Abstract
Background
Some studies have evaluated the association between the Ubiquilin 1 (UBQLN1) gene UBQ-8i polymorphism and Alzheimer’s disease (AD). However, the results remain uncertain. We carried out a meta-analysis to derive a more comprehensive estimation of this association.
Material/Methods
Case-control studies were identified by searching databases of PubMed, EMBASE, Web of Science, CNKI, CBM, Wanfang, and VIP. Odds ratios (ORs) with 95% confidence intervals (CIs) were used to assess the strength of the association.
Results
The UBQ-8i polymorphism was significantly associated with an increased AD risk (OR=1.15; 95%CI 1.05–1.25; P=0.002). The combination of adjusted ORs also found UBQ-8i polymorphism was significantly associated with AD risk (OR=1.15; 95%CI 1.02–1.30; P=0.02). When stratified by APOE ɛ4 status, both APOE ɛ4 carriers and APOE non-ɛ4 carriers with UBQ-8i polymorphism had significantly increased AD risk (OR=1.28; 95%CI 1.05–1.56; P=0.01 and OR=1.25; 95%CI 1.04–1.50; P=0.02). In the subgroup analysis according to age, UBQ-8i polymorphism was significantly associated with LOAD risk (OR=1.17; 95%CI 1.05–1.31; P=0.005), but not with EOAD risk (OR=1.12; 95%CI 0.95–1.31; P=0.17).
Conclusions
These results suggest that the UBQ-8i polymorphism is associated with AD risk.
MeSH Keywords: Alzheimer’s Disease; Genetics; Polymorphism, Single Nucleotide; Risk
Background
Alzheimer’s disease (AD), the most common form of irreversible dementia, is placing a considerable and increasing burden on patients, caregivers, and society, as more people live long enough to become affected [1]. AD is clinically characterized by a progression from episodic memory problems to a slow global decline of cognitive function that leaves patients with end-stage AD bedridden and dependent on custodial care, with death occurring on average 9 years after diagnosis [2–4]. Studies have demonstrated that AD is a result of intricate interactions between interior and environmental factors, such as genetics, race, age, family history, obesity, and diet [5–8].
Ubiquilin 1 (UBQLN1) is an ubiquitin-like protein that has been shown to play a central role in regulating the proteasomal degradation of various proteins, including the presenilins (PSs). PS plays a fundamental role in AD pathogenesis [9]. UBQLN1 has previously been shown to interact with PS1 and PS2; overexpression of UBQLN1 was reported to enhance the accumulation of PS holoproteins [10,11]. More recently, down-regulation of UBQLN1 was reported to modulate PS1 endoproteolysis, along with protein levels of nicastrin and PEN-2 in non-neuronal cell lines [12].
The UBQLN1 gene on chromosome 9q22 lies in a region that has been implicated in several linkage studies of AD [13–20]. Several studies suggested that a single intronic C/T polymorphism, UBQ-8i (rs12344615), contributed to AD risk. This variant seemed to be less effective than the wild type due to variation in the central domain of ubiquilin. Ford et al. [21] suggested that variations in the central part of the protein could facilitate its ability to form dimers, which in turn could be less capable of binding to PS1 than monomers. If ubiquilin does not bind to PS1, a gamma-secretase complex can form and become involved in the processing of the amyloid precursor protein. This entails the production of the peptides Aβ40 and Aβ42, which contribute to neurotoxicity. However, the previous results were inconsistent [13–20]. These disparate findings may be partly due to limited sample size, false-positive results, and publication bias. In the present study, a meta-analysis based on all published studies was performed to estimate the UBQ-8i polymorphism with AD susceptibility.
Material and Methods
Identification and search of relevant studies
To search for all the studies that examined the association of UBQ-8i with AD susceptibility, we conducted a comprehensive literature search of PubMed, EMBASE, Web of Science, CNKI (China Nation Knowledge Infrastructure Platform), CBM (China Biological Medicine Database), Wanfang, and VIP databases (up to May 2014), using the following MeSH terms and keywords: ‘Ubiquilin 1’, ‘UBQLN1’, ‘UBQ-8i, ‘polymorphism’, ‘Alzheimer’s disease, ‘Alzheimer disease or AD’. We also screened all the reference lists of retrieved articles and review articles and retrieved additional studies by hand-searching of relevant journals and by correspondence with authors of included studies. If there were multiple publications from the same study group, to prevent data duplication, the most complete and recent results were kept.
Eligible studies had to meet the following criteria: (a) only case-control studies were considered and (b) the study explored the correlation between AD and UBQ-8i. Major exclusion criteria were: (a) no control population, (b) no available genotype frequency, and (c) duplication of the previous publications (the largest or most recent publication was selected).
Data extraction
Information was extracted from all eligible publications independently by 2 authors according to the inclusion criteria listed above. The following information was extracted from each study: first author, publication year, racial background, age of patients, sex, number of cases and controls, adjustment, odds ratios (ORs), and the corresponding 95% confidence intervals (CIs) of AD risk.
Statistical analysis
OR and 95% CI were applied to assess the strength of the association of UBQ-8i with AD. The dominant model (CT+CC vs. TT) was used in this meta-analysis, because most of the studies reported the results in this model. Heterogeneity among studies was calculated using the χ2-based Cochran’s Q-statistic test (P<0.10 was considered statistically significant heterogeneity), and the inconsistency index I2 statistic was also calculated to observe between-study variability that was due to heterogeneity rather than chance. This statistic, which was documented by percentage, yields result ranging from 0% to 100% (I2=0–25%, no heterogeneity; I2=25–50%, moderate heterogeneity; I2=50–75%, large heterogeneity; I2=75–100%, extreme heterogeneity). A fixed-effects model using the Mantel and Haenszel method was used in the absence of between-study heterogeneity, and a random effects model using the method of DerSimonian and Laird was used to investigate variation both from in-study and between-study. Stratified analyses were performed in accordance with APOE ɛ4 status and age. We defined the early-onset AD (EOAD) as the first event before 70 years of age. Sensitivity analyses were conducted to estimate the stability of the results, such that each study was omitted one at a time to reflect the influence of the individual data set on the pooled OR. Cumulative meta-analysis was also conducted. Potential publication bias was assessed by visual inspection of funnel plot asymmetry, the Begg’s rank correlation method, and the Egger’s weighted regression method [22]. All statistical analyses were conducted using STATA software version 11.0 and Revman 5.1. All P values were 2-sided.
Results
Studies included in the meta-analysis
After a comprehensive literature search applying our inclusion criteria, 7 relevant studies including 4780 patients and 10 028 controls were identified in the final analysis. One study reported 2 case-control studies and 1 study reported 5 case-control studies. Therefore, a total of 12 studies were included in this meta-analysis. There was only 1 study with Asians, while the rest of the studies used Caucasians. Five case-control studies reported the adjusted results. The main study characteristics are summarized in Table 1.
Table 1.
Characteristics of the case-control studies included in meta-analysis.
| First author | Year | Ethnicity | Age of patients | Sex | Case (n) | Control (n) | Adjustment |
|---|---|---|---|---|---|---|---|
| Bensemain | 2006 | Caucasian | 72.4 | Both | 613 | 653 | Yes, age and sex |
| Brouwers 1 | 2006 | Caucasian | 63.7 | Both | 182 | 183 | Yes, age, sex, and APOE |
| Brouwers 2 | 2006 | Caucasian | 57.5 | Both | 110 | 280 | Yes, age, sex, and APOE |
| Kamboh | 2006 | Caucasian | 77.7 | Both | 928 | 797 | Yes, age, sex, and APOE |
| Smemo 1 | 2006 | Caucasian | NR | Both | 160 | 112 | No |
| Smemo 2 | 2006 | Caucasian | NR | Both | 196 | 116 | No |
| Smemo 3 | 2006 | Caucasian | 76.2 | Both | 376 | 353 | No |
| Smemo 4 | 2006 | Caucasian | 77 | Both | 633 | 731 | No |
| Smemo 5 | 2006 | Caucasian | 87 | Both | 179 | 330 | No |
| Arias-Vasquez | 2007 | Caucasian | 62 | Both | 549 | 5728 | Yes, age, sex, and APOE |
| Golan | 2008 | Caucasian | 76.1 | Both | 407 | 407 | No |
| Chuo | 2011 | Asian | 77.9 | Both | 100 | 100 | No |
| Martín | 2012 | Caucasian | 72 | Both | 347 | 238 | No |
NR – not reported.
Quantitative synthesis
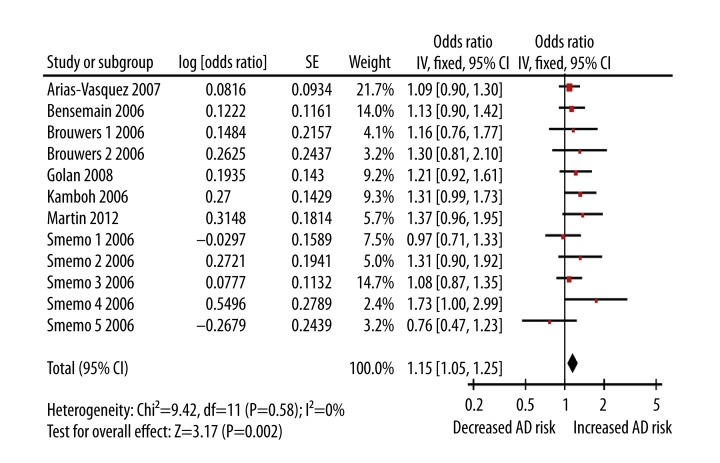
Because of the rarity of the UBQ-8i polymorphism in the Chinese population, the study with Asians was not included in the quantitative synthesis. Table 2 lists the main results of the meta-analysis. The CC+CT genotypes of UBQ-8i was significantly associated with an increased AD risk (OR=1.15; 95%CI 1.05–1.25; P=0.002; Figure 1). The combination of adjusted ORs also found UBQ-8i polymorphism was significantly associated with AD risk (OR=1.15; 95%CI 1.02–1.30; P=0.02). When stratified by APOE ɛ4 status, APOE ɛ4 carriers with CC+CT genotypes had a statistically significant increased AD risk (OR=1.28; 95%CI 1.05–1.56; P=0.01). In addition, APOE non-ɛ4 carriers with CC+CT genotypes also showed an increased AD risk (OR=1.25; 95%CI 1.04–1.50; P=0.02). In the subgroup analysis according to age, UBQ-8i polymorphism was significantly associated with LOAD risk (OR=1.17; 95%CI 1.05–1.31; P=0.005), but not with EOAD risk (OR=1.12; 95%CI 0.95–1.31; P=0.17).
Table 2.
Meta-analysis for the association of UBQ-8i polymorphism with AD risk.
| No. of studies | OR (95% CI) | P value | I2 (%) | Pheterogeneity | |
|---|---|---|---|---|---|
| Overall | 12 | 1.15 (1.05–1.25) | 0.002 | 0 | 0.58 |
| Adjusted | 5 | 1.15 (1.12–1.30) | 0.02 | 0 | 0.83 |
| APOE ɛ4 carriers | 8 | 1.28 (1.05–1.56) | 0.01 | 4 | 0.40 |
| APOE non-ɛ4 carriers | 8 | 1.25 (1.04–1.50) | 0.02 | 0 | 0.85 |
| EOAD | 3 | 1.12 (0.95–1.31) | 0.17 | 0 | 0.77 |
| LOAD | 7 | 1.17 (1.05–1.31) | 0.005 | 15 | 0.32 |
EOAD – early-onset Alzheimer’s disease; LOAD – late-onset Alzheimer’s disease.
Figure 1.
Forest plot of Alzheimer’s disease risk associated with the UBQ-8i polymorphism.
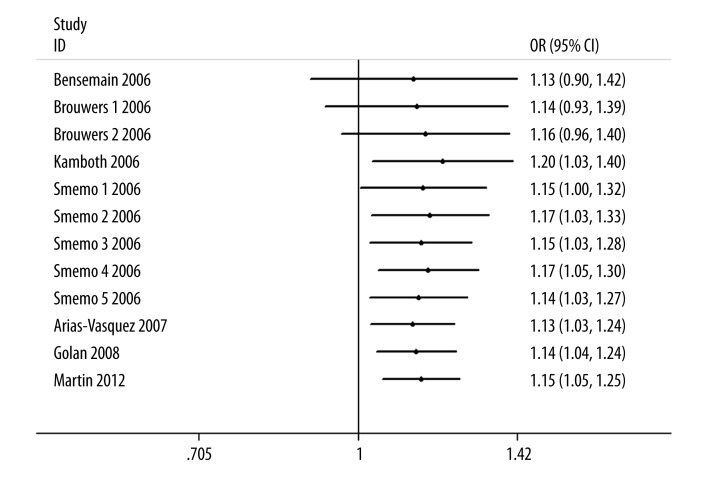
The influence analysis showed that no particular study affected the overall significance of the pooled estimates. After removing each study and recalculating the ORs, overall estimates, as well as their significance, remained nearly unchanged (data not shown). As shown in Figure 2, significant associations were evident with each addition of more data over time.
Figure 2.
Cumulative meta-analysis of Alzheimer’s disease risk associated with the UBQ-8i polymorphism.
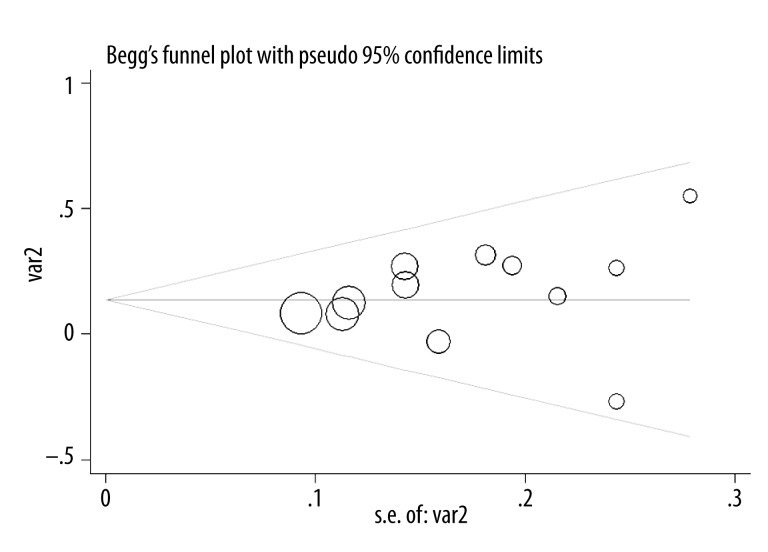
The shape of the funnel plot did not display any evidence of apparent asymmetry (Figure 3). Furthermore, the test also showed no evidence of substantial publication bias (P=0.336 for the Egger test).
Figure 3.
Funnel plot of association between Alzheimer’s disease risk associated with the UBQ-8i polymorphism.
Discussion
To our knowledge, our meta-analysis of 12 case-control studies, with a total of 4780 cases and 10 028 controls, is the most comprehensive assessment of the association between UBQ-8i polymorphism and AD. Our meta-analysis indicated that UBQ-8i CC+CT genotypes carriers had a significantly increased AD risk than the TT genotype carriers. APOE ɛ4 carriers showed increased AD risk in previous studies [23,24]. Despite the clear significance of the APOE ɛ4 allele with regards to risk of AD development, only about 30–40% of patients carry this allele. Therefore, other genetic risk factors have been suspected of influencing the incidence of AD. Thus, it was interesting to investigate the interactions between APOE ɛ4 allele and UBQ-8i polymorphisms. In the subgroup analysis by APOE ɛ4 status, an increased AD risk was observed in both APOE ɛ4 carriers and APOE non-ɛ4 carriers. Actually, when we limited the meta-analysis to studies that controlled for APOE ɛ4 status, a significant association between UBQ-8i polymorphism and AD risk was found (OR=1.16; 95%CI 1.01–1.33; P=0.03). This result indicates that the role of UBQ-8i polymorphism was not selective by APOE ɛ4 status. No significant statistical interaction was observed between the APOE ɛ4 allele and UBQ-8i polymorphism by previous studies. It is possible that studies with small sample sizes may have had insufficient statistical power to detect a slight effect. The subgroup analysis based on age found that this polymorphism showed increased LOAD risk, but not EOAD risk. The lack of an observed association may be due to the limited number of studies included in this meta-analysis.
UBQLN1 protein function could be modified by UBQ-8i polymorphism, which generates an alternative splicing variant [25]. UBQ-8i is close to exon 8, 70 bp downstream of its 3′splice site. Exon 8 is absent in an alternative, in-frame transcript, denoted as transcript variant 2 (TV2). Bertram et al. assessed whether the UBQ-8i C allele affects the splicing of this exon in the UBQLN1 message in the brain [25]. They measured the relative abundance of the TV2 and TV1 in RNA extracts from brain tissue of 25 patients with neuropathologically confirmed AD and 17 age-matched controls. They found that TV1 levels were generally 10-fold higher than those of TV2 [25]. In addition, the variant UBQ-8i C allele harbors a deletion of the exon 8, which eliminates a central region of the protein containing Sti1-like repeats involved in protein binding and dimerization [26]. Therefore, UBQ-8i C allele might alter the monomer/dimer ratio of UBQLN1, thereby changing its binding capacities. In Drosophila, expression of human UBQ-8i C allele leads to more severe degeneration than the comparable expression of the human wild-type ubiquilin [27]. Thus, the change originated by the UBQ-8i C allele may be a risk factor for the development of AD.
The present meta-analysis should be interpreted with caution, and several limitations merit consideration. First, due to lack of the original data of the eligible studies, we could not perform other subgroup analyses based on factors such as sex and smoking. Second, the number of published studies was not sufficient for a comprehensive analysis, particularly for Asians and Africans. However, our meta-analysis also had some merits. First, when the adjusted ORs were combined, the result was still positive. Second, there was low heterogeneity in this meta-analysis.
Conclusions
The results of this meta-analysis suggest that the UBQ-8i polymorphism may be associated with AD development. Further studies with larger sample sizes are needed to further assess the presence of an association.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Citron M. Alzheimer’s disease: strategies for disease modification. Nat Rev Drug Discov. 2010;9:387–98. doi: 10.1038/nrd2896. [DOI] [PubMed] [Google Scholar]
- 2.Postula M, Janicki PK, Rosiak M, et al. Effect of common single-nucleotide polymorphisms in acetylsalicylic acid metabolic pathway genes on platelet reactivity in patients with diabetes. Med Sci Monit. 2013;19:394–408. doi: 10.12659/MSM.883922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pac-Kożuchowska E, Krawiec P. Cholesterol ester transfer protein (CETP) gene polymorphism and selected parameters of lipid metabolism in children from families with history of cardiovascular system diseases. Med Sci Monit. 2013;19:818–25. doi: 10.12659/MSM.889550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gloria-Bottini F, Banci M, Saccucci P, et al. p53 codon 72 polymorphism and coronary artery disease: evidence of interaction with ACP1. Med Sci Monit. 2012;18(12):CR712–15. doi: 10.12659/MSM.883597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price DL, Tanzi RE, Borchelt DR, Sisodia SS. Alzheimer’s disease: genetic studies and transgenic models. Annu Rev Genet. 1998;32:461–93. doi: 10.1146/annurev.genet.32.1.461. [DOI] [PubMed] [Google Scholar]
- 6.Zimny A, Bladowska J, Neska M, et al. Quantitative MR evaluation of atrophy, as well as perfusion and diffusion alterations within hippocampi in patients with Alzheimer’s disease and mild cognitive impairment. Med Sci Monit. 2013;19:86–94. doi: 10.12659/MSM.883757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bidzan L, Bidzan M, Pąchalska M. Aggressive and impulsive behavior in Alzheimer’s disease and progression of dementia. Med Sci Monit. 2012;18(3):CR182–89. doi: 10.12659/MSM.882523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zvěřová M, Fišar Z, Jirák R, et al. Plasma cortisol in Alzheimer’s disease with or without depressive symptoms. Med Sci Monit. 2013;19:681–89. doi: 10.12659/MSM.889110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haapasalo A, Viswanathan J, Bertram L, et al. Emerging role of Alzheimer’s disease-associated ubiquilin-1 in protein aggregation. Biochem Soc Trans. 2010;38:150–55. doi: 10.1042/BST0380150. [DOI] [PubMed] [Google Scholar]
- 10.Mah AL, Perry G, Smith MA, Monteiro MJ. Identification of ubiquilin, a novel presenilin interactor that increases presenilin protein accumulation. J Cell Biol. 2000;151:847–62. doi: 10.1083/jcb.151.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massey LK, Mah AL, Ford DL, et al. Overexpression of ubiquilin decreases ubiquitination and degradation of presenilin proteins. J Alzheimers Dis. 2004;6:79–92. doi: 10.3233/jad-2004-6109. [DOI] [PubMed] [Google Scholar]
- 12.Massey LK, Mah AL, Monteiro MJ. Ubiquilin regulates presenilin endoproteolysis and modulates gamma-secretase components, Pen-2 and nicastrin. Biochem J. 2005;391:513–25. doi: 10.1042/BJ20050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bensemain F, Chapuis J, Tian J, et al. Association study of the Ubiquilin gene with Alzheimer’s disease. Neurobiol Dis. 2006;22:691–93. doi: 10.1016/j.nbd.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Brouwers N, Sleegers K, Engelborghs S, et al. The UBQLN1 polymorphism, UBQ-8i, at 9q22 is not associated with Alzheimer’s disease with onset before 70 years. Neurosci Lett. 2006;392:72–74. doi: 10.1016/j.neulet.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 15.Kamboh MI, Minster RL, Feingold E, DeKosky ST. Genetic association of ubiquilin with Alzheimer’s disease and related quantitative measures. Mol Psychiatry. 2006;11:273–79. doi: 10.1038/sj.mp.4001775. [DOI] [PubMed] [Google Scholar]
- 16.Smemo S, Nowotny P, Hinrichs AL, et al. Ubiquilin 1 polymorphisms are not associated with late-onset Alzheimer’s disease. Ann Neurol. 2006;59:21–26. doi: 10.1002/ana.20673. [DOI] [PubMed] [Google Scholar]
- 17.Arias-Vásquez A, de Lau L, Pardo L, et al. Relationship of the Ubiquilin 1 gene with Alzheimer’s and Parkinson’s disease and cognitive function. Neurosci Lett. 2007;424:1–5. doi: 10.1016/j.neulet.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Golan MP, Melquist S, Safranow K, et al. Analysis of UBQLN1 variants in a Polish Alzheimer’s disease patient: control series. Dement Geriatr Cogn Disord. 2008;25:366–71. doi: 10.1159/000121006. [DOI] [PubMed] [Google Scholar]
- 19.Chuo LJ, Wu ST, Chang HI, Kuo YM. Extremely rare incidence of the UBQLN1 polymorphism (UBQ-8i) in Taiwan Chinese with Alzheimer’s disease. Neurosci Lett. 2010;475:108–9. doi: 10.1016/j.neulet.2010.03.061. [DOI] [PubMed] [Google Scholar]
- 20.Martin XE, Martinez MF, Fernandez DG, et al. UBQ-8i polymorphism is not an independent risk factor for mild cognitive impairment and Alzheimer’s disease in APOE-4 carriers. Curr Alzheimer Res. 2012;9:467–72. doi: 10.2174/156720512800492440. [DOI] [PubMed] [Google Scholar]
- 21.Ford DL, Monteiro MJ. Dimerization of ubiquilin is dependent upon the central region of the protein: evidence that the monomer, but not the dimer, is involved in binding presenilins. Biochem J. 2006;399:397–404. doi: 10.1042/BJ20060441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fei M, Jianhua W. Apolipoprotein ɛ4-allele as a significant risk factor for conversion from mild cognitive impairment to Alzheimer’s disease: a meta-analysis of prospective studies. J Mol Neurosci. 2013;50:257–63. doi: 10.1007/s12031-012-9934-y. [DOI] [PubMed] [Google Scholar]
- 24.Sadigh-Eteghad S, Talebi M, Farhoudi M. Association of apolipoprotein E epsilon 4 allele with sporadic late onset Alzheimer’s disease. A meta-analysis. Neurosciences (Riyadh) 2012;17:321–26. [PubMed] [Google Scholar]
- 25.Bertram L, Hiltunen M, Parkinson M, et al. Family-based association between Alzheimer’s disease and variants in UBQLN1. N Engl J Med. 2005;352:884–94. doi: 10.1056/NEJMoa042765. [DOI] [PubMed] [Google Scholar]
- 26.Ko HS, Uehara T, Tsuruma K, Nomura Y. Ubiquilin interacts with ubiquitylated proteins and proteasome through its ubiquitin-associated and ubiquitin-like domains. FEBS Lett. 2004;566:110–14. doi: 10.1016/j.febslet.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 27.Ganguly A, Feldman RM, Guo M. ubiquilin antagonizes presenilin and promotes neurodegeneration in Drosophila. Hum Mol Genet. 2008;17:293–302. doi: 10.1093/hmg/ddm305. [DOI] [PubMed] [Google Scholar]