Abstract
Background
Iloprost, a stable prostacyclin I2 analogue, seems to have an osteoblast-protective potential, whereas indomethacin suppresses new bone formation. The aim of this study was to investigate human bone marrow stromal cell (BMSC) proliferation and differentiation towards the osteoblastic lineage by administration of indomethacin and/or iloprost.
Material/Methods
Human bone marrow cells were obtained from 3 different donors (A=26 yrs/m; B=25 yrs/f, C=35 yrs/m) via vacuum aspiration of the iliac crest followed by density gradient centrifugation and flow cytometry with defined antigens (CD105+/73+/45−/14−). The cells were seeded and incubated as follows: without additives (Group 0; donor A/B/C), with 10−7 M iloprost only (Group 0+ilo; A/B), with indomethacin only in concentrations of 10−6 M (Group 1, A), 10−5 M (Group 2, B), 10−4 M (Group 3, A/B), and together with 10−7 M iloprost (Groups 4–6, A/B/C). On Day 10 and 28, UV/Vis spectrometric and immunocytochemical assays (4 samples per group and donor) were performed to investigate cell proliferation (cell count measurement) and differentiation towards the osteoblastic lineage (CD34−, CD45−, CD105+, type 1 collagen (Col1), osteocalcin (OC), alkaline phosphatase (ALP), Runx2, Twist, specific ALP-activity).
Results
Indomethacin alone suppressed BMSC differentiation towards the osteoblastic lineage by downregulation of Runx2, Col1, and ALP. In combination with indomethacin, iloprost increased cell proliferation and differentiation and it completely suppressed Twist expression at Day 10 and 28. Iloprost alone did not promote cell proliferation, but moderately enhanced Runx2 and Twist expression. However, the proliferative effects and the specific ALP-activity varied donor-dependently.
Conclusions
Iloprost partially antagonized the suppressing effects of indomethacin on BMSC differentiation towards the osteoblast lineage. It enhanced the expression of Runx2 and, only in the presence of indomethacin, it completely suppressed Twist. Thus, in the treatment of avascular osteonecrosis or painful bone marrow edema, the undesirable effects of indomethacin might be counterbalanced by iloprost.
MeSH Keywords: Cell Differentiation, Core Binding Factor Alpha 1 Subunit, Indomethacin, Mesenchymal Stromal Cells, Prostaglandins I, Twist Transcription Factor
Background
‘Multipotent mesenchymal stromal cells’ is the currently recommended designation for the plastic-adherent cells isolated from bone marrow, cord blood, adipose tissue, peripheral blood, and connective tissues, and minimal criteria for defining these cells were proposed by the International Society for Cellular Therapy [1,2]. Although MSC belong to the mesenchymal lineage, other studies demonstrated the high plasticity of these cells. It is evident that MSC can also differentiate into endothelial cells [3], liver [4], neuronal [5], and myocardial tissue [6]. Moreover, in the field of regenerative orthopedics it was hypothesized that patients with critical-size bone defects or impaired bone healing might profit from local bone marrow-derived MSC therapy [7]. Mechanical strain on BMSC leads to enhanced intracellular calcium concentration and activates immediate-early genes (c-fos, c-jun, and c-myc) that may initiate proliferation as well as differentiation [8].
Differentiation of MSC towards the osteoblast lineage is promoted by dexamethasone and β-glycerophosphate, whereas indomethacin, dexamethasone, and 3-isobutyl-1-methylxanthine induce differentiation towards adipocytes [9]. Wnt signaling proteins, members of the transforming growth factor beta (TGF-β) superfamily, and hormones like 1α and 25-dihydroxyvitamin D3, promote the expression of defined transcription factors such as the Runt-related transcription factor 2 (Runx2), which is essential for osteoblast differentiation. In contrast, Twist-related protein 1 (Twist) inhibits the expression of Runx2 and prevents expression of osteoblast-specific markers [10]. Furthermore, there are intertwining pathways between inflammatory mediators and osteoblast promoting factors [11]. One example is the clinical application of cyclooxygenase (COX)-inhibitors to suppress bone formation and prevent heterotopic ossifications (HO).
Indomethacin, a common NSAID, is a typical representative of these agents and is frequently used in the treatment of osteoarthritis- and osteonecrosis-related pain, as well as for HO prophylaxis after musculoskeletal surgery. It is a non-selective inhibitor of COX-1 and COX-2, thus it suppresses the production of prostaglandin H2, a precursor of various prostaglandins, including prostaglandin I2 (PGI2=prostacyclin) and E2 (PGE2). The osteoblast suppressive effects of indomethacin were demonstrated both in vivo and in vitro [9,12].
Iloprost, a stable PGI2 analogue, is used clinically in the treatment of thromboangiitis obliterans, pulmonary hypertension, and scleroderma because of its antithrombotic, vasodilatative, and antiproliferative effects [13]. Moreover, some data indicate that it reduces pain in bone marrow edema (BME) and early avascular osteonecrosis (AVN) [14]. Based on these data, it is questionable if NSAID, which suppress prostacyclin expression and inhibit osteoblast differentiation, are reasonable to use in treating AVN-related pain. The purpose of this in vitro study was to investigate the effects of indomethacin, iloprost, and the combination of both agents on human, bone marrow-derived, multipotent mesenchymal stromal cells (BMSC) in terms of proliferation and differentiation towards the osteoblastic lineage.
Material and Methods
Cell culture
Human bone marrow was harvested from the iliac crest by Jamshidi vacuum aspiration in a standardized procedure, as described previously [7]. The bone marrow donors met the criteria of the German Medical Association, written informed consent was obtained, and the study was approved by the local ethics committee (#3096/2008). Cells were isolated from 3 different donors (donor A: 26 yrs, male, investigated groups: 0, 0+ilo, 1, 3, 4, 5, and 6; donor B: 25 yrs, female, investigated groups: 0, 0+ilo, 2, 3, 4, 5, and 6; and donor C: 38 yrs, male, investigated groups: 0, 4, 5, and 6. Four samples per group and assay were measured). Donor selection criteria included the absence from systemic autoimmune or inflammatory disorders, immunosuppressive medication, or malignant diseases. To purify the mesenchymal cell fraction, cells were expanded in T25 vessels at (37°C, 5% CO2) and trypsinized after a confluent cell layer was achieved. To control the mesenchymal character of the cells, a flow cytometric analysis with defined antigens (CD105+/73+/45−14−) was performed. In an additional step as described below, CD105, CD34, and CD45 were investigated immunocytochemically.
The cells were seeded in a concentration of 5000 cells per cm2 in Dulbecco’s Modified Eagle’s Medium (low glucose) supplemented with 20% fetal calf serum (FCS) in 5% CO2 at 37°C. Culture medium was changed twice a week. During cell cultivation, optical microscopy was performed before each change of the culture medium to check for contamination, cell adherence, and proliferation. The cells were stimulated with 10−6M, 10−5M, and 10−4M indomethacin (Group 1–3) and in an additional assay with 10−7 M iloprost only (Group 0+ilo). In Group 4–6, a combination of 10−7 M iloprost and 10−6 M, 10−5 M, and 10−4 M indomethacin was administered. Cell cultures with neither indomethacin nor iloprost supplementation served as controls (Group 0). Concentrations of indomethacin and iloprost were used according to recent investigators [15,16]. All experiments were carried out in quadruplicate for each concentration and investigated group. Kinetic points for immunocytochemical, enzymatic (LDH, ALP), and protein (bicinchoninic acid assay) analysis were performed on Days 10 and 28.
Cell proliferation measurement
Assays for cell proliferation can monitor the number of cells over time, the number of cellular divisions, metabolic activity, or DNA synthesis. In this study, an LDH-based cytotoxicity assay was used to determine the number of cells without and under the influence of different concentrations of indomethacin/iloprost at Days 10 and 28 (CytoTox® 96, Promega, Mannheim, Germany). LDH is an intracellular enzyme released by damaged cells. At Days 10 and 28, cells were washed twice with PBS before they were frozen and stocked at −80°C in 12-well plates. For cell disruption and release of LDH, the cells were thawed and 200 μl of PBS were added per well and incubated on the thermoshaker for 30 s at 300 rpm at room temperature (RT). Afterwards, the lysate was centrifuged for 4 min at 250 g at RT. Duplicate measurements were performed by transferring 5 μl of the supernatant to 45 μl PBS/well of a 96-well plate. The dilution of 1:10 had shown metrological accurate absorbance ranges (0.2–0.8) during pretests. Then, 50 μl of the substrate was added to each well, which resulted in the conversion of a tetrazolium salt into a red formazan product, presuming that the amount of color formed is proportional to the amount of released LDH and subsequently to the number of lysed cells. After 30 min, the assay was stopped by adding 50 μl H2SO4 and the absorption was measured using a micro-plate reader (Cary 50 Bio, Varian, Darmstadt, Germany) at 490 nm. Cell number was calculated using a standard curve based on LDH amount for defined bone marrow cells.
Cell differentiation
a) UV/Vis spectrometry
Alkaline phosphatase (ALP, Ostase) is an enzyme that removes phosphate groups from proteins, nucleotides, and alkaloids. It is present in the entire body but is particularly concentrated in liver, bile duct, kidney, bone, and placenta. In humans, 3 major isoenzymes exist: intestinal, tissue non-specific, and placental. A high level of ALP can be found in increased bone turnover and in vitro it is associated with early osteoblast differentiation [17]. To quantify ALP content in the cultures, an enzyme assay-based on dephosphorylating p-nitrophenol phosphate to p-nitrophenol was used (Enzymatic Assay of Alkaline Phosphatase, Sigma-Aldrich Chemie GmbH, Steinheim, Germany). At Days 10 and 28, cells were lysed by adding 200 μl of aqua dest to each of 4 wells and incubated on the shaker for 30 s at 300 RPM at RT followed by centrifugation at 250 g at RT for 4 min. In duplicate measurements, 5 μl of supernatant were diluted in 45 μl aqua dest/well of a 96-well plate to reach suitable absorbance. We added 50 μl of the substrate per well and the absorption was measured every 10 min up to 1 h at a wavelength of 405 nm using the micro-plate reader to quantify the amount of the chemical product p-nitrophenol over time. The ALP activity was calculated with a standard curve calibrated by an enzyme solution with defined ALP activity per volume (Sigma P6774, 50 Units/μl). The specific enzymatic activity was calculated by setting the ALP activity in relation to the total amount of protein in the supernatant (μUnits ×60/μg protein). The total amount of protein was measured with a bicinchoninic acid assay (BCA, Pierce, Thermo Fisher Scientific Inc. Rockford, IL, USA). We added 200 μl of PBS to each of the 4 wells. After shaking for 30 s at 300 RPM, plates were centrifuged for 4 min at 250 g at RT. At Day 10, 15 μl of lysate was diluted in 135 μl of PBS/well in duplicates, and at Day 28, 30 μl were transferred to 120 μl PBS to match the ideal absorbance range. In duplicate measurements, the assay was incubated for 120 min before the optical density was measured at 562 nm in the microplate reader.
b) Immunocytochemical assays
Immunocytochemical assays were performed to detect specific osteoblast differentiation markers and to proof the presence or absence typical cell markers for multipotent bone marrow stromal cells (CD105+, CD34−, CD45−).
The triple helical-structured type 1 collagen is the major organic component in bone and it is expressed by mature osteoblasts. Its detection indicates osteoblastic activity in vitro and there is evidence that type 1 collagen itself induces the differentiation of bone marrow cells towards the osteoblastic lineage [18]. Bone gamma-carboxyglutamic acid-containing protein, also known as osteocalcin, is the major non-collagen protein in bone and accounts for 2% of bone mass. It consists of 49 amino acids, is secreted solely by osteoblasts, inhibits bone mineralization, and binds to hydroxyl apatite and calcium. DNA-binding Runt-related transcription factor 2 (Runx2) is the key transcription factor of osteoblast differentiation and it is essential for embryonic and postnatal skeletogenesis [19]. Twist is a basic helix-loop-helix transcription factor and it is involved in the development of mesodermally-derived tissue, including bone. In bone marrow-derived mesenchymal stromal cells and in contrast to Runx2, high expression of Twist displays a decreased capacity for osteogenic differentiation and an enhanced capacity to undergo adipogenesis [20].
At Days 10 and 28, cell plates were washed twice with PBS and incubated for 5 min in 0.3% H2O2/PBS to eliminate endogenous peroxidase activity. After washing with PBS, the cells were incubated with primary antibodies in dilutions from 1:20 for CD34 and CD105 to 1:500 for Runx2 at 4°C for 12 h. Subsequently, biotinylated secondary antibodies with specificity against the primary antibody were added for 60 min in a dilution of 1:200 with PBS + triton x-100 (PBST). Afterwards, the cells were washed and incubated with avidin-biotin complex (ABC, Biozol, Eching, Germany) for 40 min. This substrate included the biotinylated enzyme, horseradish-peroxidase (HRP). As negative control, secondary antibodies were added without precedent incubation with primary antibodies. For color reaction, diaminobenzidine (DAB) substrate was added for 2 min and was converted by HRP to a brownish end-product. Finally, stop solution was added for 2 min, and the cells were washed again and conserved in glycerol gelatin. The following primary antibodies were used: CD34 (pre-dilution: 1:20, DAKO, Hamburg, Germany), CD45 (1:100), CD105 (1:20), Col1 (1:100, Cosmo Bio, Tokyo, Japan), OC (1:200, Santa Cruz, Heidelberg, Germany), the transcription factors Runx2 (1:500, R&D, Wiesbaden, Germany), and Twist (1:200, Santa Cruz, Heidelberg, Germany). For ALP detection, cells were incubated after washing twice with PBS with vector blue (Biozol, Eching, Germany) for 45 min at RT. Afterwards, they were washed twice again and conserved in glycerol gelatin. A microscopy-based semiquantitative scoring system was used:
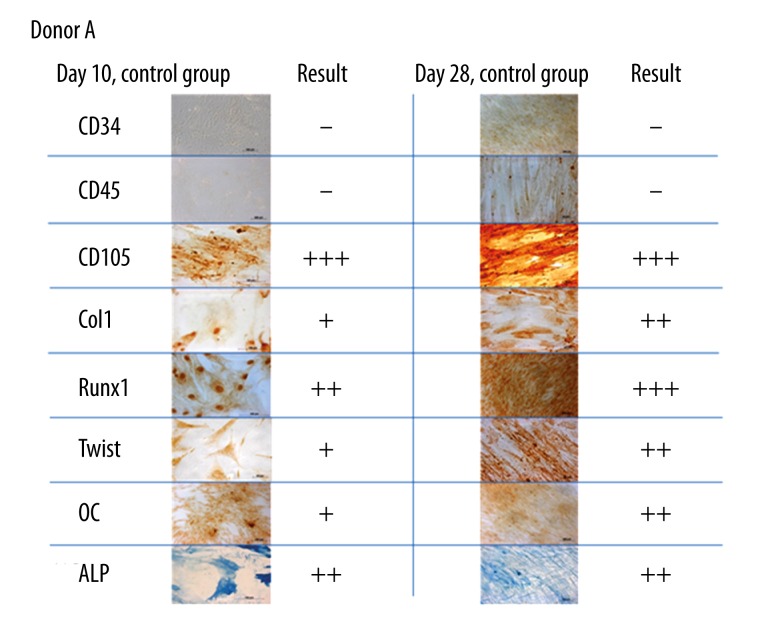
Assisted by optical microscopy, the proportion of stained cells was calculated for each marker and distributed into 4 groups from absent (−) to strong (+++) (Figure 1, − = absence of stained cells, + = up to 50% stained cells, ++ = 50–75%, +++ = >75%).
Figure 1.
Immunocytochemical staining of CD34, CD45, CD105, Col1, Runx2, Twist, OC and ALP in BMSC of donor A without administration of iloprost or indomethacin at Days 10 and 28. The hematopoietic markers CD34 and CD45 could not be detected in almost any assay (−), whereas CD105 was always present (+++). Graduation according to the proportion of stained cells as described in the running text. Optical magnification: 400×.
Statistical analysis
Statistical analysis was supported by SPSS (Version 18.0, SPSS Inc., Chicago, USA) and included descriptive parameters (X, SD) and the paired 2-sample t test. P values below 0.05 were considered as statistically significant and p values below 0.01 as highly significant.
Results
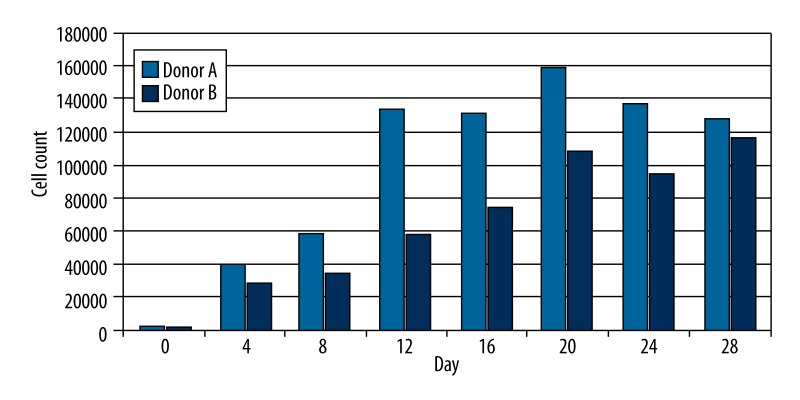
Within donors, different cell concentrations during cultivation were apparent even between nearly coeval donors and after the same number of cell passages. Donors A and B served as control (0) to proof cell proliferation without supplementation of indomethacin or iloprost every 4 days up to Day 28 (Figure 2). BMSC proliferation without supplementation showed an average logistic growth rate of 1.48 for donor A and 1.41 for donor B from Day 4 up to Day 20. Afterwards, cell proliferation decelerated significantly or even arrested, with lower cell counts. A flow cytometric analysis of the culture (Donor A) was done previously to verify the mesenchymal character. However, the cell culture from bone marrow aspiration concentrate did not meet the criteria of the International Society of Cellular Therapy (ISCT) for defining multipotent mesenchymal stromal cells: 98.7% of our cells expressed CD105, 99.7% expressed CD73, and 73.5% did not show expression of the hematopoietic markers CD45 and CD14.
Figure 2.
LDH-Assay and calculated cell count of Donors A and B without supplementation of iloprost or indomethacin at different points in time.
Influence of indomethacin and iloprost on BMSC proliferation
Using the CytoTox assay, cell count was defined at Day 10 and Day 28. For each trial, the mean cell count of 4 wells was calculated. Mean cell count values for different donors are listed in Table 1. Our results did not clearly show that increasing concentrations of indomethacin alone lead to suppression of proliferation at Days 10 and 28 compared to the control group. Analyzing the results of Donor A, one might speculate that at Day 28 indomethacin suppresses proliferation dose-dependently. However, compared to the control group of Donor A, even higher cell counts could be measured in the presence of indomethacin.
Table 1.
Mean cell count (in thousands) for Donors A, B, and C with/without administration of indomethacin and/or iloprost at Days 10 and 28. Comparisons of intraindividual values (Donors A and B) were performed and statistically analyzed: * for p<0.05 and ** for p<0.01. Displayed significances refer to the control group ‘0’ in case of a value on regular font or to group ‘0 + ilo’ on a bold font.
| Group | 0 control | 1 10−6 M indo |
2 10−5 M indo |
3 10−4 M indo |
0+ilo 10−7 M ilo |
4 10−6 M+ilo |
5 10−5 M+ilo |
6 10−4 M+ilo |
|
|---|---|---|---|---|---|---|---|---|---|
| Donor A | Day 10 | 63.2 | 41.1** | – | 58.6 | 60.5 | 48.6** | 41.0 | 25.6* |
| Day 28 | 175.8 | 284.8** | – | 242.6* | 170.7 | 153.2 | 152.0 | 162.9 | |
| Donor B | Day 10 | 47.3 | – | 52.3 | 38.9 | 79.4 | 157.1* | 134.0** | 103.4 |
| Day 28 | 212.1 | – | 202.9 | 196.6 | 160.1 | 396.4** | 384.1** | 310.2* | |
| Donor C | Day 10 | 5.0 | – | – | – | – | 6.4 | 6.3 | 8.6 |
| Day 28 | 29.3 | – | – | – | – | 38.5 | 35.9 | 18.1 |
Proliferative effects of iloprost alone compared to the control group (0) could not be observed at Days 10 and 28 for Donors A and B. In combination with indomethacin, iloprost might have a proliferative effect on BMSC at both points in time (Donor B). Indomethacin in combination with iloprost reduced cell count at Day 10 dose-dependently in Donors A and B and at Day 28 in Donors B and C. However, between different donors, our data are partially inconsistent.
The BCA assay, which we used to determine the specific ALP activity, revealed that the expression of proteins increased almost linearly up to Day 28 without any supplement in Donors A and B (by measurement every 4th day). Indomethacin did not alter this tendency at Days 10 and 28. Additional administration of iloprost increased total protein expression significantly with or without the influence of indomethacin.
Effects of indomethacin and iloprost on BMSC differentiation
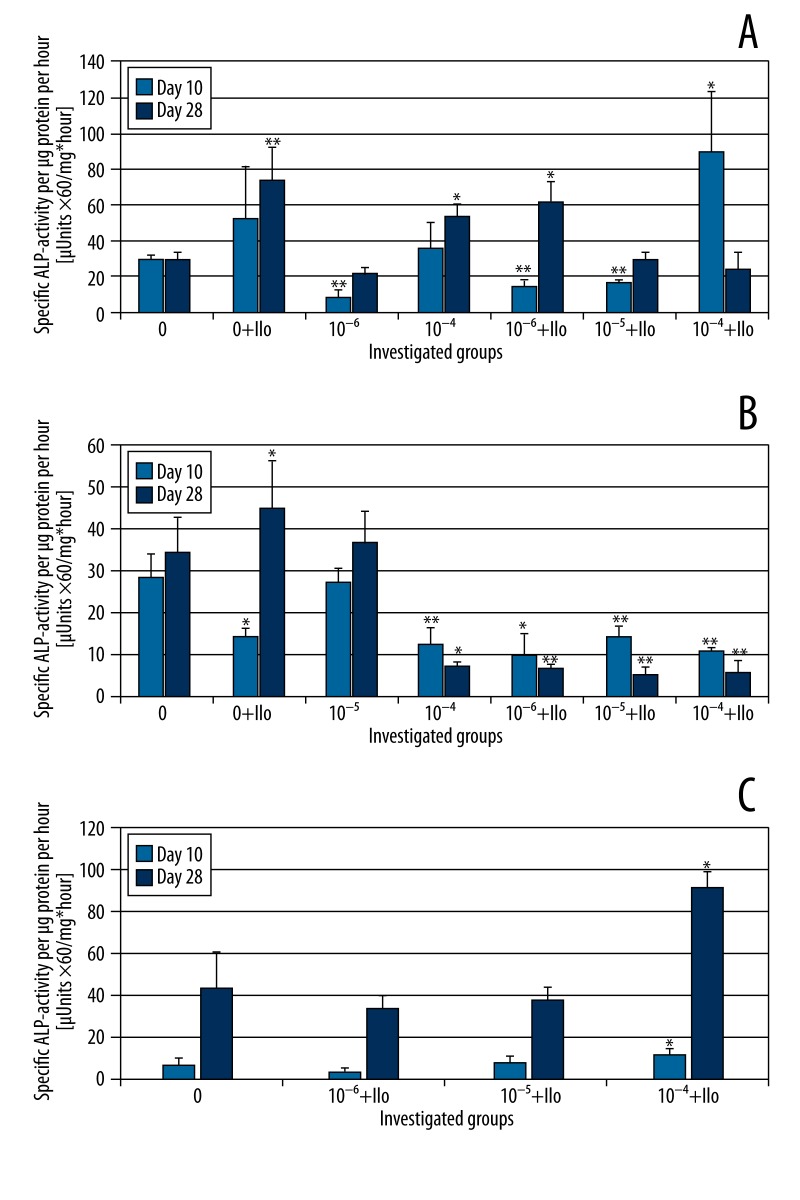
Immunocytochemical staining revealed that at Days 10 and 28, Runx2 expression is inhibited by indomethacin dose-dependently with or without iloprost (Table 2, Donors A and B). The expression of Col1 and ALP was also inhibited by indomethacin in comparison to the control group at Days 10 and 28, but our results did not show a strict dose-dependency for Runx2. OC expression was not lessened significantly by indomethacin alone. Iloprost alone led to enhanced Runx2 expression and it counterbalanced the suppressive effect of indomethacin on Runx2, which was more distinct at Day 28 than at Day 10. A similar counter-effect was found for ALP at Day 10 and Col1 at Day 28, as additive iloprost nearly normalized the expression of these markers. Remarkably, Twist could not be detected under administration of both agents at Days 10 and 28. However, BMSC showed enhanced expression of Twist under the influence of iloprost alone compared to the control group, and Twist was also detected when indomethacin alone was administered. Moreover, Twist expression was suppressed dose-dependently by indomethacin alone at Day 28 but not at Day 10. The BCA assays showed that indomethacin did not influence the total protein concentration at Days 10 and 28, whereas additional administration of iloprost led to enhanced protein expression. The results of the ALP and BCA assay were used to calculate the specific ALP activity (Figure 3A–3C). In Donors A and B, iloprost enhanced specific ALP activity at Day 28 compared to the control group. Our data did not show an obvious relationship of indomethacin and a reduction of specific ALP activity. However, a significant reduction was found for Donor A and 10−6 M indomethacin (Day 10) as well as Donor B and 10−4 M indomethacin (Days 10 and 28). Additional administration of iloprost showed donor-dependent tendencies: in Donor A a slight enhancement was detected, whereas in Donor B a decrease of specific ALP-activity was found compared to the corresponding concentration of indomethacin alone. Moreover, after testing every 4th day, the ALP assay revealed a cyclical ALP activity curve in non-incubated cells of Donors A and B. We therefore speculate that our inconsistent results of the specific ALP activity could be a result of different phases of the cell cycle.
Table 2.
Graduation of immunohistochemical staining according to the mean proportion of stained cells for Donors A and B (− = absent, + = weak, ++ = moderate, +++ = strong) and for different osteoblast differentiation markers with/without administration of indomethacin and/or iloprost (concentrations of 10−6 M for Donor B solely and 10−5 M for Donor A, respectively). No substantial variations in the expression of these markers between Donors A and B could be found.
| 0 | 10−6 M | 10−5 M | 10−4 M | 0+Ilo | 10−6 M+Ilo | 10−5 M+Ilo | 10−4 M+Ilo | |
|---|---|---|---|---|---|---|---|---|
| Day 10 | ||||||||
| Col1 | + | + | − | ++ | + | + | + | + |
| Runx2 | ++ | ++ | ++ | + | +++ | ++ | ++ | ++ |
| Twist | + | + | ++ | + | ++ | − | − | − |
| OC | + | ++ | ++ | + | + | + | + | + |
| ALP | ++ | + | + | + | ++ | + | ++ | ++ |
| Day 28 | ||||||||
| Col1 | ++ | + | + | + | ++ | ++ | ++ | + |
| Runx2 | +++ | ++ | ++ | + | +++ | +++ | ++ | ++ |
| Twist | ++ | + | + | − | +++ | − | − | − |
| OC | ++ | ++ | +++ | + | ++ | ++ | ++ | ++ |
| ALP | +++ | ++ | +++ | ++ | ++ | ++ | +++ | ++ |
Figure 3.
Specific ALP activity of Donor A (A), B (B) and C (C) at Days 10 and 28 with and without additional administration of indomethacin and/or iloprost. Standard deviations and statistical significances are displayed: * for p<0.05 and ** for p<0.01 [paired two-sample t test for Days 10 and 28, respectively, compared to the control group (0)]. Concentrations of 10−5 M (A) and 10−6 M (B) indomethacin alone were not investigated.
Discussion
The average number of population doublings for marrow-derived adult human MSCs was determined to be 38±4 [21]. BMSC proliferation without supplementation of the 2 agents (Figure 2) showed an average logistic growth rate of 1.48 for Donor A and 1.41 for Donor B from Day 4 up to Day 20. Thereafter, proliferation decelerated significantly. Banfi et al. also found that BMSC slowed their proliferation rate considerably after Day 20 [22]. One explanation is that the cell population was beginning to exhaust its environment until a steady cell count is reached, according to the logistic growth model of Lotka-Volterra. In this scenario, the concentration of mitogenic factors may become rate-limiting as the cells metabolize the culture medium [21]. However, Bruder et al. also found that with increasing passages the fraction of daughter cells with rapidly dividing spindle shape decreases in favor of cells with broad morphology and reduced replication rate [21].
Indomethacin is clinically used to prevent HO after musculoskeletal surgery. It is known to inhibit bone formation when given before mechanical stimulation [23]. As a non-selective inhibitor of COX-1 and COX-2 it suppresses the production of prostaglandin H2, a precursor of different prostaglandins, including PGI2 and PGE2. It is evident that PGE2 promotes osteogenic differentiation in BMSC mainly via activation of the EP2 and EP4 receptor [24] followed by cAMP-dependent PKA-/MAPK signalling, which ultimately leads to enhanced expression of osteoblast-associated transcription factors such as Runx2, Osterix, and Dlx5 [11]. In human osteoblasts, the following functional prostanoid receptors have been identified: EP4, IP, FP, and TP [25]. Prostacyclin is known to be the most effective prostanoid in inhibition of bone resorption and osteoclast activity [26–28]. It has the capacity to induce COX activity in MC3T3-E1 osteoblasts and therefore seems to act in the reverse fashion of indomethacin [29]. Alternatively, it was shown that adult prostacyclin-deficient mice showed increased bone mass compared to controls [30]. The production of prostacyclin is induced by mechanical stress in osteocytes and osteoblasts [31]. Our results show that suppression of PGE2 production by indomethacin inhibits proliferation [16] and osteogenic differentiation of BMSC. Our results are in concordance with other investigations that have shown that NSAID diminish Runx2 expression [32,33]. Application of iloprost leads to enhanced activation of the IP receptor in BMSC, whereas indomethacin suppresses the stimulation of other prostaglandin receptors such as EP4. Therefore, isolated effects of IP receptor signalling pathways in BMSC were investigated in this study with coeval elimination of other prostanoid receptor effects. Interestingly, in this scenario we found that prostacyclin not only increased Runx2 but also completely suppressed Twist expression in the hypothesized absence of other prostanoid effects. It is even more remarkable that prostacyclin without indomethacin, and presumably together with other prostanoids, further increased Twist expression compared to the control group. There is evidence that overexpression of Twist decreased gene expression of osteoblast-associated markers, bone morphogenic protein-2, bone sialoprotein, osteopontin, alkaline phosphatase, and osteocalcin in human mesenchymal stem cells [20]. Overexpression of Twist is associated with decreased capacity for osteo-/chondrogenic differentiation and an enhanced capacity to undergo adipogenesis. Moreover, it enhances the life span human mesenchymal stromal cells and it maintains an immature stromal phenotype [20]. We speculate that intertwining pathways of intracellular signalling cascades are responsible for enhanced expression of Twist under administration of iloprost alone, presumably due to high stimulation of IP receptors together with moderate or low stimulation of other prostanoid receptors.
In murine mesenchymal stem cells, silencing of Twist led to increased osteogenic differentiation [34]. There is evidence that during early stages of fetal enchondral and intramembranous bone development, Twist is downregulated [35]. Bialek et al. reported in a murine calvarial study that Twist does not affect the expression of Runx2, but it inhibits its DNA-binding function via a novel antiosteogenic domain called Twist box [10]. Analyzing our results, iloprost and indomethacin together may increase osteogenic differentiation via downregulation of Twist and upregulation of Runx2. However, in the absence of Twist, an increased marker expression for differentiated osteoblasts like Col1, OC, and ALP was not observed. Prostacyclin mainly exerts its effects cAMP-dependently via protein kinase A signalling [28,36]. High cAMP levels are linked to anabolic bone remodelling [37] and they can be found after stimulation of several prostanoid receptors, including IP, EP2, and EP4 [38]. Moreover, in mechanically-stimulated MC3T3-E1 osteoblasts, cAMP is linked to enhanced expression of the proto-oncogene c-fos, an element of the activator protein 1 (AP-1) that modulates the expression of Col1, OC, and ALP and thus might promote osteoblastic proliferation and differentiation [39]. However, there is evidence that cAMP/PKA and other signalling cascades are mediated by IP receptor activation, including Ca2+-increase and phosphatidylinositol breakdown in different cell lines [40,41]. We speculate that signalling pathways other than cAMP/PKA are responsible for the suppression of Twist by prostacyclin in the coeval absence of other prostanoid effects.
In this study, we found that ALP expression decreased with increasing concentrations of indomethacin at Days 10 and 28. This effect was more distinctive at Day 10 than at Day 28. In MC3T3-E1 osteoblasts, Igarashi et al. found that indomethacin boosts ALP activity up to Day 15 and that this activity was maintained until Day 27, whereas the control group showed a decrease of ALP activity after Day 15 [42]. Other authors did not find clear effects of indomethacin on ALP activity in the same cell line [43], whereas in human BMSC, Chang et al. found a decrease of ALP activity after 1 week of incubation with different NSAIDs. Presumably due to different cell cycle phases and an inhomogeneous donor population, the specific ALP activity in BMSC after incubation with/without indomethacin or iloprost showed inconsistent results in this study compared to the results of ALP immunohistochemistry. Thus, the various groups in this study should only be compared intraindividually.
It is evident that the capacity of proliferation and differentiation varies with age [44,45] and that indomethacin inhibits proliferation and arrests cell cycle [16]. It was demonstrated that prostaglandin E1, E2, and F2alpha were not able to rescue the suppressive effects of NSAIDs on BMSC proliferation [16]. Our study did not show clear effects of either agent on the expression of OC, but we observed a moderate suppression of Col1 at Day 28 by administration of indomethacin. Chang et al. investigated the influence of different NSAIDs on BMSC and found no changes in OC, Col1, or Runx2 expression in the first 8 days [16]. Compared to our study, in this early interval we found that the expression of Runx2 was only slightly diminished, and like Chang et al., we did not find significant changes in OC and Col1 expression at Day 10. Other authors have published similar results in human MG-63 osteosarcoma cell lines and in MC3T3-E1 osteoblasts [43,46].
Taken together, the literature suggests that bone healing is compromised by high doses of NSAIDs in orthopaedic patients because these agents inhibit the osteogenic potential of BMSC.
Even in the presence of iloprost, indomethacin was able to inhibit proliferation with increasing doses.
In the literature to date, there are no studies describing the effects of iloprost on Twist expression in osteoblasts. Hence, further research is needed on intracellular signalling cascades mediating the suppression of Twist by iloprost and indomethacin.
Limitations of our study include inconsistent results in the investigation of BMSC proliferation and, in part, for differentiation (notably specific ALP activity compared to the results of ALP immunocytochemistry), which are likely attributable to different age groups, sex, different phases of cell cycle, and to an inhomogeneous donor population. Due to a low cell number, the analysis of differentiation was only performed at 2 points in time: Day 10 for early differentiation in the phase of matrix deposition and Day 28 for late differentiation progress. However, analysis of BMSC differentiation at Day 28 is a commonly used point in time, according to other investigators [47,48]. Over-interpretation of statistical significances should be avoided due to a small sample size, which results in low statistical power. Furthermore, the cells of the bone marrow aspiration concentrate that were used for our study did not meet the minimum criteria for defining pure multipotent mesenchymal stromal cells according to the ISCT.
Conclusions
Iloprost seems to have osteoblast protective potential. In this study, it partially antagonized the suppressing effects of indomethacin on BMSC differentiation towards the osteoblast lineage. Iloprost enhanced the expression of Runx2, and it completely suppressed Twist only in the presence of indomethacin. Thus, in the treatment of avascular osteonecrosis or painful bone marrow edema, the undesirable effects of indomethacin might be counterbalanced by iloprost.
Footnotes
Source of support: Departmental sources
References
- 1.Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99(13):8932–37. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–17. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 3.Portalska KJ, Groen N, Krenning G, et al. The effect of donor variation and senescence on endothelial differentiation of human mesenchymal stromal cells. Tissue Eng Part A. 2013;19(21–22):2318–29. doi: 10.1089/ten.TEA.2012.0646. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Zhao T, Xu F, et al. How important is differentiation in the therapeutic effect of mesenchymal stromal cells in liver disease? Cytotherapy. 2014;16(3):309–18. doi: 10.1016/j.jcyt.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61(4):364–70. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 6.Yang S, Piao J, Jin L, Zhou Y. Does pretreatment of bone marrow mesenchymal stem cells with 5-azacytidine or double intravenous infusion improve their therapeutic potential for dilated cardiomyopathy? Med Sci Monit Basic Res. 2013;19:20–31. doi: 10.12659/MSMBR.883737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jager M, Herten M, Fochtmann U, et al. Bridging the gap: bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. J Orthop Res. 2011;29(2):173–80. doi: 10.1002/jor.21230. [DOI] [PubMed] [Google Scholar]
- 8.Li R, Wei M, Shao J. Effects of verapamil on the immediate-early gene expression of bone marrow mesenchymal stem cells stimulated by mechanical strain in vitro. Med Sci Monit Basic Res. 2013;19:68–75. doi: 10.12659/MSMBR.883790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruedigam C, Driel M, Koedam M, et al. Basic techniques in human mesenchymal stem cell cultures: differentiation into osteogenic and adipogenic lineages, genetic perturbations, and phenotypic analyses. Curr Protoc Stem Cell Biol. 2011;Chapter 1(Unit1H.3) doi: 10.1002/9780470151808.sc01h03s17. [DOI] [PubMed] [Google Scholar]
- 10.Bialek P, Kern B, Yang X, et al. A twist code determines the onset of osteoblast differentiation. Dev Cell. 2004;6(3):423–35. doi: 10.1016/s1534-5807(04)00058-9. [DOI] [PubMed] [Google Scholar]
- 11.Haversath M, Catelas I, Li X, et al. PGE(2) and BMP-2 in bone and cartilage metabolism: 2 intertwining pathways. Can J Physiol Pharmacol. 2012;90(11):1434–45. doi: 10.1139/y2012-123. [DOI] [PubMed] [Google Scholar]
- 12.Persson PE, Nilsson OS, Berggren AM. Do non-steroidal anti-inflammatory drugs cause endoprosthetic loosening? A 10-year follow-up of a randomized trial on ibuprofen for prevention of heterotopic ossification after hip arthroplasty. Acta Orthop. 2005;76(6):735–40. doi: 10.1080/17453670510045309. [DOI] [PubMed] [Google Scholar]
- 13.Schermuly RT, Kreisselmeier KP, Ghofrani HA, et al. Antiremodeling effects of iloprost and the dual-selective phosphodiesterase 3/4 inhibitor tolafentrine in chronic experimental pulmonary hypertension. Circ Res. 2004;94(8):1101–8. doi: 10.1161/01.RES.0000126050.41296.8E. [DOI] [PubMed] [Google Scholar]
- 14.Jager M, Zilkens C, Bittersohl B, et al. Efficiency of iloprost treatment for osseous malperfusion. Int Orthop. 2011;35(5):761–65. doi: 10.1007/s00264-010-0998-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fetalvero KM, Shyu M, Nomikos AP, et al. The prostacyclin receptor induces human vascular smooth muscle cell differentiation via the protein kinase A pathway. Am J Physiol Heart Circ Physiol. 2006;290(4):H1337–46. doi: 10.1152/ajpheart.00936.2005. [DOI] [PubMed] [Google Scholar]
- 16.Chang JK, Li CJ, Wu SC, et al. Effects of anti-inflammatory drugs on proliferation, cytotoxicity and osteogenesis in bone marrow mesenchymal stem cells. Biochem Pharmacol. 2007;74(9):1371–82. doi: 10.1016/j.bcp.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 17.Hoemann CD, El-Gabalawy H, McKee MD. In vitro osteogenesis assays: influence of the primary cell source on alkaline phosphatase activity and mineralization. Pathol Biol (Paris) 2009;57(4):318–23. doi: 10.1016/j.patbio.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Mizuno M, Fujisawa R, Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J Cell Physiol. 2000;184(2):207–13. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 19.Takarada T, Hinoi E, Nakazato R, et al. An analysis of skeletal development in osteoblast-specific and chondrocyte-specific runt-related transcription factor-2 (Runx2) knockout mice. J Bone Miner Res. 2013;28(10):2064–69. doi: 10.1002/jbmr.1945. [DOI] [PubMed] [Google Scholar]
- 20.Isenmann S, Arthur A, Zannettino AC, et al. TWIST family of basic helix-loop-helix transcription factors mediate human mesenchymal stem cell growth and commitment. Stem Cells. 2009;27(10):2457–68. doi: 10.1002/stem.181. [DOI] [PubMed] [Google Scholar]
- 21.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64(2):278–94. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 22.Banfi A, Muraglia A, Dozin B, et al. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol. 2000;28(6):707–15. doi: 10.1016/s0301-472x(00)00160-0. [DOI] [PubMed] [Google Scholar]
- 23.Chow JW, Chambers TJ. Indomethacin has distinct early and late actions on bone formation induced by mechanical stimulation. Am J Physiol. 1994;267( 2 Pt 1):E287–92. doi: 10.1152/ajpendo.1994.267.2.E287. [DOI] [PubMed] [Google Scholar]
- 24.Blackwell KA, Raisz LG, Pilbeam CC. Prostaglandins in bone: bad cop, good cop? Trends Endocrinol Metab. 2010;21(5):294–301. doi: 10.1016/j.tem.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarrazin P, Bkaily G, Hache R, et al. Characterization of the prostaglandin receptors in human osteoblasts in culture. Prostaglandins Leukot Essent Fatty Acids. 2001;64(3):203–10. doi: 10.1054/plef.1999.0127. [DOI] [PubMed] [Google Scholar]
- 26.Chambers TJ, Fuller K, Athanasou NA. The effect of prostaglandins I2, E1, E2 and dibutyryl cyclic AMP on the cytoplasmic spreading of rat osteoclasts. Br J Exp Pathol. 1984;65(5):557–66. [PMC free article] [PubMed] [Google Scholar]
- 27.Zaidi M. Modularity of osteoclast behaviour and “mode-specific” inhibition of osteoclast function. Biosci Rep. 1990;10(6):547–56. doi: 10.1007/BF01116615. [DOI] [PubMed] [Google Scholar]
- 28.Fortier I, Patry C, Lora M, et al. Immunohistochemical localization of the prostacyclin receptor (IP) human bone. Prostaglandins Leukot Essent Fatty Acids. 2001;65(2):79–83. doi: 10.1054/plef.2001.0292. [DOI] [PubMed] [Google Scholar]
- 29.Sakuma Y, Li Z, Pilbeam CC, et al. Stimulation of cAMP production and cyclooxygenase-2 by prostaglandin E(2) and selective prostaglandin receptor agonists in murine osteoblastic cells. Bone. 2004;34(5):827–34. doi: 10.1016/j.bone.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Nakalekha C, Yokoyama C, Miura H, et al. Increased bone mass in adult prostacyclin-deficient mice. J Endocrinol. 2010;204(2):125–33. doi: 10.1677/JOE-09-0376. [DOI] [PubMed] [Google Scholar]
- 31.Lanyon LE. Control of bone architecture by functional load bearing. J Bone Miner Res. 1992;7(Suppl 2):S369–75. doi: 10.1002/jbmr.5650071403. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Yamamoto K, Sugimoto Y, et al. Induction of prostaglandin I(2) receptor by tumor necrosis factor alpha in osteoblastic MC3T3-E1 cells. Biochim Biophys Acta. 1999;1441(1):69–76. doi: 10.1016/s1388-1981(99)00139-0. [DOI] [PubMed] [Google Scholar]
- 33.Yoon DS, Yoo JH, Kim YH, et al. The effects of COX-2 inhibitor during osteogenic differentiation of bone marrow-derived human mesenchymal stem cells. Stem Cells Dev. 2010;19(10):1523–33. doi: 10.1089/scd.2009.0393. [DOI] [PubMed] [Google Scholar]
- 34.Miraoui H, Severe N, Vaudin P, et al. Molecular silencing of Twist1 enhances osteogenic differentiation of murine mesenchymal stem cells: implication of FGFR2 signaling. J Cell Biochem. 2010;110(5):1147–54. doi: 10.1002/jcb.22628. [DOI] [PubMed] [Google Scholar]
- 35.Alborzi A, Mac K, Glackin CA, et al. Endochondral and intramembranous fetal bone development: osteoblastic cell proliferation, and expression of alkaline phosphatase, m-twist, and histone H4. J Craniofac Genet Dev Biol. 1996;16(2):94–106. [PubMed] [Google Scholar]
- 36.Robin JC, Brown MJ, Weinfeld N, Dziak R. Prostacyclin: effects on cyclic AMP in bone cells. Res Commun Chem Pathol Pharmacol. 1982;35(1):43–49. [PubMed] [Google Scholar]
- 37.Hakeda Y, Yoshino T, Natakani Y, et al. Prostaglandin E2 stimulates DNA synthesis by a cyclic AMP-independent pathway in osteoblastic clone MC3T3-E1 cells. J Cell Physiol. 1986;128(2):155–61. doi: 10.1002/jcp.1041280204. [DOI] [PubMed] [Google Scholar]
- 38.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79(4):1193–26. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 39.Fitzgerald J, Hughes-Fulford M. Mechanically induced c-fos expression is mediated by cAMP in MC3T3-E1 osteoblasts. FASEB J. 1999;13(3):553–57. doi: 10.1096/fasebj.13.3.553. [DOI] [PubMed] [Google Scholar]
- 40.Namba T, Oida H, Sugimoto Y, et al. cDNA cloning of a mouse prostacyclin receptor. Multiple signaling pathways and expression in thymic medulla. J Biol Chem. 1994;269(13):9986–92. [PubMed] [Google Scholar]
- 41.Watanabe T, Yatomi Y, Sunaga S, et al. Characterization of prostaglandin and thromboxane receptors expressed on a megakaryoblastic leukemia cell line, MEG-01s. Blood. 1991;78(9):2328–36. [PubMed] [Google Scholar]
- 42.Igarashi K, Hirafuji M, Adachi H, et al. Role of endogenous PGE2 in osteoblastic functions of a clonal osteoblast-like cell, MC3T3-E1. Prostaglandins Leukot Essent Fatty Acids. 1994;50(4):169–72. doi: 10.1016/0952-3278(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 43.Arpornmaeklong P, Akarawatcharangura B, Pripatnanont P. Factors influencing effects of specific COX-2 inhibitor NSAIDs on growth and differentiation of mouse osteoblasts on titanium surfaces. Int J Oral Maxillofac Implants. 2008;23(6):1071–81. [PubMed] [Google Scholar]
- 44.Hermann A, List C, Habisch HJ, et al. Age-dependent neuroectodermal differentiation capacity of human mesenchymal stromal cells: limitations for autologous cell replacement strategies. Cytotherapy. 2010;12(1):17–30. doi: 10.3109/14653240903313941. [DOI] [PubMed] [Google Scholar]
- 45.Khan M, Mohsin S, Khan SN, Riazuddin S. Repair of senescent myocardium by mesenchymal stem cells is dependent on the age of donor mice. J Cell Mol Med. 2011;15(7):1515–27. doi: 10.1111/j.1582-4934.2009.00998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diaz-Rodriguez L, Garcia-Martinez O, Morales MA, et al. Effects of indomethacin, nimesulide, and diclofenac on human MG-63 osteosarcoma cell line. Biol Res Nurs. 2012;14(1):98–107. doi: 10.1177/1099800411398933. [DOI] [PubMed] [Google Scholar]
- 47.Hakelien AM, Bryne JC, Harstad KG, et al. The regulatory landscape of osteogenic differentiation. Stem Cells. 2014 doi: 10.1002/stem.1759. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Walsh S, Jordan GR, Jefferiss C, et al. High concentrations of dexamethasone suppress the proliferation but not the differentiation or further maturation of human osteoblast precursors in vitro: relevance to glucocorticoid-induced osteoporosis. Rheumatology (Oxford) 2001;40(1):74–83. doi: 10.1093/rheumatology/40.1.74. [DOI] [PubMed] [Google Scholar]