Key messages
29% of Brent HIV patients have at least one comorbid disease.
Some of the most common comorbidities are hepatitis, mental health disorders and cardiovascular disease.
Male, White and older patients are more likely to have a comorbidity.
Co-morbidities appear to be largely independent of HIV duration.
Why this matters to us
This project was undertaken due to changes in the National Health Service (NHS) HIV services, leading to patients being transferred between services commissioned by three different organisations (Local Authority, NHS England and Clinical Commissioning Groups). Given the change in the course of disease from a terminal to a chronic condition, we were keen to contribute to the existing knowledge base regarding changes in healthcare needs among HIV-positive patients that also consider a wider range of comorbidities.
Keywords: AIDS, clinical audit, comorbidity, HIV
Abstract
Background HIV has changed from a rapidly deteriorating illness to a complex chronic disease, with increasing incidences of comorbidity, including cancer, and liver, lung and cardiovascular diseases. North West London has 6719 individuals living with the human immunodeficiency virus (HIV), 873 of whom reside in the London Borough of Brent. Traditionally, commissioning services have focused on HIV therapy alone without considering how comorbidity affects treatment outcome and total service costs.
Setting The setting for the study was NHS Brent Primary Care Trust, London UK.
Question What associated comorbidities are present in people in Brent (London, UK) living with HIV, and how common are they?
Methods A point-prevalence audit of retrospective data was conducted on all HIV-positive patients in Brent (financial year 2011/12). Data were collected from genito-urinary medicine (GUM) services, community services and general practitioners (GPs) on HIV diagnosis, patient demographics and past/current comorbidities: hepatitis B and C, cardiovascular disease, diabetes and mental health disorders.
Results This study identified that 29% of people living with HIV/AIDS (PLWHA) in Brent have at least one comorbidity. The most common was hepatitis, followed by mental health disorders and cardiovascular disease (CVD). Comorbidity was more likely in older male patients (in particular CVD and diabetes) and White patients (except for diabetes which was more common in Asian groups).
Discussion/Conclusion Many PLWHA in Brent suffer from a number of other conditions, which appear largely independent of HIV. Findings confirm the need to treat HIV as a long-term condition, including patient education, empowerment and encouraging self-management. The multi-morbidity of many PLWHA suggests a role for both primary care and collaborative, holistic, patient-centred and individualised healthcare. Service providers and commissioners need to consider comorbidities in their treatment of and provision of services for PLWHA. This study also highlighted the need for services to address limitations of their data collection systems.
Background
North West London (Brent, Ealing, Hammersmith & Fulham, Harrow, Hillingdon, Hounslow, Kensington & Chelsea and Westminster) has 6719 individuals living with the human immunodeficiency virus (HIV) infection; 873 of whom reside in the London Borough of Brent.1 With the advent of antiretroviral therapies (ART) the disease has changed from a rapidly deteriorating condition with significant mortality to a complex, chronic condition with individuals now expecting to live a normal lifespan. However, it is unclear whether this prolonged lifespan and extended disease exposure can affect the risk of developing other conditions including heart and liver disease, cancer and neurocognitive impairments for which there is no direct causal relationship with HIV.
Comorbidity in HIV can be defined as a disease outside the scope of an acquired immunodeficiency syndrome-associated (AIDS-defining) illness. The mean number of general- and HIV-associated comorbidities amongst HIV patients is 1.1 and 1.4, respectively.2 The most common comorbidities amongst patients with HIV include: diabetes mellitus, cardiovascular disease (CVD, e.g. hypertension), respiratory diseases (e.g. chronic obstructive pulmonary diseases and pneumonia), and hepatic diseases (hepatitis B and C).1,3–5 Liver disease, renal disease, substance dependence and abuse, sexually transmitted infections (herpes simplex, syphilis, gonorrhoea and Mycoplasma genitalium) and psychiatric disorders (including depression, anxiety, schizophrenia and cognitive impairment) are also greater among HIV-positive individuals.1,6,7
Mortality amongst HIV-positive individuals is primarily due to liver disease (hepatitis B and C, alcoholassociated or antiretroviral toxicity), vascular disease (associated with smoking, alcohol, antiretrovirals), AIDS-related conditions (due to non-adherence or intolerance of antiretroviral regimens), lung disease (due to smoking and alcohol), cancer (due to smoking, alcohol, HCV, possibly antiretrovirals) and violence (associated with alcohol and drug use).8
Such comorbidities can occur by chance, but are more often due to the HIV infection and its associated risk factors.9 Comorbidity increases with HIV severity and the greater prevalence of comorbidities among people living with HIV/AIDS (PLWHA) may be attributed to antiretroviral toxicity (diabetes, vascular disease and liver disease) or caused by the HIV infection itself (vascular, pulmonary and renal diseases).10 Comorbidity may also be due to potential coinfection through overlapping risk factors such as intravenous drug use (HCV).3 Because of the effects of the infection on the immune system, ageing HIV-positive individuals may have a higher disease burden than those who do not have the infection. Other concomitant conditions, often related to lifestyle (such as alcoholism or co-infection with viral hepatitis) can also affect outcomes and increase the risk of adverse effects. Some associated disorders are also often associated with ethnicity, gender and economic variation. Recent literature recommends that patients living with HIV should be managed independently of general population guidelines for common medical conditions6, and that early diagnosis of HIV may be possible in patients presenting with symptoms of associated diseases (e.g. tuberculosis).11
There is an increased need to evaluate the burden of HIV care and its associated diseases in the community, so that commissioning services respond adequately to the changing needs of patients living in the community with HIV and accompanying comorbidity.
We aimed to undertake a point prevalence audit for 2011/12 of the number of people in Brent, London UK with HIV with one or more comorbidity. We also aimed to explore sociodemographic differences regarding HIV comorbidity.
Methods
Setting
There were 311 000 individuals living in Brent as of 201112, of whom 873 resident patients were treated with HIV. Of these, 30% were White, 38% Black African, and 37% were aged 35–44. The majority of individuals (67%) are situated in the south of the Brent borough (postcodes NW10, NW6 and NW2), with a further 30% in HA9, HA0 and NW9.1,13,14
Data collection
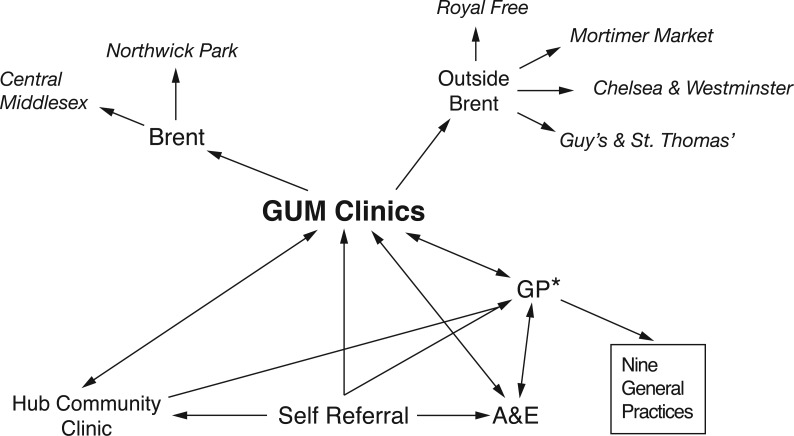
This audit aimed to collect available data on all episodes of healthcare service use amongst HIV-positive residents in Brent PCT for the financial year April 2011 to April 2012, including those seen in services outside of Brent (Figure 1), as follows:
outpatients (GUM Acute Services at Central Middlesex Hospital and Northwick Park Hospital);
Brent patients presenting in 20111 to GUM services at St Mary's Hospital, London (n = 211), Royal Free Hospital, London (n = 75), Mortimer Market Centre, London (n = 63), Chelsea & Westminster Hospital, London (n = 56), Guy's Hospital and St Thomas's Hospital, London (n = 27);
HIV community services: ‘The Hub’;
general practitioner (GP) practices with > 10 HIV patients in Brent (NW10, NW6, NW2, HA0 and HA9).
Figure 1.
HIV service organisation in Brent. *Only those clinics/GPs with more than 10 Brent HIV patients in 2011 are included (only 41% of Brent HIV patients were actually treated at NP/CMH).
Inclusion criteria were HIV-positive residents of Brent (postcodes NW2, NW6*, NW9*, NW10*, HA0*, HA1*, HA3, HA8*, HA9, W9*) using the service between 5 April 2011 and 4 April 2012. Data were collected from medical records using read codes on HIV diagnosis, demographics, and current and past comorbidities: hepatitis B and C, CVD, diabetes and mental health. We excluded practices containing fewer than ten HIV positive patients, because extraction from such practices would offer little further advantage given the extra effort and time.
Identifiable information was anonymised at the point of data extraction so that only aggregated HIV status and comorbidity data were obtained. A partial postcode and year of birth was also obtained as anonymised identifiers to eliminate duplicate patient data and to anonymously map a particular patient's progression through healthcare services. All data were tabulated and stored in an encrypted Microsoft Excel document stored on departmental servers. Analysis was undertaken using SPSS v22.0.
Descriptive analysis and bivariate statistics (chisquared and t-test) were used to explore sociodemographic differences, including relative prevalence of comorbidity in HIV and ethnic group variation. The relationship between HIV and comorbidities were explored through comparison of diagnosis dates in each patient.
Results
Data were collected relating to 982 patients: 487 from Brent GUM outpatients, 335 from community clinics, 150 from GUM services outside Brent, and 10 from one eligible GP practice; 502 duplicates were removed. We were unable to collect data from A&E departments, St Mary's Hospital, Chelsea & Westminster Hospital, and a further eight GP practices due to staffing limitations and time constraints.
Of the patients, 55% (n = 537) were male, and 83% (n = 798) were aged between 30 and 59 years. The most common ethnic group was Black/Black British (Table 1). Two hundred and eighty-five patients (29%) had at least one comorbidity. The most common comorbidity was hepatitis, followed by mental health issues and CVD problems (Table 2). Data analysis suggested that comorbidity among HIV-positive patients was more likely in males (chi-squared, p = 0.001), and those from White ethnic backgrounds (40.8% had a comorbidity; chi-squared p < 0.001). However, Asians were more likely to have diabetes (p = 0.020). Being born in the UK or elsewhere was not associated with any mean differences in the likelihood of comorbidity.
Table 1.
Demographic details of HIV patients living in Brent
| n (Total n = 982) | % | |
|---|---|---|
| Gender | ||
| Female | 444 | 45.2 |
| Male | 537 | 54.7 |
| Missing | 1 | |
| Age | ||
| 19 or under | 2 | 0.2 |
| 20 to 29 | 71 | 7.2 |
| 30 to 39 | 211 | 21.5 |
| 40 to 49 | 377 | 38.4 |
| 50 to 59 | 210 | 21.4 |
| 60 to 69 | 64 | 6.5 |
| 70 to 79 | 20 | 2.0 |
| 80 or over | 4 | 0.4 |
| Missing | 23 | |
| Ethnicity | ||
| White | 228 | 23.2 |
| Mixed | 18 | 1.8 |
| Asian | 96 | 9.8 |
| Black | 595 | 60.6 |
| Chinese/Other | 21 | 2.1 |
| Missing | 24 | |
Table 2.
Comorbidities among HIV patients in Brent.
| n (Total n = 285) | % | |
|---|---|---|
| Hepatitis | 147 | 51.6 |
| Hep B | 69 | |
| Hep C | 41 | |
| Unspecified | 37 | |
| CVD | 58 | 20.4 |
| Cardiac failure/cardiomyopathy | 2 | |
| CVA | 3 | |
| Hyperlipidaemia | 12 | |
| Hypertension | 14 | |
| LVH | 1 | |
| MI | 4 | |
| Stroke/TIA | 2 | |
| Unspecified | 20 | |
| Diabetes | 32 | 11.2 |
| Mental Health | 64 | 22.5 |
| Liver disease | 16 | 5.6 |
The average age of those with comorbidities was higher than those without (46.7 versus 44.2, t-test p = 0.001). This was also apparent among individual comorbidities including CVD (52.6 versus 45.3, p < 0.001) and diabetes (52.6 versus 46.0, p = 0.001). However, patients with a comorbid mental illness were on average 3.6 years younger than those without (p = 0.024). No comorbidities were associated with the year of HIV diagnosis. For the 110 for whom data were available, hepatitis was diagnosed on average 4.6 years after HIV.
Discussion
This audit identified that 29% of PLWHA in Brent have one or more comorbidity. In line with previous data,1,3–5 we identified hepatitis as the most common comorbidity followed by CVD. Mental health issues were also common.
However, our study was subject to a number of limitations. Because to a lack of resources, disparity in data recording and competing staff priorities, we were unable to collect data from two GUM services, eight GP practices and those with small HIV patient lists and A&E departments. High heterogeneity between data recorded by different sites also limited the reliability of collected data and the extent to which patient data could be analysed. Given the time restraints and confidential nature of extracted data, we were limited in our ability to map patients across services, identify duplicate patient data and fully explore all known comorbidities (such as COPD). Additional research is therefore warranted, including further collection of routine data in a wider range of comorbidities.
Comorbidities did not appear to be associated with the time elapsed since an individual's HIV diagnosis, suggesting that differences were due to additional, associated risk factors or an independent comorbidity, e.g. psychosocial factors modulating CVD risk, or unsafe sexual behaviour and intravenous drug use affecting hepatitis risk.9,15–18 Furthermore, comorbidities may be related to the presence of the HIV infection itself, although it is thought that for some conditions, independent risk factors play a greater role than those that are HIV-related.16 Diabetes and CVD may be related to antiretroviral toxicity.10 We did not collect data on intravenous drug use, but the prevalence of hepatitis alongside HIV may be due to this common risk factor.3
The likelihood of comorbidity was greater in male, older (especially CVD and diabetes) and White patients (except for diabetes, which was more common in Asian groups), while the increased prevalence of hepatitis and mental health issues in White groups reflects the general population.19,20 The higher prevalence of CVD21 and diabetes22 in older patients and males is also comparable with trends in the general population, as is the higher prevalence of diabetes in Asian groups.23 However, the higher prevalence of CVD in White patients contradicts existing evidence, which suggests relevant risk factors (including hypertension) are more common in Black and Minority Ethnic (BME) groups.23–25 This instead may be related to age or poor socio-economic status, which are risk factors for CVD.26,27 Studies also confirm that polypathology (more than comorbidities) is more common in older, male HIV patients.15,28 The prevalence of comorbidity, particularly polypathology, in older patients confirms the need to treat HIV similarly to other chronic diseases: for example, considering routine monitoring in primary care in partnership with secondary care and exploring the use of a range of services including nursing, preventive and rehabilitation services, as well as home health and nursing homes.29,30 There is also a clear need to tailor care provision for people with HIV to reduce mortality and morbidity risks by preventing comorbidities; through encouragement of lifestyle changes, screening,29 education about comorbidity risks15 and widening knowledge about self-management in those with multiple long-term conditions.31
Practitioners need to be more aware of the risk of comorbidities in people with HIV, in particular depression,32 hepatitis and tuberculosis,33 and the safety implications of interactions between HIV and other medications.34 Treating comorbidities early may be beneficial, for example, the early treatment of HCV in PLWHA may improve clinical outcomes35 and treating mental health issues may improve adherence to ART.16 Primary care may have a fundamental role to play in managing the co-morbid diseases of people with HIV8,34 particularly mental and emotional issues.31
Recent changes in the NHS increasingly emphasise shared care models and moving HIV patient care to GPs, particularly in treating non-HIV conditions.36 However, the issue of non-disclosure of HIV status to GPs remains a huge barrier.37 There perhaps needs to be a programme of standardising knowledge and practices targeted at healthcare professionals who manage long-term conditions including HIV. GPs, GUM clinicians and specialist nurses may be the most appropriate target as they are likely to engage with these patients.
Although there is little agreement of what ‘good care’ for chronic conditions (such as HIV) constitutes, there may be an important role for services which are collaborative, multidisciplinary, holistic, patient-centred and individualised,31,38 for example ‘The Hub’ in Brent.14 Such services may improve patient engagement with services, improve adherence to medication, help PLWHA cope with HIV and manage their own health.39 An individualised case-management approach is useful in managing patients with multiple comorbidities, reducing unmet needs (income assistance, health insurance, home healthcare, emotional counselling) and improves adherence to HIV medications.31,40–42 Multidisciplinary services may improve detection of comorbidities, improve GP liaison and improve patient satisfaction.43 Such holistic services also provide the opportunity to combine HIV treatment with other diseases such as other infections like hepatitis.44
The experience of extracting the data for this audit highlighted the need for systems to routinely record comorbidities. Further, many services still used paper records. Community services are currently considering adding a dropdown list to their current system. A standardised HIV–comorbidity EMIS template could be created for GP practices and GUM clinics, although the limitations associated with the linkage and confidentiality of patient-sensitive data would need to be addressed.
ACKNOWLEDGEMENTS
We would like to thank all the services that provided data and Brent CCG for funding the study.
Contributor Information
Ava Lorenc, Research Fellow, London South Bank University, London, UK.
Piriyankan Ananthavarathan, Clinical Research Officer.
Mohamade Jowata, Community Services, Brent CCG, London, UK.
Gary Brook, North West London Hospitals NHS Trust, London, UK.
Ricky Banarsee, Director, Applied Research Unit.
ETHICAL APPROVAL
The audit was commissioned by NHS Brent Primary Care Trust (PCT) whilst under transition to Brent Clinical Commissioning Group (CCG). As this was an audit of existing data, research ethics committee approval was not needed. NHS Brent PCT provided governance for all ethical issues associated with the research project. All data collected were anonymised onsite at the location from which the data were extracted.
COMPETING INTERESTS
All authors declare no competing interests.
FUNDING
This study was funded by NHS Brent PCT, commissioned by JL who was on the study steering group and also contributed to the writing of the paper.
REFERENCES
- 1.Health Protection Agency. HIV-diagnosed Persons Seen for Care – Survey of Prevalent HIV Infections Diagnosed (SOPHID) North West London. HPA: London, nd. www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1221482345789 (accessed 23/06/2014). [Google Scholar]
- 2.Kilbourne AM, Justice AC, Rabeneck L, Rodriguez-Barradas M, Weissman S. VACS 3 Project Team. General medical and psychiatric comorbidity among HIV-infected veterans in the post-HAART era. Journal of Clinical Epidemiology 2001;54 (Suppl 1):S22–8. [DOI] [PubMed] [Google Scholar]
- 3.Turner J, Bansi L, Gilson R. et al. The prevalence of hepatitis C virus (HCV) infection in HIV-positive individuals in the UK – trends in HCV testing and the impact of HCV on HIV treatment outcomes. Journal Viral Hepatitis 2010;17:569–77. [DOI] [PubMed] [Google Scholar]
- 4.Sudano I, Spieker LE, Noll G, Corti R, Weber R, Luscher TF. Cardiovascular disease in HIV infection. American Heart Journal 2006;151:1147–55. [DOI] [PubMed] [Google Scholar]
- 5.Crothers K, Butt AA, Gibert CL. et al. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest 2006;130:1326–33. [DOI] [PubMed] [Google Scholar]
- 6.Wilson M, Chambers L, Bacon J, Rueda S, Ragan M, Rourke S. Issues of Comorbidity in HIV/AIDS: An Overview of Systematic Reviews. Ontario HIV Treatment Network: Toronto, 2010. [Google Scholar]
- 7.Gray R, Brewin E, Noak J, Wyke-Joseph J, Sonik B. A review of the literature on HIV infection and schizophrenia: implications for research, policy and clinical practice. Journal of Psychiatric and Mental Health Nursing 2002;9:405–9. [DOI] [PubMed] [Google Scholar]
- 8.Justice AC. Prioritizing primary care in HIV: comorbidity, toxicity, and demography. Topics in HIV Medicine 2006;14:159–63. [PubMed] [Google Scholar]
- 9.Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Annals of Family Medicine 2009;7:357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goulet JL, Fultz SL, Rimland D. et al. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clinical Infectous Diseases 2007;45:1593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan AK, Curtis H, Sabin CA, Johnson MA. Newly diagnosed HIV infections: review in UK and Ireland. BMJ 2005;330(7503):1301–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greater London Authority. 2011 Census First Results: London Boroughs' Population by Age by Sex. CIS2012–01 GLA: London, 2012. //data.london.gov.uk/datastore files/documents/2011-census-first-results.pdf (accessed XX/XX/XX). [Google Scholar]
- 13.Health Protection Agency: Northwest London. New HIV Diagnoses to End of June 2012. HPA: London, 2012. [Google Scholar]
- 14.NHS Brent. NHS Brent Sexual Health and Substance Misuse Services. NHS: London, 2014. www.sexualhealthbrent.org.uk/Home/HIV/HIVServices/tabid/871/language/en-GB/Default.aspx (accessed XX/XX/XX). [Google Scholar]
- 15.Weiss JJ, Osorio G, Ryan E, Marcus SM, Fishbein DA. Prevalence and patient awareness of medical comorbidities in an urban AIDS clinic. AIDS Patient Care and STDs 2010;24:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchacz K, Rangel M, Blacher R, Brooks JT. Changes in the clinical epidemiology of HIV infection in the United States: implications for the clinician. Current Infectious Disease Reports 2009;11:75–83. [DOI] [PubMed] [Google Scholar]
- 17.Hemingway H, Marmot M. Evidence based cardiology: psychosocial factors in the aetiology and prognosis of coronary heart disease. Systematic review of prospective cohort studies. BMJ 1999;318(7196):1460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alter MJ, Kruszon-Moran D, Nainan OV. et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. New England Journal of Medicine 1999;341:556–62. [DOI] [PubMed] [Google Scholar]
- 19.Riolo SA, Nguyen TA, Greden JF, King CA. Prevalence of depression by race/ethnicity: findings from the national health and nutrition examination survey III. American Journal of Public Health 2005;95:998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alter MJ. Epidemiology of hepatitis C. Hepatology 1997;26(3 Suppl 1):62S–65S. [DOI] [PubMed] [Google Scholar]
- 21.Mittelmark MB, Psaty BM, Rautaharju PM. et al. Prevalence of cardiovascular diseases among older adults. The Cardiovascular Health Study. American Journal of Epidemiology 1993;137:311–17. [DOI] [PubMed] [Google Scholar]
- 22.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–53. [DOI] [PubMed] [Google Scholar]
- 23.Cappuccio FP, Cook DG, Atkinson RW, Strazzullo P. Prevalence, detection, and management of cardiovascular risk factors in different ethnic groups in South London. Heart 1997;78:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethnicity & Disease 2007;17:143–52. [PubMed] [Google Scholar]
- 25.Winkleby MA, Kraemer HC, Ahn DK, Varady AN. Ethnic and socioeconomic differences in cardiovascular disease risk factors: findings for women from the third national health and nutrition examination survey, 1988–1994. JAMA 1998;280:356–62. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation 1993;88(4 Pt 1):1973–98. [DOI] [PubMed] [Google Scholar]
- 27.Smith GD, Hart C, Watt G, Hole D, Hawthorne V. Individual social class, area-based deprivation, cardiovascular disease risk factors, and mortality: the Renfrew and Paisley study. Journal of Epidemiology and Community Health 1998;52:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guaraldi G, Orlando G, Zona S. et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clinical Infectious Diseases 2011;53:1120–6. [DOI] [PubMed] [Google Scholar]
- 29.Singh S, Dunford A, Carter YH. Routine care of people with HIV infection and AIDS: Should interested general practitioners take the lead? British Journal of General Practice 2001;51(466):399–403. [PMC free article] [PubMed] [Google Scholar]
- 30.Uphold CR, Mkanta WN. Review. Use of health care services among persons living with HIV infection: state of the science and future directions. AIDS Patient Care and STDs 2005;19:473–85. [DOI] [PubMed] [Google Scholar]
- 31.Goodwin N, Curry N, Naylor C, Ross S, Duldig W. Managing People with Long-term Conditions: An Inquiry into the Quality of General Practice in England. The King's Fund: London, 2010. [Google Scholar]
- 32.Asch SM, Kilbourne AM, Gifford AL. et al. Underdiagnosis of depression in HIV: who are we missing? Journal of General Internal Medicine 2003;18:450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aberg JA, Kaplan JE, Libman H. et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clinical Infectous Diseases 2009;49:651–81. [DOI] [PubMed] [Google Scholar]
- 34.Evans HE, Tsourapas A, Mercer CH. et al. Primary care consultations and costs among HIV-positive individuals in UK primary care 1995–2005: a cohort study. Sexually Transmitted Infections 2009;85:543–9. [DOI] [PubMed] [Google Scholar]
- 35.Marine-Barjoan E, Saint-Paul MC, Pradier C. et al. Impact of antiretroviral treatment on progression of hepatic fibrosis in HIV/hepatitis C virus co-infected patients. AIDS 2004;18:2163–70. [DOI] [PubMed] [Google Scholar]
- 36.British HIV Association. BHIVA Position Statement: The future role of primary and community care in HIV. BHIVA: London, 2014. www.bhiva.org/documents/Publications/Position Statement.pdf (accessed XX/XX/XX). [Google Scholar]
- 37.Evans HE, Mercer CH, Rait G. et al. Trends in HIV testing and recording of HIV status in the UK primary care setting: a retrospective cohort study 1995–2005. Sexually Transmitted Infections 2009;85:520–6. [DOI] [PubMed] [Google Scholar]
- 38.Long-term Conditions NSF Team. The National Service Framework for Long-term Conditions. Gateway Reference 4377 Department of Health: London, 2005. [Google Scholar]
- 39.Lorenc A, Banarsee R, Robinson N. Complementary therapy provision in a London community clinic for people living with HIV/AIDS: a case study. Complementary Therapies in Clinical Practice 2014;20(1):65–9. [DOI] [PubMed] [Google Scholar]
- 40.Murphy R, Tobias C, Rajabiun S, Abuchar V. HIV case management: A review of the literature. AIDS Education and Prevention 2003;15:93–108.12627746 [Google Scholar]
- 41.Katz MH, Cunningham WE, Fleishman JA. et al. Effect of case management on unmet needs and utilization of medical care and medications among HIV-infected persons. Annals of Internal Medicine 2001;135(8 Pt 1):557–65. [DOI] [PubMed] [Google Scholar]
- 42.Kushel MB, Colfax G, Ragland K, Heineman A, Palacio H, Bangsberg DR. Case management is associated with improved antiretroviral adherence and CD4+ cell counts in homeless and marginally housed individuals with HIV infection. Clinical Infectous Diseases 2006;43:234–42. [DOI] [PubMed] [Google Scholar]
- 43.Waters L, Patterson B, Scourfield A. et al. A dedicated clinic for HIV-positive individuals over 50 years of age: a multidisciplinary experience. International Journal of STD & AIDS 2012;23:546–52. [DOI] [PubMed] [Google Scholar]
- 44.Sylla L, Bruce RD, Kamarulzaman A, Altice FL. Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. International Journal of Drug Policy 2007;18:306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]