Abstract
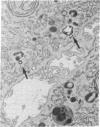
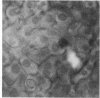
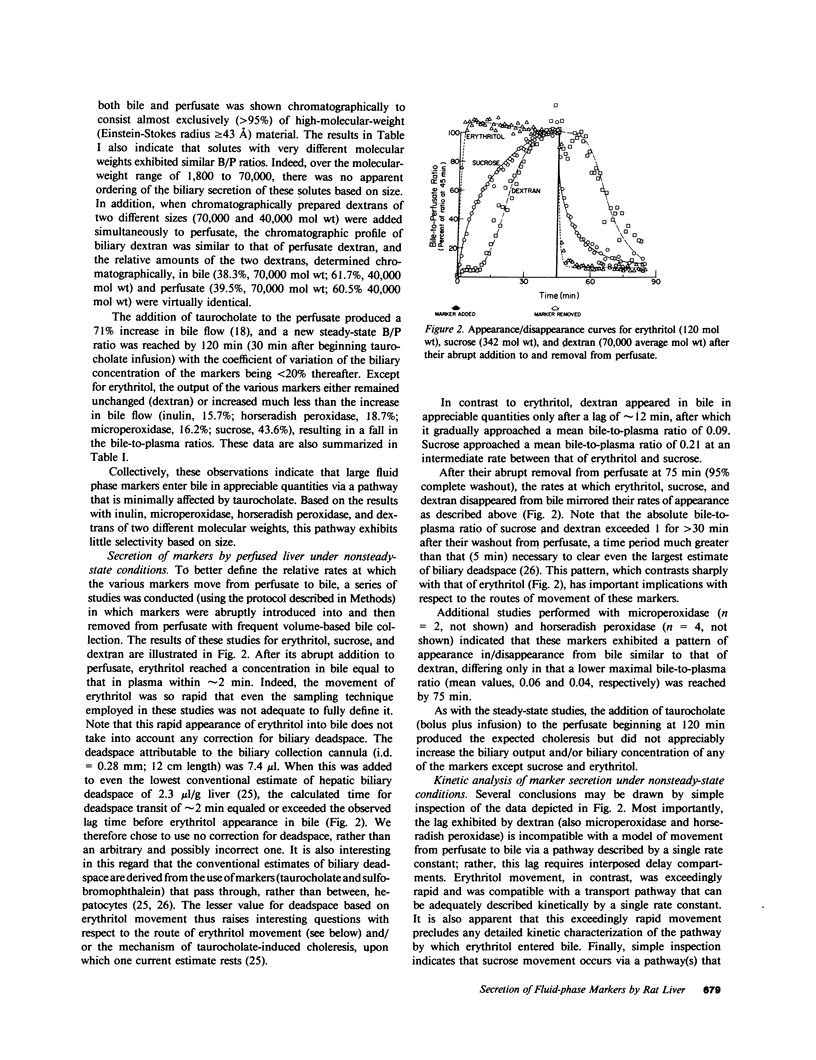
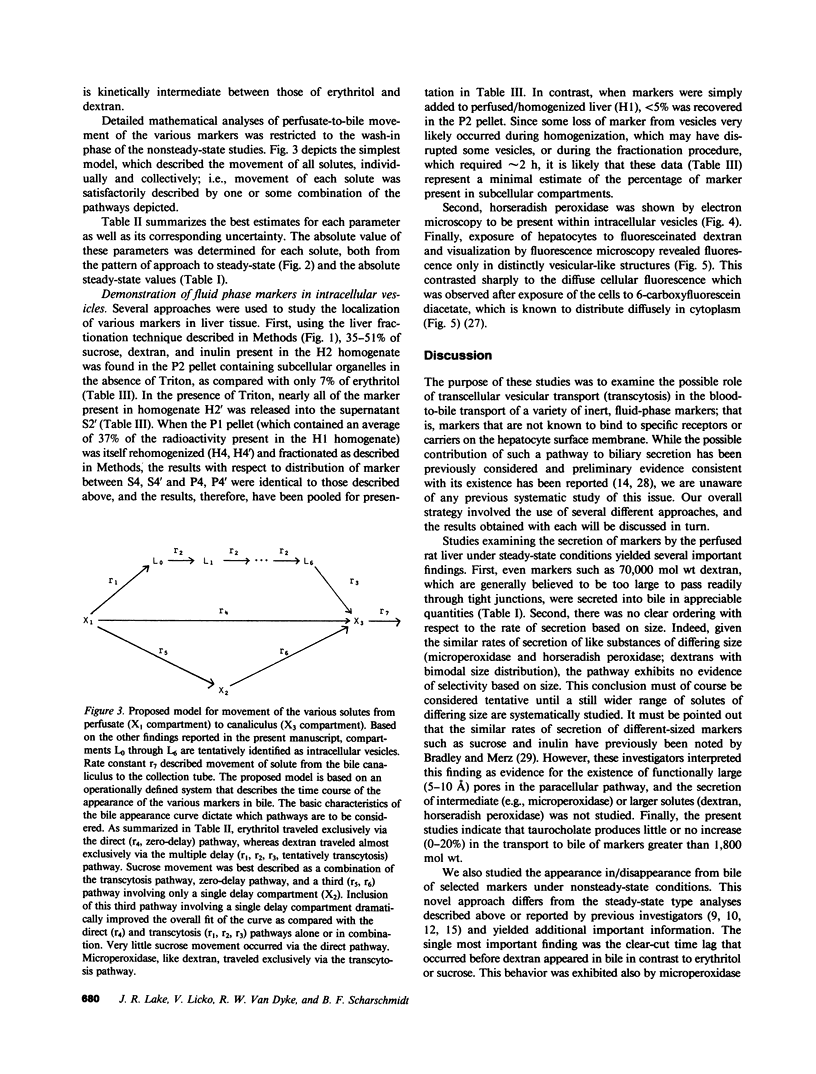
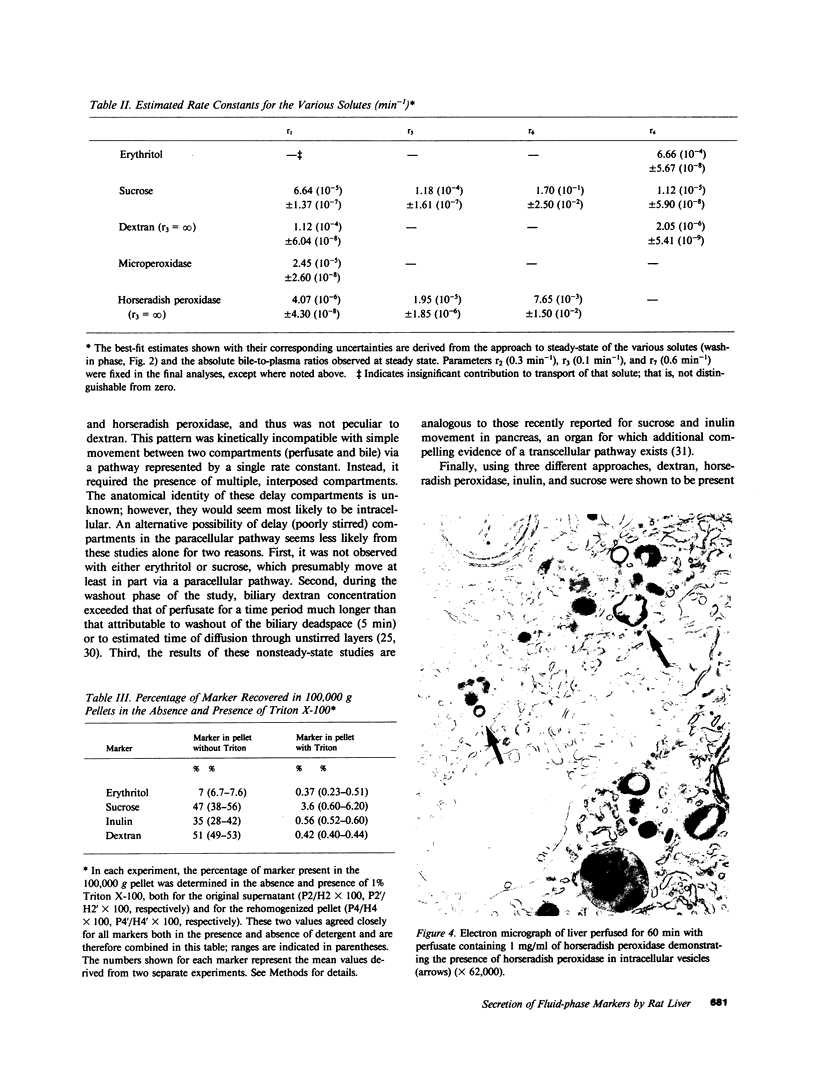
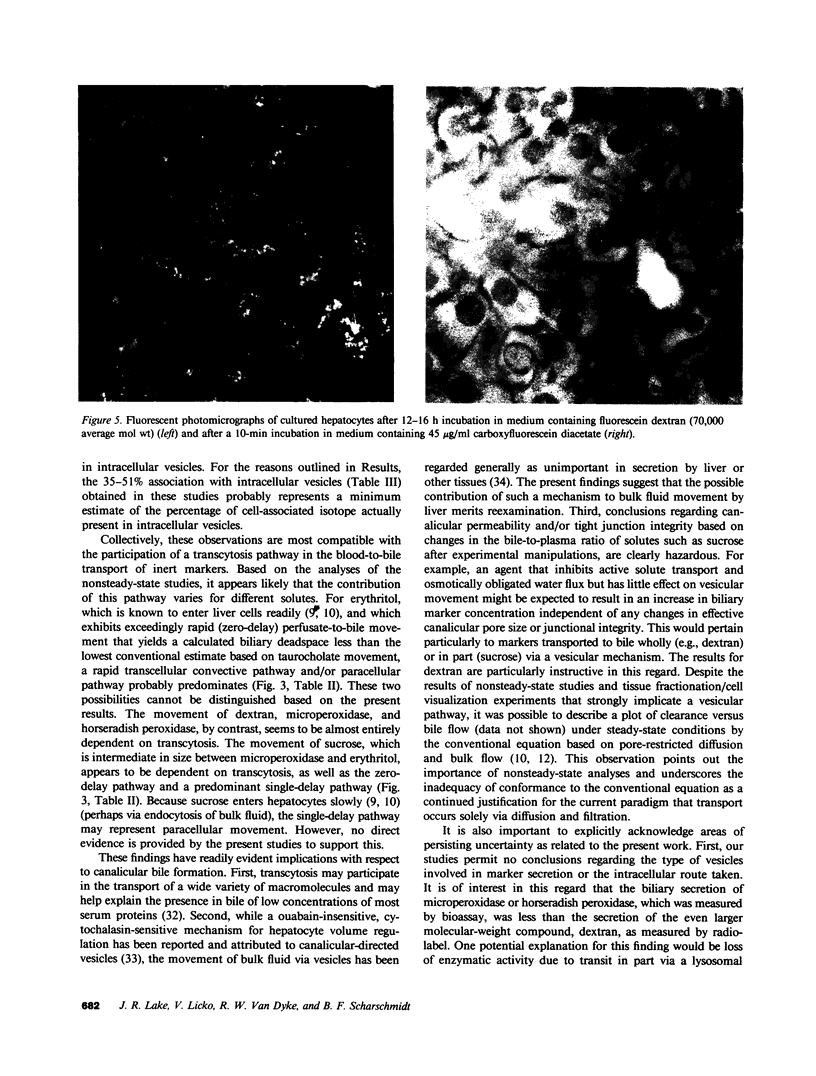
In these studies, we have used several approaches to systematically explore the contribution of transcellular vesicular transport (transcytosis) to the blood-to-bile movement of inert fluid-phase markers of widely varying molecular weight. First, under steady-state conditions, the perfused rat liver secreted even large markers in appreciable amounts. The bile-to-plasma (B/P) ratio of these different markers, including microperoxidase (B/P ratio = 0.06; mol wt = 1,879), insulin (B/P ratio = 0.09, mol wt = 5,000), horseradish peroxidase (B/P ratio = 0.04, mol wt = 40,000), and dextran (B/P ratio = 0.09, mol wt = 70,000), exhibited no clear ordering based on size alone, and when dextrans of two different sizes (40,000 and 70,000 mol wt) were studied simultaneously, the relative amounts of the two dextran species in bile were the same as in perfusate. Taurocholate administration produced a 71% increase in bile flow but little or no (0-20%) increase in the output of horseradish peroxidase, microperoxidase, inulin, and dextran. Second, under nonsteady-state conditions in which the appearance in or disappearance from bile of selected markers was studied after their abrupt addition to or removal from perfusate, erythritol reached a B/P ratio of 1 within 2 min. Microperoxidase and dextran appeared in bile only after a lag period of approximately 12 min and then slowly approached maximal values, whereas sucrose exhibited kinetically intermediate behavior. A similar pattern was observed after removal of greater than 95% of the marker from the perfusate. Erythritol rapidly reapproached a B/P ratio of 1, whereas the B/P ratio for sucrose, dextran, and microperoxidase fell much more slowly and exceeded 1 for a full 30 min after perfusate washout. Finally, electron microscopy and fluorescence microscopy of cultured hepatocytes demonstrated the presence of horseradish peroxidase and fluorescein-dextran, respectively, in intracellular vesicles, and fractionation of perfused liver homogenates revealed that at least 35-50% of sucrose, inulin, and dextran was associated with subcellular organelles. Collectively, these observations are most compatible with a transcytosis pathway that contributes minimally to the secretion of erythritol, but accounts for a substantial fraction of sucrose secretion and virtually all (greater than 95%) of the blood-to-bile transport of microperoxidase and larger markers. These findings have important implications with respect to current concepts of canalicular bile formation as well as with respect to the conventional use of solutes such as sucrose as markers of canalicular or paracellular pathway permeability.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- Barnhart J. L., Combes B. Erythritol and mannitol clearances with taurocholate and secretin-induced cholereses. Am J Physiol. 1978 Feb;234(2):E146–E156. doi: 10.1152/ajpendo.1978.234.2.E146. [DOI] [PubMed] [Google Scholar]
- Barth C. A., Schwarz L. R. Transcellular transport of fluorescein in hepatocyte monolayers: evidence for functional polarity of cells in culture. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4985–4987. doi: 10.1073/pnas.79.16.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L. E., Meyer U. A. Parenchymal cells from adult rat liver in nonproliferating monolayer culture. I. Functional studies. J Cell Biol. 1973 Dec;59(3):722–734. doi: 10.1083/jcb.59.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley S. E., Herz R. Permselectivity of biliary canalicular membrane in rats: clearance probe analysis. Am J Physiol. 1978 Nov;235(5):E570–E576. doi: 10.1152/ajpendo.1978.235.5.E570. [DOI] [PubMed] [Google Scholar]
- Elias E., Hruban Z., Wade J. B., Boyer J. L. Phalloidin-induced cholestasis: a microfilament-mediated change in junctional complex permeability. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2229–2233. doi: 10.1073/pnas.77.4.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgac M., Cantley L., Wiedenmann B., Altstiel L., Branton D. Clathrin-coated vesicles contain an ATP-dependent proton pump. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1300–1303. doi: 10.1073/pnas.80.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forker E. L. Bile formation in guinea pigs: analysis with inert solutes of graded molecular radius. Am J Physiol. 1968 Jul;215(1):56–62. doi: 10.1152/ajplegacy.1968.215.1.56. [DOI] [PubMed] [Google Scholar]
- Forker E. L. Two sites of bile formation as determined by mannitol and erythritol clearance in the guinea pig. J Clin Invest. 1967 Jul;46(7):1189–1195. doi: 10.1172/JCI105612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway C. J., Dean G. E., Marsh M., Rudnick G., Mellman I. Acidification of macrophage and fibroblast endocytic vesicles in vitro. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3334–3338. doi: 10.1073/pnas.80.11.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Granath K. A., Kvist B. E. Molecular weight distribution analysis by gel chromatography on Sephadex. J Chromatogr. 1967 May;28(1):69–81. doi: 10.1016/s0021-9673(01)85930-6. [DOI] [PubMed] [Google Scholar]
- Häcki W., Paumgartner G. Determination of the biliary dead space using 14C-taurocholate as a marker. Experientia. 1973 Sep 15;29(9):1091–1093. doi: 10.1007/BF01946737. [DOI] [PubMed] [Google Scholar]
- Kacich R. L., Renston R. H., Jones A. L. Effects of cytochalasin D and colchicine on the uptake, translocation, and biliary secretion of horseradish peroxidase and [14C]sodium taurocholate in the rat. Gastroenterology. 1983 Aug;85(2):385–394. [PubMed] [Google Scholar]
- LaRusso N. F., Fowler S. Coordinate secretion of acid hydrolases in rat bile. J Clin Invest. 1979 Oct;64(4):948–954. doi: 10.1172/JCI109561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullock B. M., Dobrota M., Hinton R. H. Sources of the proteins of rat bile. Biochim Biophys Acta. 1978 Nov 1;543(4):497–507. doi: 10.1016/0304-4165(78)90304-5. [DOI] [PubMed] [Google Scholar]
- Myers B. D., Chui F., Hilberman M., Michaels A. S. Transtubular leakage of glomerular filtrate in human acute renal failure. Am J Physiol. 1979 Oct;237(4):F319–F325. doi: 10.1152/ajprenal.1979.237.4.F319. [DOI] [PubMed] [Google Scholar]
- Mélèse T., Rothman S. S. Pancreatic epithelium is permeable to sucrose and inulin across secretory cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4870–4874. doi: 10.1073/pnas.80.15.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENKIN E. M. Filtration, diffusion, and molecular sieving through porous cellulose membranes. J Gen Physiol. 1954 Nov 20;38(2):225–243. [PMC free article] [PubMed] [Google Scholar]
- Reichen J., Le M. Taurocholate, but not taurodehydrocholate, increases biliary permeability to sucrose. Am J Physiol. 1983 Nov;245(5 Pt 1):G651–G655. doi: 10.1152/ajpgi.1983.245.5.G651. [DOI] [PubMed] [Google Scholar]
- Renston R. H., Jones A. L., Christiansen W. D., Hradek G. T., Underdown B. J. Evidence for a vesicular transport mechanism in hepatocytes for biliary secretion of immunoglobulin A. Science. 1980 Jun 13;208(4449):1276–1278. doi: 10.1126/science.7375938. [DOI] [PubMed] [Google Scholar]
- Scharschmidt B. F., Stephens J. E. Transport of sodium, chloride, and taurocholate by cultured rat hepatocytes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):986–990. doi: 10.1073/pnas.78.2.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharschmidt B. F., Van Dyke R. W. Mechanisms of hepatic electrolyte transport. Gastroenterology. 1983 Nov;85(5):1199–1214. [PubMed] [Google Scholar]
- Scharschmidt B. F., Van Dyke R. W., Stephens J. E. Chloride transport by intact rat liver and cultured rat hepatocytes. Am J Physiol. 1982 Jun;242(6):G628–G633. doi: 10.1152/ajpgi.1982.242.6.G628. [DOI] [PubMed] [Google Scholar]
- Schiff J. M., Fisher M. M., Underdown B. J. Receptor-mediated biliary transport of immunoglobulin A and asialoglycoprotein: sorting and missorting of ligands revealed by two radiolabeling methods. J Cell Biol. 1984 Jan;98(1):79–89. doi: 10.1083/jcb.98.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M., Palade G. E. Differentiated microdomains on the luminal surface of the capillary endothelium. I. Preferential distribution of anionic sites. J Cell Biol. 1981 Sep;90(3):605–613. doi: 10.1083/jcb.90.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N. D., Boyer J. L. Permeability characteristics of bile duct in the rat. Am J Physiol. 1982 Jan;242(1):G52–G57. doi: 10.1152/ajpgi.1982.242.1.G52. [DOI] [PubMed] [Google Scholar]
- Strasberg S. M., Petrunka C. N., Ilson R. G., Paloheimo J. E. Characteristics of inert solute clearance by the monkey liver. Gastroenterology. 1979 Feb;76(2):259–266. [PubMed] [Google Scholar]
- Tipping E., Ketterer B. The influence of soluble binding proteins on lipophile transport and metabolism in hepatocytes. Biochem J. 1981 May 1;195(2):441–452. doi: 10.1042/bj1950441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke R. W., Steer C. J., Scharschmidt B. F. Clathrin-coated vesicles from rat liver: enzymatic profile and characterization of ATP-dependent proton transport. Proc Natl Acad Sci U S A. 1984 May;81(10):3108–3112. doi: 10.1073/pnas.81.10.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke R. W., Stephens J. E., Scharschmidt B. F. Effects of ion substitution on bile acid-dependent and -independent bile formation by rat liver. J Clin Invest. 1982 Sep;70(3):505–517. doi: 10.1172/JCI110642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dyke R. W., Gollan J. L., Scharschmidt B. F. Oxygen consumption by rat liver: effects of taurocholate and sulfobromophthalein transport, glucagon, and cation substitution. Am J Physiol. 1983 May;244(5):G523–G531. doi: 10.1152/ajpgi.1983.244.5.G523. [DOI] [PubMed] [Google Scholar]
- van Rossum G. D., Russo M. A. Ouabain-resistant mechanism of volume control and the ultrastructural organization of liver slices recovering from swelling in vitro. J Membr Biol. 1981 Apr 30;59(3):191–209. doi: 10.1007/BF01875425. [DOI] [PubMed] [Google Scholar]