Abstract
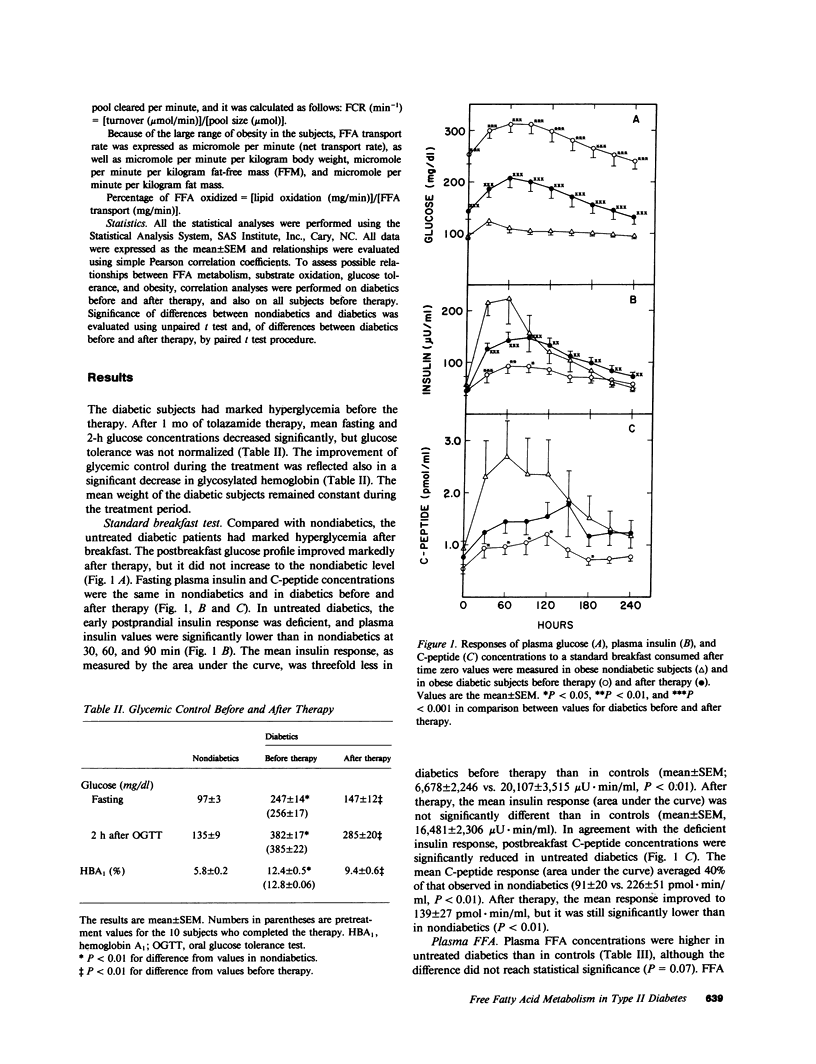
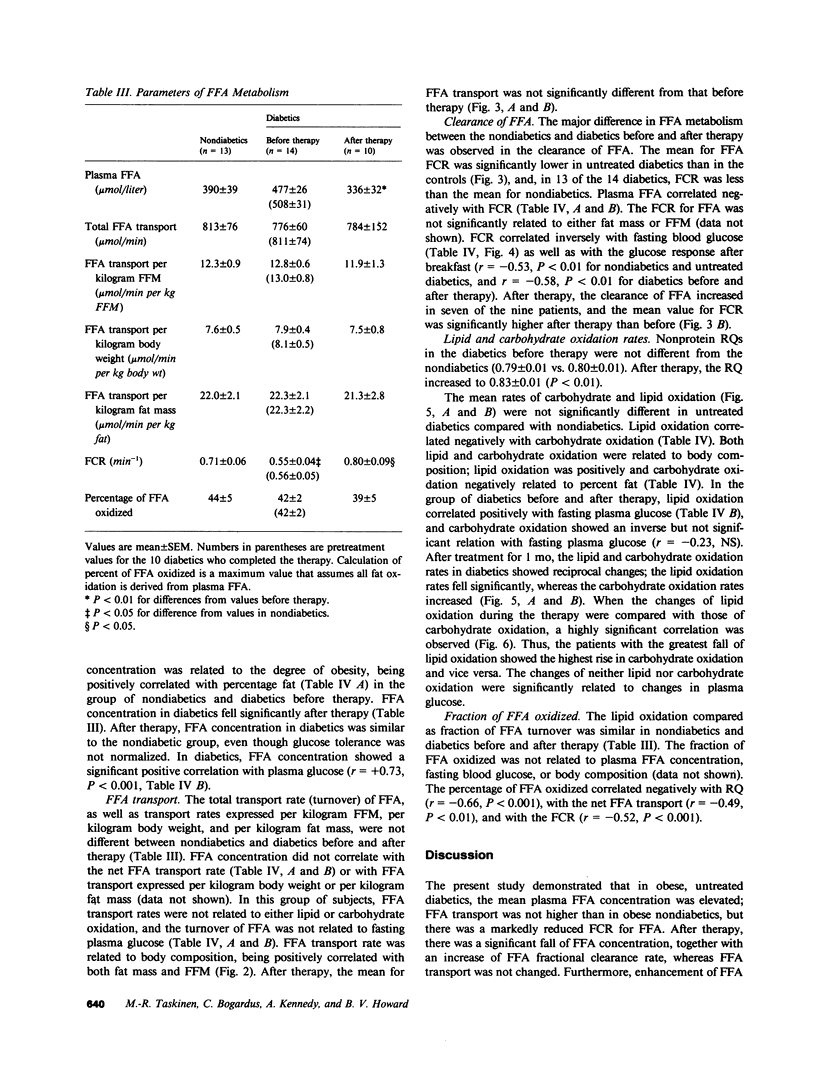
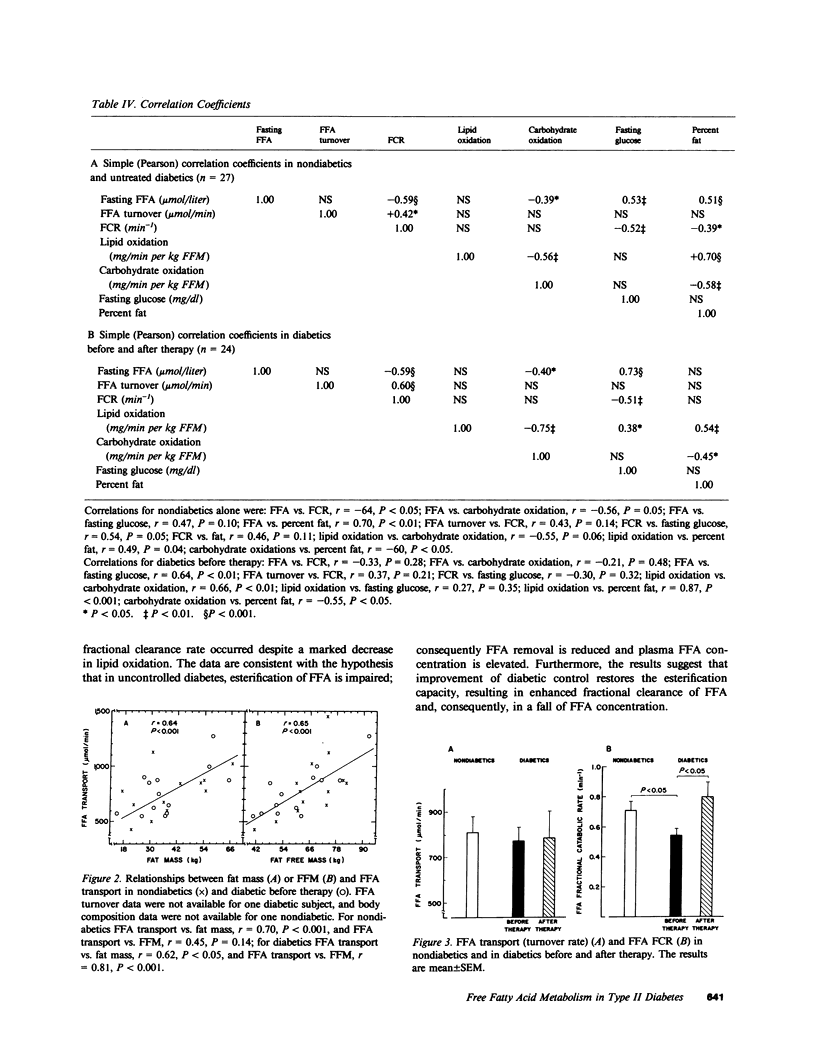
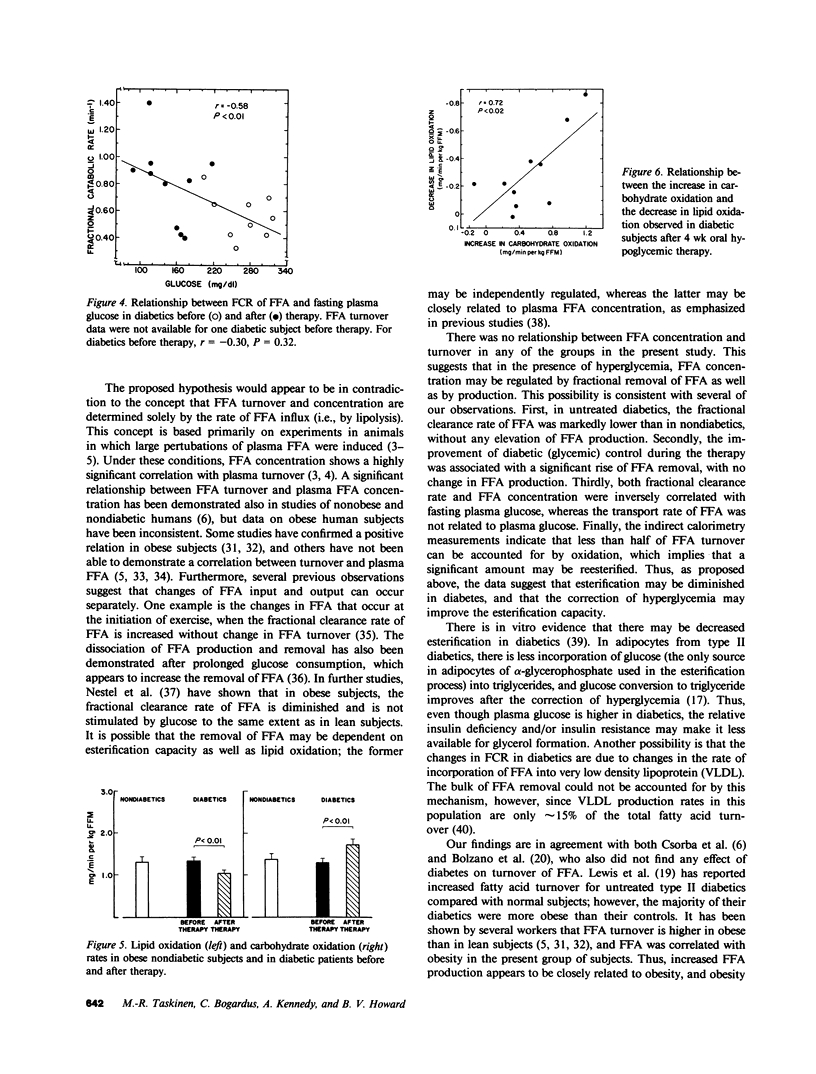
To assess the mechanisms for the elevation of free fatty acids in noninsulin-dependent diabetes, free fatty acid metabolism and lipid and carbohydrate oxidation were compared in 14 obese diabetic Pima Indians and in 13 age-, sex-, and weight-matched nondiabetics. The studies were repeated in 10 of the diabetics after 1 mo of oral hypoglycemic therapy. Fasting plasma glucose concentrations were elevated in diabetics (242 +/- 14 vs. 97 +/- 3 mg/dl, P less than 0.01) and decreased to 142 +/- 12 (P less than 0.01) after therapy. Fasting free fatty acid concentrations were elevated in diabetics (477 +/- 26 vs. 390 +/- 39 mumol/liter, P less than 0.01) and declined to normal values after therapy (336 +/- 32, P less than 0.01). Although free fatty acid transport rate was correlated with obesity (r = 0.75, P less than 0.001), the transport of free fatty acid was not higher in diabetics than in nondiabetics and did not change after therapy. On the other hand, the fractional catabolic rate for free fatty acid was significantly lower in untreated diabetics (0.55 +/- 0.04 vs. 0.71 +/- 0.06 min-1, P less than 0.05); it increased after therapy to 0.80 +/- 0.09 min-1, P less than 0.05, and was inversely correlated with fasting glucose (r = -0.52, P less than 0.01). In diabetics after therapy, lipid oxidation rates fell significantly (from 1.35 +/- 0.06 to 1.05 +/- 0.01 mg/min per kg fat-free mass, P less than 0.01), whereas carbohydrate oxidation increased (from 1.21 +/- 0.10 to 1.73 +/- 0.13 mg/min per kg fat-free mass, P less than 0.01); changes in lipid and carbohydrate oxidation were correlated (r = 0.72, P less than 0.02), and in all subjects lipid oxidation accounted for only approximately 40% of free fatty acid transport. The data suggest that in noninsulin-dependent diabetics, although free fatty acid production may be elevated because of obesity, the elevations in plasma free fatty acid concentrations are also a result of reduced removal, and fractional clearance of free fatty acid appears to be closely related to diabetic control. Furthermore, the increase in fractional clearance rate, despite a marked decrease in lipid oxidation, suggests that the clearance defect in the diabetics is due to an impairment in reesterification, which is restored after therapy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG D. T., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S., DE BODO R. C. Regulation of plasma free fatty acid turnover. Am J Physiol. 1961 Jul;201:9–15. doi: 10.1152/ajplegacy.1961.201.1.9. [DOI] [PubMed] [Google Scholar]
- Arner P., Bolinder J., Engfeldt P., Ostman J. The antilipolytic effect of insulin in human adipose tissue in obesity, diabetes mellitus, hyperinsulinemia, and starvation. Metabolism. 1981 Aug;30(8):753–760. doi: 10.1016/0026-0495(81)90020-2. [DOI] [PubMed] [Google Scholar]
- BIERMAN E. L., DOLE V. P., ROBERTS T. N. An abnormality of nonesterified fatty acid metabolism in diabetes mellitus. Diabetes. 1957 Nov-Dec;6(6):475–479. doi: 10.2337/diab.6.6.475. [DOI] [PubMed] [Google Scholar]
- Bagdade J. D., Porte D., Jr, Bierman E. L. The interaction of diabetes and obesity on the regulation of fat mobilization in man. Diabetes. 1969 Nov;18(11):759–772. doi: 10.2337/diab.18.11.759. [DOI] [PubMed] [Google Scholar]
- Barter P. J., Nestel P. J. Plasma free fatty acid transport during prolonged glucose consumption and its relationship to plasma triglyceride fatty acids in man. J Lipid Res. 1972 Jul;13(4):483–490. [PubMed] [Google Scholar]
- Birkenhäger J. C., Tjabbes T. Turnover rate of plasma FFA and rate of esterification of plasma FFA to plasma triglycerides in obese humans before and after weight reduction. Metabolism. 1969 Jan;18(1):18–32. doi: 10.1016/0026-0495(69)90129-2. [DOI] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Howard B. V., Reaven G., Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. 1984 Oct;74(4):1238–1246. doi: 10.1172/JCI111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolzano K., Sandhofer F., Sailer S., Braunsteiner H. The effect of oral administration of sucrose on the turnover rate of plasma free fatty acids and on the esterification rate of plasma free fatty acids to plasma triglycerides in normal subjects, patients with primary endogenous hypertriglyceridemia, and patients with well controlled diabetes mellitus. Horm Metab Res. 1972 Nov;4(6):439–446. doi: 10.1055/s-0028-1094002. [DOI] [PubMed] [Google Scholar]
- Csorba T. R., Matsuda I., Kalant N. Effects of insulin and diabetes on flux rates of plasma glucose and free fatty acids. Metabolism. 1966 Mar;15(3):262–270. doi: 10.1016/0026-0495(66)90024-2. [DOI] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E., Barrett E. J., Bevilacqua S., DeFronzo R. A. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983 Nov;72(5):1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J. E., Kashiwagi A., Verso M. A., Reaven G., Andrews J. Improvement in in vitro insulin action after one month of insulin therapy in obese noninsulin-dependent diabetics. Measurements of glucose transport and metabolism, insulin binding, and lipolysis in isolated adipocytes. J Clin Invest. 1983 Dec;72(6):1901–1909. doi: 10.1172/JCI111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golay A., Felber J. P., Meyer H. U., Curchod B., Maeder E., Jéquier E. Study on lipid metabolism in obesity diabetes. Metabolism. 1984 Feb;33(2):111–116. doi: 10.1016/0026-0495(84)90121-5. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., NAIMARK A., BORCHGREVINK C. F. Turnover rate and oxidation of free fatty acids of blood plasma in man during exercise: studies during continuous infusion of palmitate-1-C14. J Clin Invest. 1963 Jul;42:1054–1063. doi: 10.1172/JCI104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heding L. G. Radioimmunological determination of human C-peptide in serum. Diabetologia. 1975 Dec;11(6):541–548. doi: 10.1007/BF01222104. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Ho R. J. Radiochemical assay of long-chain fatty acids using 63Ni as tracer. Anal Biochem. 1970 Jul;36(1):105–113. doi: 10.1016/0003-2697(70)90337-4. [DOI] [PubMed] [Google Scholar]
- Howard B. V., Reitman J. S., Vasquez B., Zech L. Very-low-density lipoprotein triglyceride metabolism in non-insulin-dependent diabetes mellitus. Relationship to plasma insulin and free fatty acids. Diabetes. 1983 Mar;32(3):271–276. doi: 10.2337/diab.32.3.271. [DOI] [PubMed] [Google Scholar]
- Howard B. V., Savage P. J., Nagulesparan M., Bennion L. J., Unger R. H., Bennett P. H. Evidence for marked sensitivity to the antilipolytic action of insulin in obese maturity-onset diabetics. Metabolism. 1979 Jul;28(7):744–750. doi: 10.1016/0026-0495(79)90180-x. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Bortz W. M., Miller H. I., Paul P. Turnover rate of plasma FFA in humans and in dogs. Metabolism. 1967 Nov;16(11):1001–1009. doi: 10.1016/0026-0495(67)90093-5. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Paul P., Miller H. I., Bortz W. M. Oxidation of plasma FFA in lean and obese humans. Metabolism. 1968 Jan;17(1):62–73. doi: 10.1016/s0026-0495(68)80008-3. [DOI] [PubMed] [Google Scholar]
- Kashiwagi A., Verso M. A., Andrews J., Vasquez B., Reaven G., Foley J. E. In vitro insulin resistance of human adipocytes isolated from subjects with noninsulin-dependent diabetes mellitus. J Clin Invest. 1983 Oct;72(4):1246–1254. doi: 10.1172/JCI111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B., Mancini M., Mattock M., Chait A., Fraser T. R. Plasma triglyceride and fatty acid metabolism in diabetes mellitus. Eur J Clin Invest. 1972 Nov;2(6):445–453. doi: 10.1111/j.1365-2362.1972.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Lillioja S., Bogardus C., Mott D. M., Kennedy A. L., Knowler W. C., Howard B. V. Relationship between insulin-mediated glucose disposal and lipid metabolism in man. J Clin Invest. 1985 Apr;75(4):1106–1115. doi: 10.1172/JCI111804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles J., Glasscock R., Aikens J., Gerich J., Haymond M. A microfluorometric method for the determination of free fatty acids in plasma. J Lipid Res. 1983 Jan;24(1):96–99. [PubMed] [Google Scholar]
- Nestel P. J., Ishikawa T., Goldrick R. B. Diminished plasma free fatty acid clearance in obese subjects. Metabolism. 1978 May;27(5):589–597. doi: 10.1016/0026-0495(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Nestel P. J. Relationship between FFA flux and TGFA influx in plasma before and during the infusion of insulin. Metabolism. 1967 Dec;16(12):1123–1132. doi: 10.1016/0026-0495(67)90058-3. [DOI] [PubMed] [Google Scholar]
- Nestel P. J., Whyte H. M. Plasma free fatty acid and triglyceride turnover in obesity. Metabolism. 1968 Dec;17(12):1122–1128. doi: 10.1016/0026-0495(68)90092-9. [DOI] [PubMed] [Google Scholar]
- Paul P., Issekutz B., Jr, Miller H. I. Interrelationship of free fatty acids and glucose metabolism in the dog. Am J Physiol. 1966 Dec;211(6):1313–1320. doi: 10.1152/ajplegacy.1966.211.6.1313. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Garland P. B., Newsholme E. A., Hales C. N. The glucose fatty acid cycle in obesity and maturity onset diabetes mellitus. Ann N Y Acad Sci. 1965 Oct 8;131(1):324–333. doi: 10.1111/j.1749-6632.1965.tb34800.x. [DOI] [PubMed] [Google Scholar]
- Reitsma W. D. The relationship between serum free fatty acids and blood sugar in non-obese and obese diabetics. Acta Med Scand. 1967 Sep;182(3):353–361. doi: 10.1111/j.0954-6820.1967.tb11535.x. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Toews C. J., Shafrir E. Role of free fatty acids in glucose homeostasis. Arch Intern Med. 1969 Mar;123(3):299–313. [PubMed] [Google Scholar]
- Ryan W. G., Schwartz T. B. Dynamics of plasma triglyceride turnover in man. Metabolism. 1965 Dec;14(12):1243–1254. doi: 10.1016/s0026-0495(65)80004-x. [DOI] [PubMed] [Google Scholar]
- Thiébaud D., DeFronzo R. A., Jacot E., Golay A., Acheson K., Maeder E., Jéquier E., Felber J. P. Effect of long chain triglyceride infusion on glucose metabolism in man. Metabolism. 1982 Nov;31(11):1128–1136. doi: 10.1016/0026-0495(82)90163-9. [DOI] [PubMed] [Google Scholar]
- Welch S. G., Boucher B. J. A rapid micro-scale method for the measurement of Haemoglobin A1(a+b+c). Diabetologia. 1978 Mar;14(3):209–211. doi: 10.1007/BF00429782. [DOI] [PubMed] [Google Scholar]
- Zweens J., Frankena H. An improved method for the determination of the plasma volume with Evans Blue. J Clin Chem Clin Biochem. 1981 Sep;19(9):919–924. doi: 10.1515/cclm.1981.19.9.919. [DOI] [PubMed] [Google Scholar]



