Abstract
Environmental exposures that affect accumulation of polychlorinated biphenyls (PCBs) in humans are complex and not fully understood. One challenge in linking environmental exposure to accumulation is determining variability of PCB concentrations in samples collected from the same person at different times. We hypothesized that PCBs in human blood serum are consistent from year to year in people who live in the same environment between sampling. We analyzed blood serum from children and their mothers from urban and rural U.S. communities (n = 200) for all 209 PCBs (median ∑PCBs = 45 ng/g lw) and 12 hydroxylated PCBs (median ∑OH-PCBs = 0.09 ng/g fw). A subset of these participants (n = 155) also had blood PCB and OH-PCB concentrations analyzed during the previous calendar year. Although many participants had similar levels of PCBs and OH-PCBs in their blood from one year to the next, some participants had surprisingly different levels. Year-to-year variability in ∑PCBs ranged from −87% to 567% and in ∑OH-PCBs ranged from −51 to 358% (5th–95th percentile). This is the first study to report variability of all PCBs and major metabolites in two generations of people and suggests short-term exposures to PCBs may be a significant component of what is measured in human serum.
Introduction
Polychlorinated biphenyls (PCBs) are a class of 209 anthropogenic, chlorinated organic compounds that were widely manufactured and used around the world in a variety of industries and products like electrical components and building materials.1 Production of commercial mixtures of PCBs ended in the United States in 1977, but PCBs are still measured in humans2,3 due to their persistent and bioaccumulative properties and exposure to current inadvertent and legacy sources. A recent study from Nost et al. found that the relative contribution of PCBs to total chlorinated persistent organic pollutants in human serum has increased,4 indicating the continued need to monitor PCBs in people. Humans metabolize PCBs to hydroxylated PCBs (OH-PCBs) via cytochrome P450 enzymes, and OH-PCBs have also been measured in people.2,5 An International Agency for Research on Cancer Working Group recently classified PCBs as human carcinogens; both PCBs and OH-PCBs also target the endocrine system;6,7 and in some instances OH-PCBs were shown to be more toxic than their precursor PCB.8−11 PCBs are neurotoxic and have been implicated in developmental problems,12−16 so detection of PCBs in children is of particular concern.
Several cross-sectional population studies have shown that PCBs in serum, especially the middle and higher chlorinated congeners, demonstrate strong positive correlations with age;3,17−20 yet other congeners show no correlation.21 Using the CoZMoMAN model Quinn and Wania found that cross-sectional concentration-age relationships are not the same as concentration-age relationships of individuals over time.21 A major finding of this study was that PCB bioaccumulation does not actually increase monotonically with age and that the previously observed correlations with age were likely due to a combination of the amount of time elapsed after peak emissions and human metabolic and environmental degradation rates.
There are few recent studies of PCBs with repeat sampling of the same participants over time using congener-specific analysis, and no studies have evaluated all 209 PCBs and OH-PCBs over time. These few studies found an overall decrease in selected PCBs over periods ranging from 4 to 28 years, though trends for individual congeners and participants varied.4,22−25 In most cases, a major source of exposure (e.g., a nearby chemical plant or fish consumption) was identified as having been removed or reduced between the first and last sampling date.
Congener-specific analysis for all 209 PCBs is optimal for determining the variability of PCBs in human serum over time because of humans’ exposure to a range of low to high chlorinated current and legacy PCBs and because PCB metabolism in the body is congener-specific depending on the number and position of chlorines.26 In this study we quantified blood concentrations of two groups of target analytes, 209 PCBs and 12 OH-PCBs, in mother/child pairs living in urban or rural locations.
Residents of our urban cohort live in East Chicago, Indiana. East Chicago was incorporated in 1893 as a railroad and steel community and is still heavily industrialized. East Chicago is bisected by the Indiana Harbor and Ship Canal (IHSC), an artificial waterway created to serve the manufacturing industries. The IHSC also flows near junior and senior high schools. Volatilization from IHSC contributes about 7.5 kg/yr of PCBs to the air.27
Residents of our rural cohort live in the Columbus Community School District, which includes Columbus Junction, Columbus City, Conesville, Cotter, Fredonia, and surrounding rural areas with the schools located in Columbus Junction, IA. Columbus Junction was incorporated in 1874 as a railroad and steel town but in contrast to East Chicago, Columbus Junction is now predominantly an agricultural setting. The Columbus Community School District has no known current or historical industrial sources of PCBs.
Dietary habits, environmental exposures, and physiological changes like body composition and metabolism are expected to remain fairly consistent in a shorter period of time, and therefore we assumed that PCB concentration in an individual does not change significantly in a short period of time. We hypothesized that little variability from year to year would be measured in human serum. To address this hypothesis, we characterize the second annual data set of PCBs and OH-PCBs from children and their mothers living in East Chicago and the Columbus Junction area and compare them with the first annual data set, previously reported,2 in order to quantify the variability from one year to the next. We are the first to quantify variability of all PCBs and the major OH-PCBs in the same people.
Materials and Methods
Sample Collection, Extraction, and Instrument Analysis
Serum samples and survey data were collected from junior high school-aged students and their mothers who were enrolled in the Airborne Exposures to Semivolatile Organic Pollutants (AESOP) Study between April 2009 and March 2010. In this second year of the study, serum was analyzed from 50 East Chicago mothers and their 50 enrolled children and from 46 Columbus Junction area mothers and their 54 enrolled children. Of those 200 participants, 155 had also provided blood for the year 1 (April 2008-March 2009) data set. Nine families enrolled more than one child. All AESOP subjects gave informed consent or assent in English or Spanish according to an established Institutional Review Board protocol. Participants generally did not fast prior to giving blood. The sample collection, extraction, separation, and cleanup methods are described in detail elsewhere,2 with minor improvements included here. Briefly, sera were weighed (∼4 g) and spiked with 5 ng 13C-labeled PCBs and 4′-OH-PCB 159 (Supporting Information (SI) Table S1). The OH-PCB extract was derivatized to the methoxylated form (MeO-PCBs) using diazomethane. Immediately prior to instrument analysis, PCB extracts were spiked with 2 ng 13C-labeled internal standards and OH-PCB extracts were spiked with 5 ng PCB 209 (SI Table S1). Nine samples were removed from the PCB and OH-PCB data sets for having less than 4 g serum available for extraction, and 33 samples were removed from the OH-PCB data set following extraction errors.
GC-MS/MS (Agilent 7000 and Agilent 6890N with Waters Micromass MS) in multiple reaction monitoring mode was used for identification and quantification of 209 PCB congeners as 159 chromatographic peaks. GC-ECD was used for identification and quantification of 12 OH-PCB congeners as MeO-PCBs. Instrument operating parameters are in the SI. Instrument blanks of hexane were analyzed with each instrument run before and after the calibration and after the samples to ensure no cross-contamination.
Calibration standards were purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA) and AccuStandard, Inc. (New Haven, CT, USA). The OH-PCB congeners were chosen based on the known metabolic pathways for the most common PCB congeners detected in the year 1 serum samples and commercial availability (as MeO-PCBs). Congener mass was calculated by applying a relative response factor obtained from each congener in the calibration.
A common congener list (SI and Table S7) was used when comparing the two data sets. Median change in PCB concentration from year 1 to year 2 was 8 ng/g lw (28%) considering all congeners and 6 ng/g lw (14%) using the common congener list. Median change in OH-PCB concentration from year 1 to year 2 was 0.032 ng/g fw (54%) considering all congeners and 0.004 ng/g fw (4%) using the common congener list.
Statistics
The concentration data set was first dichotomized at the threshold of the congener-specific LOQ (SI Tables S3–S4). Distribution of sum and individual congener concentrations were skewed to the right, and data did not exhibit a normal distribution following logarithmic transformation. Therefore, the nonparametric Wilcoxon Rank Sum test and Wilcoxon Signed Ranks test were used to compare sum and individual congener concentrations and paired mother-child sum concentrations, respectively.
Statistical analysis was carried out in R 2.13.128 and Minitab 16 (7.14.0.739). In all statistical tests, the level of significance was α = 0.05.
Quality Control
Data were evaluated for representativeness, precision, reproducibility, and accuracy using a suite of quality control measures including method blanks, surrogate standards, and replicates of Standard Reference Material from the National Institute of Standards and Technology (NIST SRM 1957: Organic Contaminants in Non-Fortified Human Serum).
Method blanks consisting of 4 mL potassium chloride (1% w/w KCl in reagent water) were extracted, analyzed, and quantified with each batch of 10 samples. Most congeners were detected in the method blanks at low levels below 0.05 ng representing background noise (mean 0.012 ± 0.028 ng and mean 0.016 ± 0.042 ng for PCBs and OH-PCBs, respectively). A limit of quantification (LOQ) for each congener was determined as the upper limit of the 95% confidence interval (average mass in the method blanks plus two times the standard deviation). The ∑PCBs in five batches were higher than in the blank mass in the other 20 batches (p = 0.0001); consequently a separate LOQ was determined for those batches. PCB LOQ ranged from 0.0021 ng for PCB 24 to 0.68 ng for PCB 52 (mean 0.035 ± 0.067 ng). OH-PCB LOQ ranged from 0.0039 ng for 3′-OH-PCB 118 to 0.066 ng for 4′-OH-PCB 107 (mean 0.025 ± 0.021 ng).
Surrogate standards (SI Table S1) were used to evaluate extraction efficiency, and sample mass was corrected according to surrogate recoveries. Recovery of 13C-PCB 194 on the Agilent GC-MS/MS was consistently poor compared to the unlabeled standard, and therefore the 13C-labeled hepta-chlorinated surrogate standard (13C-PCB 180) was used instead of 13C-PCB 194 to correct the mass of octa-chlorinated PCBs. Quantification of the SRM confirmed the appropriateness of this substitution. Recovery of the remaining nine PCB surrogate standards ranged from 22 to 213% (mean 85 ± 25%). Recovery of the OH-PCB surrogate standard ranged from 40 to 113% (mean 70 ± 11%) (SI Table S5).
Analysis of PCBs in the SRM using the same extraction and quantification as the samples (SI Figure S1) resulted in a mean difference of 6 ± 17% between the NIST certified or reference values and our measured values for 22 congeners. Analytical variability of measurements between year 1 and year 2 can be approximated by the 22% difference in ∑PCBs in the NIST SRM 1957 extracted and analyzed in year 1 and again extracted and analyzed in year 2. Although their identity and concentration are not certified by NIST, we report values for OH-PCBs detected in the SRM (SI Table S6).
Results and Discussion
PCBs and OH-PCBs in Year 2 Participants
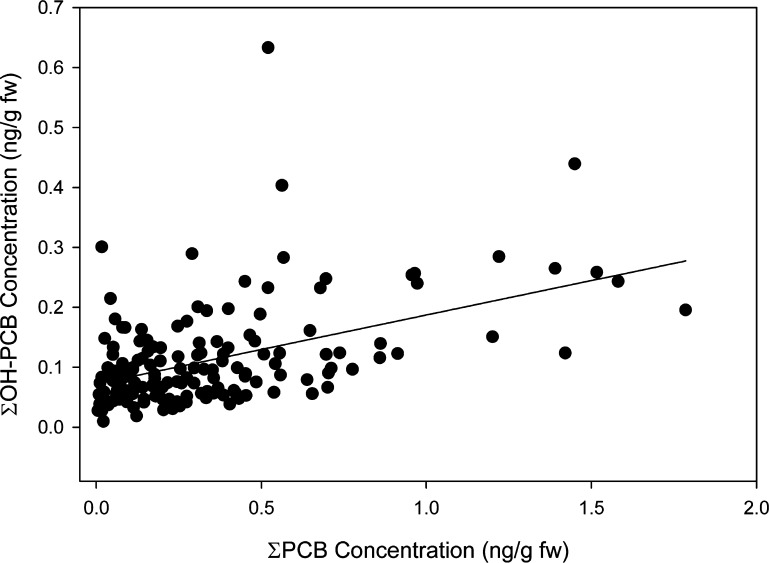
202 PCB congeners as 152 unique chromatographic peaks and all 11 OH-PCBs were detected in the samples (SI Tables S9–S11). Frequently detected PCBs included dioxin-like congeners 105, 118, 156 + 157, and 167. ∑PCB concentrations ranged from 4 to 199 ng/g lipid weight (5th–95th percentile; median 45 ng/g lw). ∑OH-PCB concentrations ranged from 0.04 to 0.27 ng/g fresh weight (5th–95th percentile; median 0.09 ng/g fw). After removing one leverage point, a Columbus Junction mother with ∑PCBs and ∑OH-PCBs much higher than the other participants, there was a significant positive correlation between ∑PCBs and ∑ΣOH-PCBs (Figure 1, R = 0.48, p < 0.0001).
Figure 1.
∑PCBs correlate with ∑OH-PCBs (R = 0.48, p < 0.0001). Each point represents one participant. One leverage point with ∑PCBs and ∑OH-PCBs much higher than the other samples was removed. Concentrations are given in units of ng/g fresh weight so PCB and OH-PCB concentration could be compared.
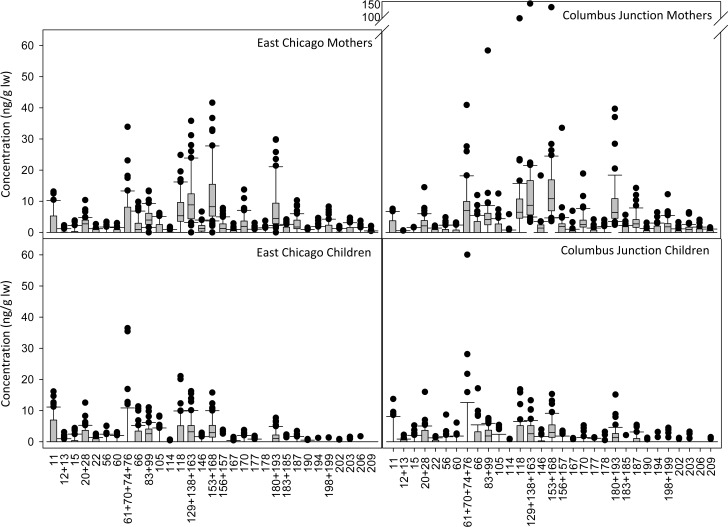
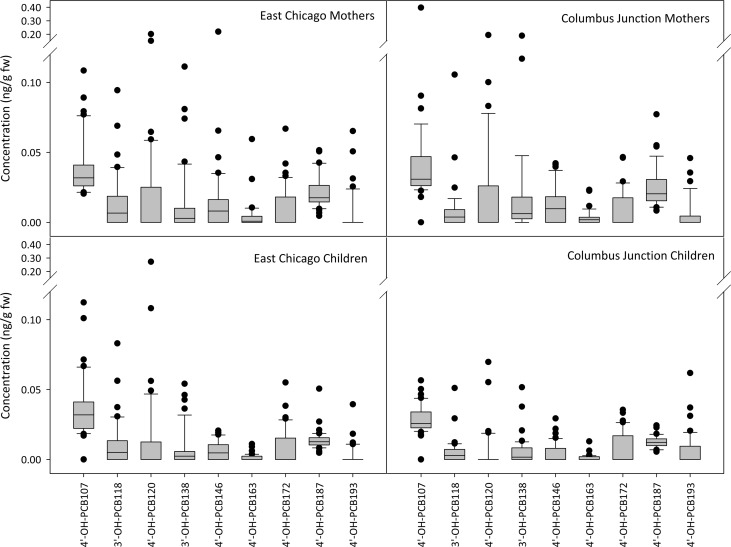
Concentrations of sum and individual PCBs and OH-PCBs were not statistically significantly different between East Chicago and Columbus Junction participants except PCBs 11, 61 + 70 + 74 + 76, 178, 180 + 193, 194, 203, and 3′-OH-PCB 118 and 4′-OH-PCB 193 (SI Table S8). Concentrations of the 31 PCBs and 9 OH-PCBs that were detected in at least 20% of participants are shown in Figures 2 and 3, respectively. Our finding of similar concentrations between the urban and rural locations is consistent with the results from the first year of sample analysis.2
Figure 2.
PCB concentrations are similar between East Chicago and Columbus Junction mothers and East Chicago and Columbus Junction children. The 31 congeners detected in at least 20% of a subgroup are shown. Concentrations are given in units of ng/g lipid weight.
Figure 3.
OH-PCB concentrations are similar between East Chicago and Columbus Junction mothers and East Chicago and Columbus Junction children. The nine congeners detected in at least 20% of a subgroup are shown. Concentrations are given in units of ng/g fresh weight.
Children had lower levels of OH-PCBs in their blood than their mothers (p < 0.0001) and much lower levels of PCBs (p < 0.0001). East Chicago and Columbus Junction children had median ∑PCBs of 46% (8–155%, 5th–95th percentile) and 30% (2–110%, 5th–95th percentile), respectively of their mothers. In contrast, East Chicago and Columbus Junction children had median ∑OH-PCBs of 79% (28–181%, 5th–95th percentile) and 62% (13–140%, 5th–95th percentile), respectively of their mothers. This result could be due to children’s faster metabolism26 or our focus on higher molecular weight OH-PCBs with five to seven chlorines.
Children are enriched in low molecular weight PCBs (homologues 1–5) compared to their mothers. An average of 64% and 59% of ∑PCBs are from low molecular weight PCBs in East Chicago and Columbus Junction children, respectively, compared with an average of 42% and 40% in East Chicago and Columbus Junction mothers, respectively. Unlike their mothers, we presume the children have not yet accumulated the higher molecular weight PCBs associated with dietary intake. Therefore, low molecular weight PCB exposure in children is important to their blood PCB levels.
Comparison between Year 1 and Year 2
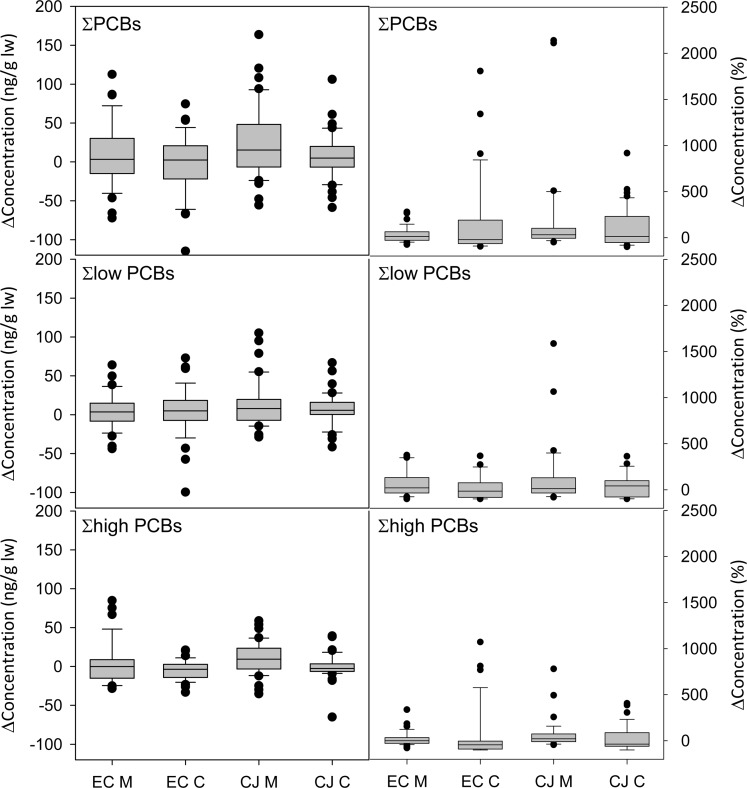
A subset of participants (n = 155) also had blood PCB and OH-PCB concentration analyzed during the previous calendar year (April 2008 to March 2009). Correlations between year 1 and year 2 concentrations are shown in SI Table S12. The median change in ∑PCBs from year 1 to year 2 was 6 ng/g lw but ranged from −115 to 164 ng/g lw (5th–95th percentile) indicating high variability in some participants (Figure 4). After removing seven participants with ∑PCBs < LOQ in year 1, this change represented a median of 14% of the participants’ year 1 ∑PCBs and ranged from −87% to 567% (5th–95th percentile). Of all participants, 27% lost or gained more than 40 ng/g lw ∑PCBs (the median ∑PCBs in year 2). Of the 148 AESOP participants with ∑PCBs > LOQ in both year 1 and year 2, the vast majority (82%) had a change more significant than the estimated analytical variability. Concentrations of PCBs significantly changed from the first year to the second year in more children (88%) than mothers (76%), suggesting that children’s serum PCB concentrations especially reflect short-term exposures compared with their mothers. There is no meaningful correlation of percent change between mothers and their children (R2 = 0.061, p = 0.05).
Figure 4.
Change in concentration (left: ng/g lw; right: %) of total, low, and high PCBs, where low PCBs are the sum of homologues 1–5 and high PCBs are the sum of homologues 6–10. A positive value indicates an increase in concentration from year 1 to year 2. EC M and EC C represent East Chicago mothers and children, respectively. CJ M and CJ C represent Columbus Junction mothers and children, respectively.
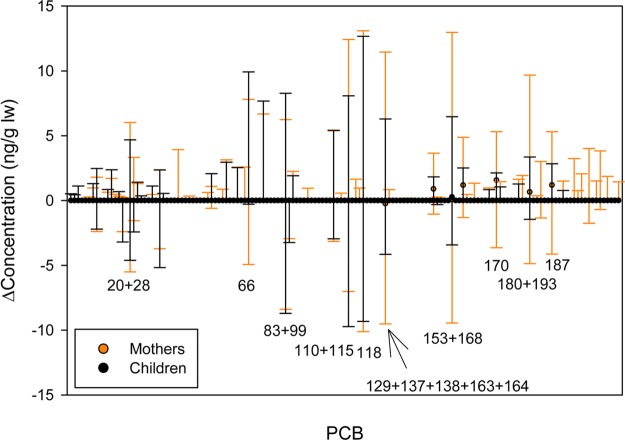
While the median variability for most PCBs was zero, large variability was found in several congeners (Figure 5). PCBs with the largest range of variability in concentration are shown in Table 1 along with the percent of mothers and children whose variability was greater than the estimated analytical variability. These congeners include higher molecular weight PCBs commonly reported in people (118, 138, 153, 180, and 187) that have been associated with dietary intake. It is possible that the large variability associated with these congeners reflects day-to-day variability from a large dietary intake of PCBs prior to sampling, although daily or monthly short-term variability is unexplored in the peer-reviewed literature. For all but one of these high variability congeners, more mothers had significant variability in concentration than children, and mothers gained more high molecular weight PCBs than their children. These differences between mothers and children could be a reflection of exposure or metabolism differences, or a combination. No difference in PCB concentration changes between boys and girls were observed.
Figure 5.
Change from year 1 to year 2 of each PCB congener in mothers and children. A positive value indicates concentration increased. Error bars represent the 5th–95th percentile ranges of change in concentration.
Table 1. Sum and Individual PCBs with Largest Change from Year 1 to Year 2a.
| PCB | change in concentration (fifth to 95th) | % change (fifth to 95th) | estimated % change due to analytical variability | % mothers, with Δ > analytical variability | % children with Δ > analytical variability |
|---|---|---|---|---|---|
| 20 + 28 | –6 to 5 | –32 to 29 | 87 | 10 | 3 |
| 66 | –5 to 8 | –13 to 66 | 136 | 1 | 1 |
| 83 + 99 | –9 to 7 | –85 to 58 | –2 | 85 | 64 |
| 105 | –3 to 5 | –20 to 46 | 58 | 10 | 1 |
| 110 + 115 | –8 to 12 | –26 to 39 | N/A | N/A | N/A |
| 118 | –10 to 13 | –57 to 99 | 8 | 71 | 29 |
| 129 + 137 + 138 + 163 + 164 | –7 to 9 | –71 to 69 | –14 | 44 | 21 |
| 146 | 0 to 3 | 0 to 61 | 50 | 8 | 2 |
| 153 + 168 | –6 to 9 | –75 to 60 | –8 | 49 | 31 |
| 156 + 157 | 0 to 4 | 0 to 53 | –1 | 60 | 8 |
| 170 | –2 to 5 | –18 to 69 | –13 | 35 | 4 |
| 180 + 193 | –3 to 7 | –30 to 93 | 4 | 71 | 32 |
| 187 | –3 to 4 | –22 to 46 | 1 | 81 | 25 |
| ΣPCBs | –50 to 83 | –87 to 567 | 22 | 76 | 88 |
Note: Concentration is in units of ng/g lipid weight. The estimated % change due to analytical variability was determined from extraction and analysis of NIST SRM 1957 in both year 1 and year 2, where N/A means not available because congener concentrations are not certified by NIST. The 10 coeluting groups (containing 19 congeners) with biggest decrease in concentration (as indicated by the 5th percentile of the difference between year 1 and year 2) and 10 coeluting groups (containing 18 congeners) with the biggest increase in concentration (as indicated by the 95th percentile of the difference between year 1 and year 2) were selected for this table. Seven coeluting groups (containing 14 congeners) had both the biggest decrease and biggest increase).
A similar median variability but smaller range compared to our participants was observed in two published studies measuring changes in PCB levels across three and nine years. In two different cohorts of pregnant Californians sampled in 2008–2009 and 2011–2012, Zota et al. found that the geometric mean of sum of five tetra- to hepta-chlorinated congeners decreased 25% (range −68 to 7%), whereas PCB 180 declined 71% (range −141 to −22%) between the earlier and later cohorts.29 Humblet et al. reported a pilot study of eight women who gave serum samples in 2000 and 2009 and found that concentration of sum of 36 PCBs decreased by an average of 19% (range −48% to 54%) during those 9 years.22 The women lived near a chlorinated chemical plant that ended operations in 2003, between the two sampling time points. These studies present important but incomplete observations about PCB trends in humans. In both cases there were externalities that may have caused a decrease in PCBs, and the decrease was observed over longer time periods. In our study examining the same cohorts, we observe significant individual variability but do not observe significant overall population declines in PCB concentration over the relatively short two year period.
A greater decrease of serum PCBs was observed in participants sampled across larger time periods of 15, 25, and 28 years. Tee et al. report a study of 179 participants in the Michigan Fisheater Cohort between 1980 and 1995. They measured a 50% decline of sum of 25 tetra- to octa-chlorinated PCBs that occurred in conjunction with an 83% decrease in mean fish consumption.24 Vo et al. found a median decline of 67% (sum of eight penta- to hepta-chlorinated PCBs) in a cohort of 123 women in the United States who were pregnant at the time of first sample collection in 1978 and then were sampled again in 2003–2004.23 In another study of fisheaters, Norwegian men sampled between 1979 and 2007, Nost et al. report that concentrations of five penta- and nine hexa-chlorinated PCBs declined, whereas six ≥hepta-chlorinated PCBs initially increased and then decreased.4 Across all 20 congeners, the median decrease was 68%. The conclusions of these studies are related to declines in the PCB levels in their food source (fish) or due to loss of PCBs through childbirth, whereas our cohorts were not selected as fisheaters or pregnant women. Furthermore, PCB decline observed in these studies were in higher molecular weight PCBs, whereas our study also included lower molecular weight PCBs.
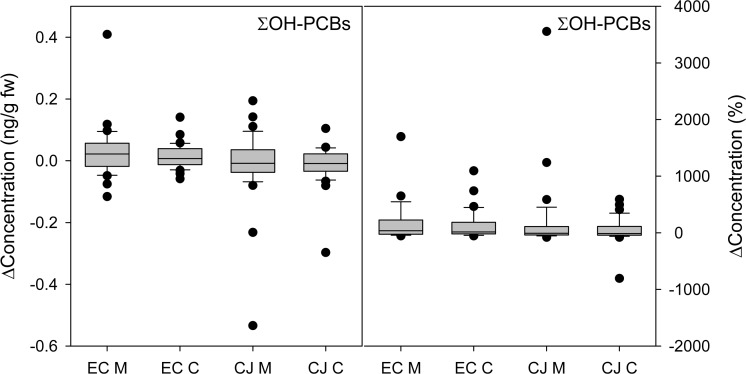
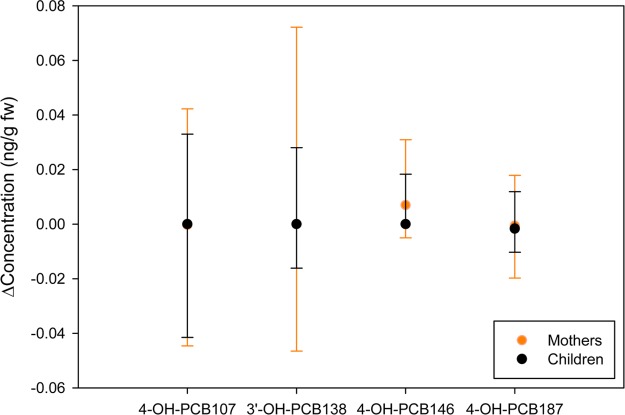
The change in ∑OH-PCBs in our cohort from year 1 to year 2 was an order of magnitude less variable than found for ∑PCBs. The median change was 0.004 ng/g fw but ranged from −0.07 to 0.10 ng/g fw (5th–95th percentile), again indicating high variability in some participants (Figure 6). After removing one participant with ∑OH-PCBs < LOQ in year 1, this change represented a median of 4% of the participants’ year 1 ∑OH-PCBs and ranged from −51 to 358% (5th–95th percentile). Of all participants, only 6% lost or gained more than 0.09 ng/g fw OH-PCBs (the median ∑OH-PCBs in year 2). The median change was nonzero for three of four OH-PCBs in mothers and only one of four OH-PCBs in children. Of the four OH-PCBs, year to year variability was largest for 3′-OH-PCB 138 for mothers and 4-OH-PCB 107 for children (Figure 7). No difference in OH-PCB concentration changes between boys and girls were observed.
Figure 6.
Change in concentration (left: ng/g fw; right: %) of sum of four OH-PCBs. A positive value indicates an increase in concentration from year 1 to year 2. EC M and EC C represent East Chicago mothers and children, respectively. CJ M and CJ C represent Columbus Junction mothers and children, respectively.
Figure 7.
Change from year 1 to year 2 of each OH-PCB congener in mothers and children. A positive value indicates concentration increased. Error bars represent the 5th–95th percentile ranges of change in concentration.
The only other study that quantified variability in OH-PCB concentrations, (although in different people from year to year) supports our finding that OH-PCB concentrations are less variable. Zota et al. found that the sum of the three measured congeners were not different between the two cohorts across three years,29 although most of the pregnant Californians had concentrations of the three OH-PCBs measured below the detection limit which makes their results harder to interpret.
A quartile analysis of the PCB and OH-PCB year to year variability within each participant subgroup was also performed. Most participants’ PCB concentrations remained in the same quartile rank from year 1 to year 2, or changed only by one quartile. A small number (8%) of East Chicago mothers’ concentrations increased or decreased more than one quartile compared with 32% of East Chicago children. PCB concentrations in Columbus Junction mothers and children were more similar year to year, with 24% and 21%, respectively increasing or decreasing by more than one quartile. Participants’ OH-PCB concentrations changed quartiles in about the same percentage in each subgroup (27% East Chicago mothers, 25% East Chicago children, 20% Columbus Junction mothers, and 20% Columbus Junction children).
We assumed that PCB concentration does not change much from year to year because dietary habits, environmental exposures, and physiological changes like body composition and metabolism are thought to remain fairly consistent in a shorter period of time. Our data show this assumption is not true for most participants in this study. In this paper we examined variability in the same population from one year to the next, and we are the first to quantify variability for all 209 PCBs and the commonly reported OH-PCBs in the same people. Although many participants had similar levels of PCBs and OH-PCBs in their blood from one year to the next, a subset of participants had surprisingly different levels, and most participants (82%) had variability in blood concentrations beyond changes due to analytical method differences. Some PCB and OH-PCB congeners had much greater variability than other congeners. This variability could be due to exposure differences, physiological changes such as metabolism and weight, or a combination, and further research to clarify the cause of the observed variability is ongoing.
Acknowledgments
We are grateful to the residents of East Chicago and the Columbus Junction area for their participation in our study. Dr. Craig Just and David Osterberg led our community engagement efforts. Barb Mendenhall and Nancy Morales recruited and enrolled subjects, collected blood and survey data, and processed samples. Dr. Hans-Joachim Lehmler synthesized the diazomethane we used to derivatize samples. We thank Nick Erdman and Collin Just for their contributions to sample analysis. This study was supported by NIH P42ES013661 and P30ES005605, and a GAANN fellowship from the Department of Education, P200A09035011. The authors declare no competing financial interests.
Supporting Information Available
Method details, quality control results, tables of congener-specific lipid weight and fresh weight PCB and OH-PCB concentrations, and correlation analysis results. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Erickson M. D.; Kaley R. G. Applications of polychlorinated biphenyls. Environ. Sci. Pollut. Res. 2011, 182135–151. [DOI] [PubMed] [Google Scholar]
- Marek R. F.; Thorne P. S.; Wang K.; DeWall J.; Hornbuckle K. C. PCBs and OH-PCBs in serum from children and mothers in urban and rural U.S. communities. Environ. Sci. Technol. 2013, 4773353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson D. G.; Wong L. Y.; Turner W. E.; Caudill S. P.; Dipietro E. S.; McClure P. C.; Cash T. P.; Osterloh J. D.; Pirkle J. L.; Sampson E. J.; Needham L. L. Levels in the US population of those persistent organic pollutants (2003–2004) included in the Stockholm Convention or in other long-range transboundary air pollution agreements. Environ. Sci. Technol. 2009, 4341211–1218. [DOI] [PubMed] [Google Scholar]
- Nost T. H.; Breivik K.; Fuskevag O.; Nieboer E.; Odland J. O.; Sandanger T. M. Persistent organic pollutants in Norwegian men from 1979 to 2007: Intraindividual changes, age-period-cohort effects, and model predictions. Environ. Health Perpsect. 2013, 10.1289/ehp.1206317 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. S.; Petreas M.; Cohn B. A.; Cirillo P. M.; Factor-Litvak P. Hydroxylated PCB metabolites (OH-PCBs) in archived serum from 1950–60s California mothers: A pilot study. Environ. Int. 2009, 356937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauby-Secretan B.; Loomis D.; Grosse Y.; El Ghissassi F.; Bouvard V.; Benbrahim-Tallaa L.; Guha N.; Baan R.; Mattock H.; Straif K. International Agency for Research on Cancer Monograph Working Group Iarc, L. F., Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol. 2013, 144287–8. [DOI] [PubMed] [Google Scholar]
- Boas M.; Feldt-Rasmussen U.; Main K. M. Thyroid effects of endocrine disrupting chemicals. Mol. Cell. Endocrinol. 2012, 3552240–248. [DOI] [PubMed] [Google Scholar]
- Stadnicki S. S.; Allen J. R. Toxicity of 2,2′,5,5′-tetrachlorobiphenyl and its metabolites, 2,2′,5,5′-tetrachlorobiphenyl-3,4-oxide and 2,2′,5,5′-tetrachlorobiphenyl-4-O1 to cultured-cells invitro. Bull. Environ. Contam. Toxicol. 1979, 236788–796. [DOI] [PubMed] [Google Scholar]
- Yamamoto H. A.; Yoshimur H. Metabolic studies on polychlorinated biphenyls 0.3. Complete structure and acute toxicity metabolites of 2,4,3′,4′-tetrachlorobiphenyl. Chem. Pharm. Bull. 1973, 21102237–2242. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Mapuskar K. A.; Marek R. F.; Xu W.; Lehmler H. J.; Robertson L. W.; Hornbuckle K. C.; Spitz D. R.; Aykin-Burns N. A new player in environmentally induced oxidative stress: Polychlorinated biphenyl congener 3,3′-dichlorobiphenyl (PCB 11). Toxicol. Sci. 2013, 10.1093/toxsci/kft186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niknam Y.; Feng W.; Cherednichenko G.; Dong Y.; Joshi S.; Vyas S.; Lehmler H. J.; Pessah I. N. Structure-activity relationship of selected meta- and para-hydroxylated non-dioxin-like polychlorinated biphenyls: From single RyR1 channels to muscle dysfunction. Toxicol. Sci. 2013, 136, 500–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. S.; Williams P. L.; Sergeyev O.; Korrick S.; Lee M. M.; Revich B.; Altshul L.; Del Prato J. T.; Humblet O.; Patterson D. G. Jr.; Turner W. E.; Needham L. L.; Starovoytov M.; Hauser R. Serum dioxins and polychlorinated biphenyls are associated with growth among Russian boys. Pediatrics 2011, 1271e59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P.; Landrigan P. J. Developmental neurotoxicity of industrial chemicals. Lancet 2006, 36895532167–2178. [DOI] [PubMed] [Google Scholar]
- Mitchell M. M.; Woods R.; Chi L. H.; Schmidt R. J.; Pessah I. N.; Kostyniak P. J.; LaSalle J. M. Levels of select PCB and PBDE congeners in human postmortem brain reveal possible environmental involvement in 15q11-q13 duplication autism spectrum disorder. Environ. Mol. Mutagen. 2012, 538589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah I. N.; Cherednichenko G.; Lein P. J. Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol. Therapeut 2010, 1252260–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sable H. J.; Monaikul S.; Poon E.; Eubig P. A.; Schantz S. L. Discriminative stimulus effects of cocaine and amphetamine in rats following developmental exposure to polychlorinated biphenyls (PCBs). Neurotoxicol. Teratol. 2011, 332255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylander C.; Lund E.; Froyland L.; Sandanger T. M. Predictors of PCP, OH-PCBs, PCBs and chlorinated pesticides in a general female Norwegian population. Environ. Int. 2012, 43, 13–20. [DOI] [PubMed] [Google Scholar]
- Cerna M.; Kratenova J.; Zejglicova K.; Brabec M.; Maly M.; Smid J.; Crhova S.; Grabic R.; Volf J. Levels of PCDDs, PCDFs, and PCBs in the blood of the non-occupationally exposed residents living in the vicinity of a chemical plant in the Czech Republic. Chemosphere 2007, 679S238–46. [DOI] [PubMed] [Google Scholar]
- Bachelet D.; Truong T.; Verner M. A.; Arveux P.; Kerbrat P.; Charlier C.; Guihenneuc-Jouyaux C.; Guenel P. Determinants of serum concentrations of 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene and polychlorinated biphenyls among French women in the CECILE study. Environ. Res. 2011, 1116861–70. [DOI] [PubMed] [Google Scholar]
- Costopoulou D.; Vassiliadou I.; Papadopoulos A.; Makropoulos V.; Leondiadis L. Levels of dioxins, furans and PCBs in human serum and milk of people living in Greece. Chemosphere 2006, 6591462–1469. [DOI] [PubMed] [Google Scholar]
- Quinn C. L.; Wania F. Understanding differences in the body burden-age relationships of bioaccumulating contaminants based on population cross sections versus individuals. Environ. Health Perspect. 2012, 1204554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humblet O.; Sergeyev O.; Altshul L.; Korrick S. A.; Williams P. L.; Emond C.; Birnbaum L. S.; Burns J. S.; Lee M. M.; Revich B.; Shelepchikov A.; Feshin D.; Hauser R., Temporal trends in serum concentrations of polychlorinated dioxins, furans, and PCBs among adult women living in Chapaevsk, Russia: A longitudinal study from 2000 to 2009. In Environ. Health, 2011; Vol. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo T. T.; Gladen B. C.; Cooper G. S.; Baird D. D.; Daniels J. L.; Gammon M. D.; Richardson D. B. Dichlorodiphenyldichloroethane and polychlorinated biphenyls: Intraindividual changes, correlations, and predictors in healthy women from the Southeastern United States. Cancer Epidem. Biomar. 2008, 17102729–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee P. G.; Sweeney A. M.; Symanski E.; Gardiner J. C.; Gasior D. M.; Schantz S. L. A longitudinal examination of factors related to changes in serum polychlorinated biphenyl levels. Environ. Health Perspect. 2003, 1115702–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmerova S.; Lancz K.; Tihanyi J.; Sovcikova E.; Kocan A.; Drobna B.; Palkovicova L.; Jureckova D.; Fabisikova A.; Conka K.; Trnovec T. Half-lives of serum PCB congener concentrations in environmentally exposed early adolescents. Chemosphere 2011, 825687–691. [DOI] [PubMed] [Google Scholar]
- Letcher R. J.; Klasson-Wehler E.; Bergman A., Methyl sulfone and hydroxylated metabolites of polychlorinated biphenyls. In New Types of Persistent Halogenated Compounds; Paasivirta J., Ed.; Springer-Verlag: Berlin-Heidelberg, 2000; Vol. 3, pp 315–359. [Google Scholar]
- Martinez A.; Wang K.; Hornbuckle K. C. Fate of PCB congeners in an industrial harbor of Lake Michigan. Environ. Sci. Technol. 2010, 4482803–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing, version 2.13.1; Vienna, Austria, 2011; ISBN 3-900051-07-0, http://www.R-project.org/.
- Zota A. R.; Linderholm L.; Park J. S.; Petreas M.; Guo T.; Privalsky M. L.; Zoeller R. T.; Woodruff T. J. Temporal comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the serum of second trimester pregnant women recruited from San Francisco General Hospital, California. Environ. Sci. Technol. 2013, 47, 11776–11784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.