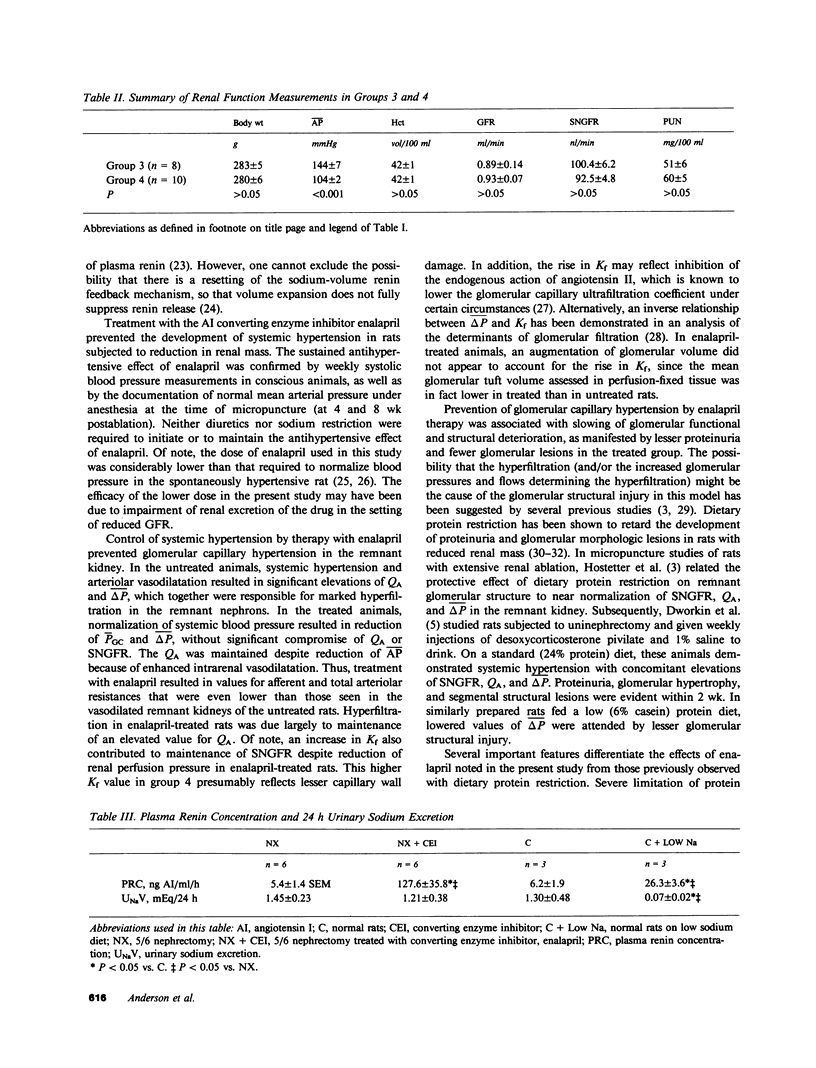
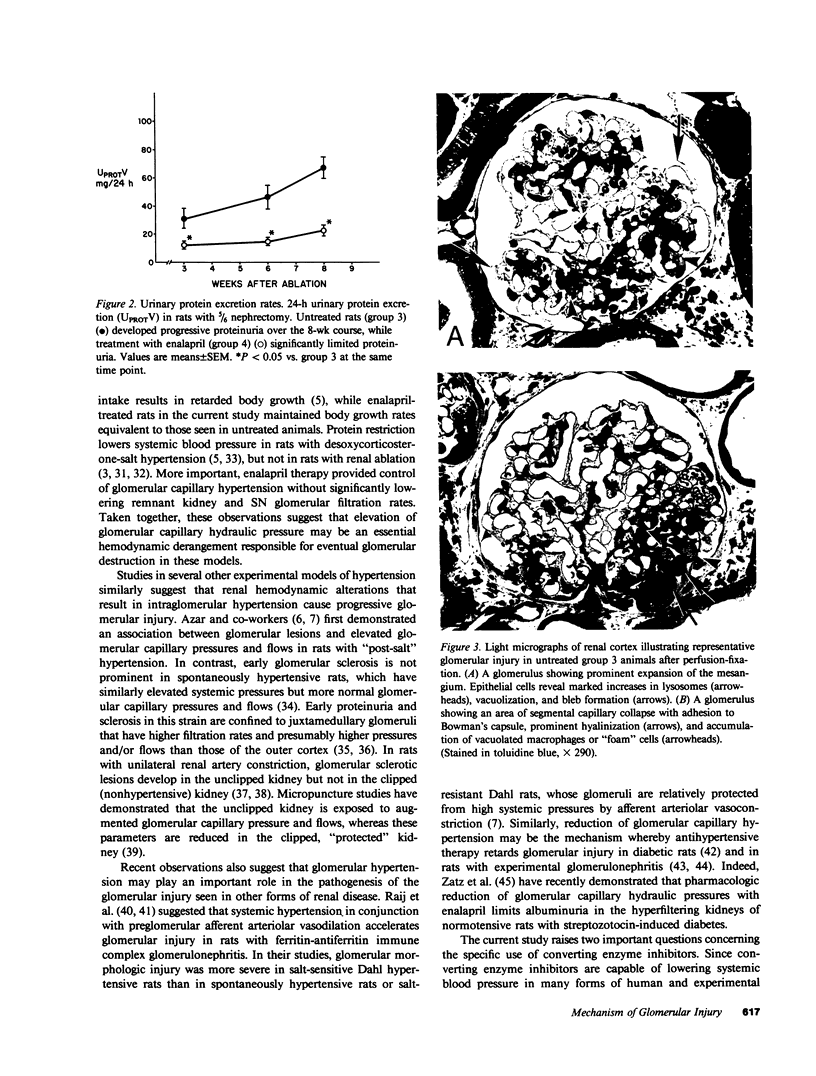
Abstract
Micropuncture and morphologic studies were performed in four groups of male Munich-Wistar rats after removal of the right kidney and segmental infarction of two-thirds of the left kidney. Groups 1 and 3 received no specific therapy. Groups 2 and 4 were treated with the angiotensin I converting enzyme inhibitor, enalapril, 50 mg/liter of which was put in their drinking water. All rats were fed standard chow. Groups 1 and 2 underwent micropuncture study 4 wk after renal ablation. Untreated group 1 rats exhibited systemic hypertension and elevation of the single nephron glomerular filtration rate (SNGFR) due to high average values for the mean glomerular transcapillary hydraulic pressure difference and glomerular plasma flow rate. In group 2 rats, treatment with enalapril prevented systemic hypertension and maintained the mean glomerular transcapillary hydraulic pressure gradient at near-normal levels without significantly compromising SNGFR and the glomerular capillary plasma flow rate, as compared with untreated group 1 rats. Groups 3 and 4 were studied 8 wk after renal ablation. Untreated group 3 rats demonstrated persistent systemic hypertension, progressive proteinuria, and glomerular structural lesions, including mesangial expansion and segmental sclerosis. In group 4 rats, treatment with enalapril maintained systemic blood pressure at normal levels over the 8-wk period and significantly limited the development of proteinuria and glomerular lesions. These studies suggest that control of glomerular hypertension effectively limits glomerular injury in rats with renal ablation, and further support the view that glomerular hemodynamic changes mediate progressive renal injury when nephron number is reduced.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arendshorst W. J., Beierwaltes W. H. Renal and nephron hemodynamics in spontaneously hypertensive rats. Am J Physiol. 1979 Mar;236(3):F246–F251. doi: 10.1152/ajprenal.1979.236.3.F246. [DOI] [PubMed] [Google Scholar]
- Azar S., Johnson M. A., Iwai J., Bruno L., Tobian L. Single-nephron dynamics in "post-salt" rats with chronic hypertension. J Lab Clin Med. 1978 Jan;91(1):156–166. [PubMed] [Google Scholar]
- Azar S., Johnson M. A., Scheinman J., Bruno L., Tobian L. Regulation of glomerular capillary pressure and filtration rate in young Kyoto hypertensive rats. Clin Sci (Lond) 1979 Mar;56(3):203–209. doi: 10.1042/cs0560203. [DOI] [PubMed] [Google Scholar]
- Bank N., Alterman L., Aynedjian H. S. Selective deep nephron hyperfiltration in uninephrectomized spontaneously hypertensive rats. Kidney Int. 1983 Aug;24(2):185–191. doi: 10.1038/ki.1983.143. [DOI] [PubMed] [Google Scholar]
- Bauer J. H. Role of angiotensin converting enzyme inhibitors in essential and renal hypertension. Effects of captopril and enalapril on renin-angiotensin-aldosterone, renal function and hemodynamics, salt and water excretion, and body fluid composition. Am J Med. 1984 Aug 20;77(2A):43–51. doi: 10.1016/s0002-9343(84)80057-1. [DOI] [PubMed] [Google Scholar]
- Blantz R. C., Konnen K. S., Tucker B. J. Angiotensin II effects upon the glomerular microcirculation and ultrafiltration coefficient of the rat. J Clin Invest. 1976 Feb;57(2):419–434. doi: 10.1172/JCI108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branca G. F., Satta A., Faedda R., Soggia G., Olmeo N. A., Vacca R., Bartoli E. Effects of blood pressure control on the progression of renal insufficiency in chronic renal failure. Panminerva Med. 1983 Oct-Dec;25(4):215–218. [PubMed] [Google Scholar]
- Brenner B. M., Meyer T. W., Hostetter T. H. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. 1982 Sep 9;307(11):652–659. doi: 10.1056/NEJM198209093071104. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Maddox D. A., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. VII. Response to reduced renal mass. Am J Physiol. 1974 Sep;227(3):556–562. doi: 10.1152/ajplegacy.1974.227.3.556. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Troy J. L., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. IV. Determination of the ultrafiltration coefficient. J Clin Invest. 1973 Jun;52(6):1500–1508. doi: 10.1172/JCI107324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin L. D., Hostetter T. H., Rennke H. G., Brenner B. M. Hemodynamic basis for glomerular injury in rats with desoxycorticosterone-salt hypertension. J Clin Invest. 1984 May;73(5):1448–1461. doi: 10.1172/JCI111349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Nahas A. M., Paraskevakou H., Zoob S., Rees A. J., Evans D. J. Effect of dietary protein restriction on the development of renal failure after subtotal nephrectomy in rats. Clin Sci (Lond) 1983 Oct;65(4):399–406. doi: 10.1042/cs0650399. [DOI] [PubMed] [Google Scholar]
- FUHR J., KACZMARCZYK J., KRUTTGEN C. D. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955 Aug 1;33(29-30):729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- Feld L. G., Van Liew J. B., Galaske R. G., Boylan J. W. Selectivity of renal injury and proteinuria in the spontaneously hypertensive rat. Kidney Int. 1977 Nov;12(5):332–343. doi: 10.1038/ki.1977.120. [DOI] [PubMed] [Google Scholar]
- Hayslett J. P. Functional adaptation to reduction in renal mass. Physiol Rev. 1979 Jan;59(1):137–164. doi: 10.1152/physrev.1979.59.1.137. [DOI] [PubMed] [Google Scholar]
- Heptinstall R. H., Hill G. S. Steroid-induced hypertension in the rat. A study of the effects of renal artery constriction on hypertension caused by deoxycorticosterone. Lab Invest. 1967 May;16(5):751–767. [PubMed] [Google Scholar]
- Hostetter T. H., Olson J. L., Rennke H. G., Venkatachalam M. A., Brenner B. M. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981 Jul;241(1):F85–F93. doi: 10.1152/ajprenal.1981.241.1.F85. [DOI] [PubMed] [Google Scholar]
- KOLETSKY S., GOODSITT A. M. Natural history and pathogenesis of renal ablation hypertension. Arch Pathol. 1960 Jun;69:654–662. [PubMed] [Google Scholar]
- Kaysen G. A., Watson J. B. Mechanism of hypoalbuminemia in the 7/8-nephrectomized rat with chronic renal failure. Am J Physiol. 1982 Oct;243(4):F372–F378. doi: 10.1152/ajprenal.1982.243.4.F372. [DOI] [PubMed] [Google Scholar]
- Lazarus J. M., Hampers C., Merrill J. P. Hypertension in chronic renal failure. Treatment with hemodialysis and nephrectomy. Arch Intern Med. 1974 Jun;133(6):1059–1066. [PubMed] [Google Scholar]
- Lindeman R. D., Tobin J. D., Shock N. W. Association between blood pressure and the rate of decline in renal function with age. Kidney Int. 1984 Dec;26(6):861–868. doi: 10.1038/ki.1984.229. [DOI] [PubMed] [Google Scholar]
- MOYER J. H., HEIDER C., PEVEY K., FORD R. V. The effect of treatment on the vascular deterioration associated with hypertension, with particular emphasis on renal function. Am J Med. 1958 Feb;24(2):177–192. doi: 10.1016/0002-9343(58)90306-1. [DOI] [PubMed] [Google Scholar]
- Maddox D. A., Price D. C., Rector F. C., Jr Effects of surgery on plasma volume and salt and water excretion in rats. Am J Physiol. 1977 Dec;233(6):F600–F606. doi: 10.1152/ajprenal.1977.233.6.F600. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. Long-term antihypertensive treatment (over six years) inhibiting the progression of diabetic nephropathy. Acta Endocrinol Suppl (Copenh) 1981;242:31–32. [PubMed] [Google Scholar]
- Okuda S., Onoyama K., Fujimi S., Oh Y., Nomoto K., Omae T. Influence of hypertension on the progression of experimental autologous immune complex nephritis. J Lab Clin Med. 1983 Mar;101(3):461–471. [PubMed] [Google Scholar]
- Parving H. H., Andersen A. R., Smidt U. M., Christiansen J. S., Oxenbøll B., Svendsen P. A. Diabetic nephropathy and arterial hypertension. The effect of antihypertensive treatment. Diabetes. 1983 May;32 (Suppl 2):83–87. doi: 10.2337/diab.32.2.s83. [DOI] [PubMed] [Google Scholar]
- Pfeffer J. M., Pfeffer M. A., Frohlich E. D. Validity of an indirect tail-cuff method for determining systolic arterial pressure in unanesthetized normotensive and spontaneously hypertensive rats. J Lab Clin Med. 1971 Dec;78(6):957–962. [PubMed] [Google Scholar]
- Purkerson M. L., Hoffsten P. E., Klahr S. Pathogenesis of the glomerulopathy associated with renal infarction in rats. Kidney Int. 1976 May;9(5):407–417. doi: 10.1038/ki.1976.50. [DOI] [PubMed] [Google Scholar]
- Raij L., Azar S., Keane W. F. Role of hypertension in progressive glomerular immune injury. Hypertension. 1985 May-Jun;7(3 Pt 1):398–404. [PubMed] [Google Scholar]
- Raij L., Azar S., Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int. 1984 Aug;26(2):137–143. doi: 10.1038/ki.1984.147. [DOI] [PubMed] [Google Scholar]
- Richer C., Doussau M. P., Giudicelli J. F. MK 421 and prevention of genetic hypertension development in young spontaneously hypertensive rats. Eur J Pharmacol. 1982 Apr 8;79(1-2):23–29. doi: 10.1016/0014-2999(82)90571-4. [DOI] [PubMed] [Google Scholar]
- Schwietzer G., Gertz K. H. Changes of hemodynamics and glomerular ultrafiltration in renal hypertension of rats. Kidney Int. 1979 Feb;15(2):134–143. doi: 10.1038/ki.1979.19. [DOI] [PubMed] [Google Scholar]
- Sweet C. S., Gross D. M., Arbegast P. T., Gaul S. L., Britt P. M., Ludden C. T., Weitz D., Stone C. A. Antihypertensive activity of N-[(S)-1-(ethoxycarbonyl)-3-phenylpropyl]-L-Ala-L-Pro (MK-421), an orally active converting enzyme inhibitor. J Pharmacol Exp Ther. 1981 Mar;216(3):558–566. [PubMed] [Google Scholar]
- Tucker B. J., Blantz R. C. Effects of glomerular filtration dynamics on the glomerular permeability coefficient. Am J Physiol. 1981 Mar;240(3):F245–F254. doi: 10.1152/ajprenal.1981.240.3.F245. [DOI] [PubMed] [Google Scholar]
- Viets J. W., Deen W. M., Troy J. L., Brenner B. M. Determination of serum protein concentration in nanoliter blood samples using fluorescamine or 9-phthalaldehyde. Anal Biochem. 1978 Aug 1;88(2):513–521. doi: 10.1016/0003-2697(78)90451-7. [DOI] [PubMed] [Google Scholar]
- Ylitalo P., Hepp R., Möhring J., Gross F. Effects of varying sodium intake on blood pressure and renin-angiotensin system in subtotally nephrectomized rats. J Lab Clin Med. 1976 Nov;88(5):807–816. [PubMed] [Google Scholar]
- Zusman R. M. Renin- and non-renin-mediated antihypertensive actions of converting enzyme inhibitors. Kidney Int. 1984 Jun;25(6):969–983. doi: 10.1038/ki.1984.119. [DOI] [PubMed] [Google Scholar]