Abstract
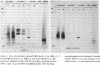
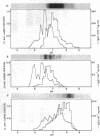
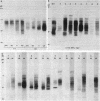
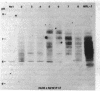
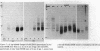
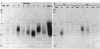
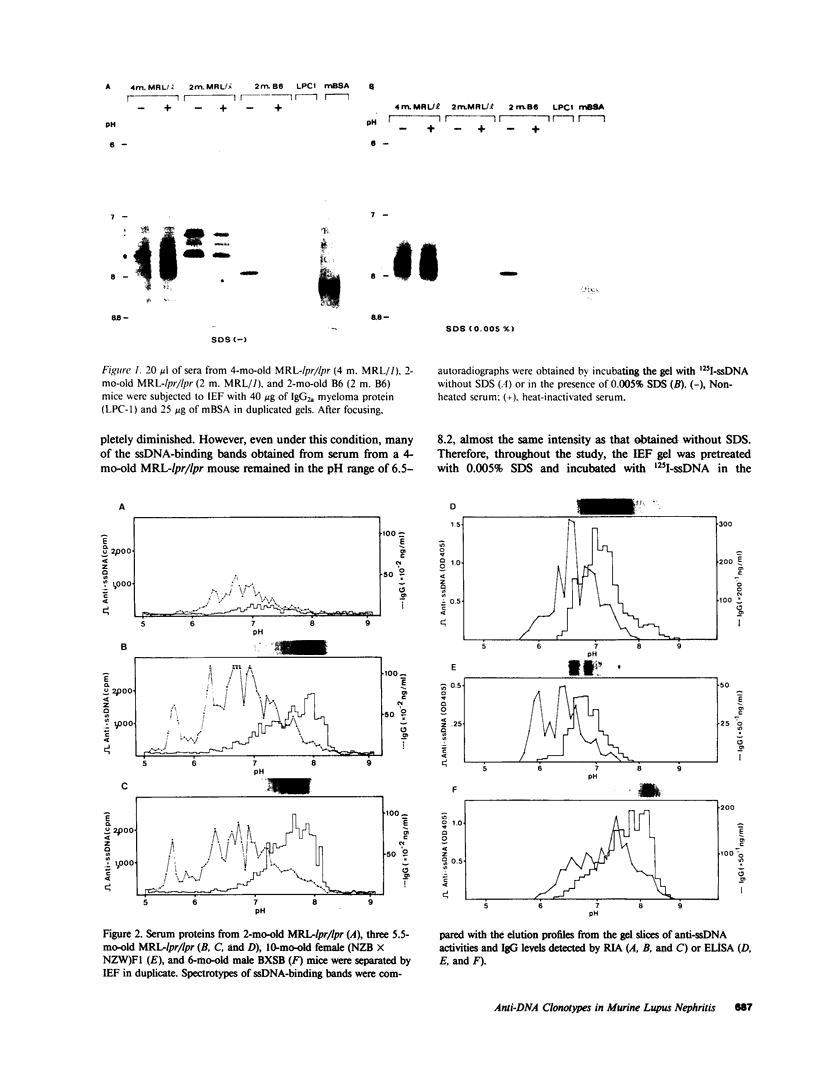
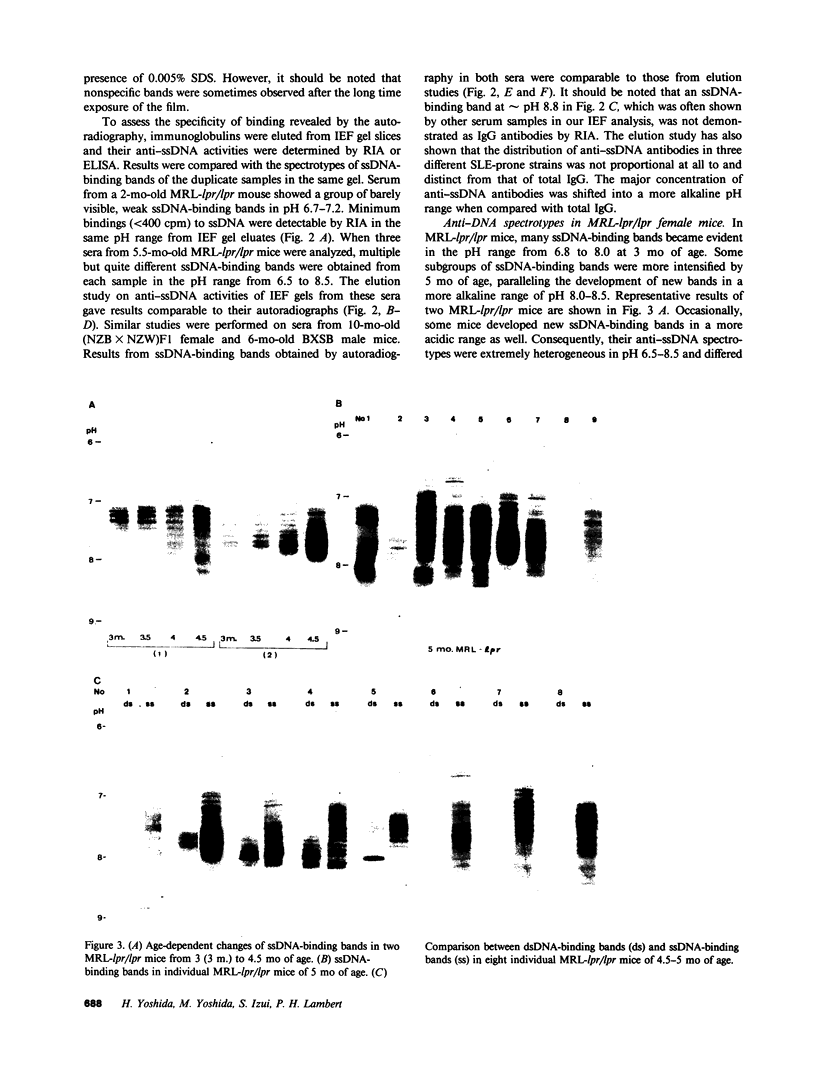
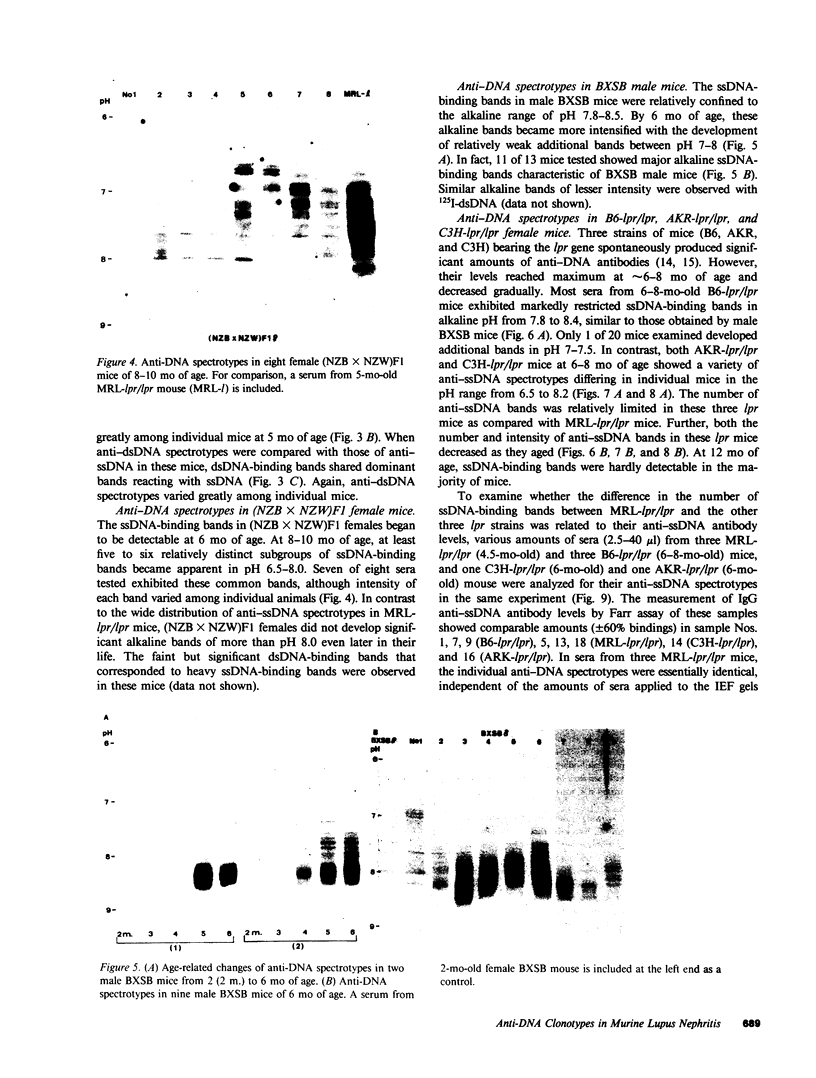
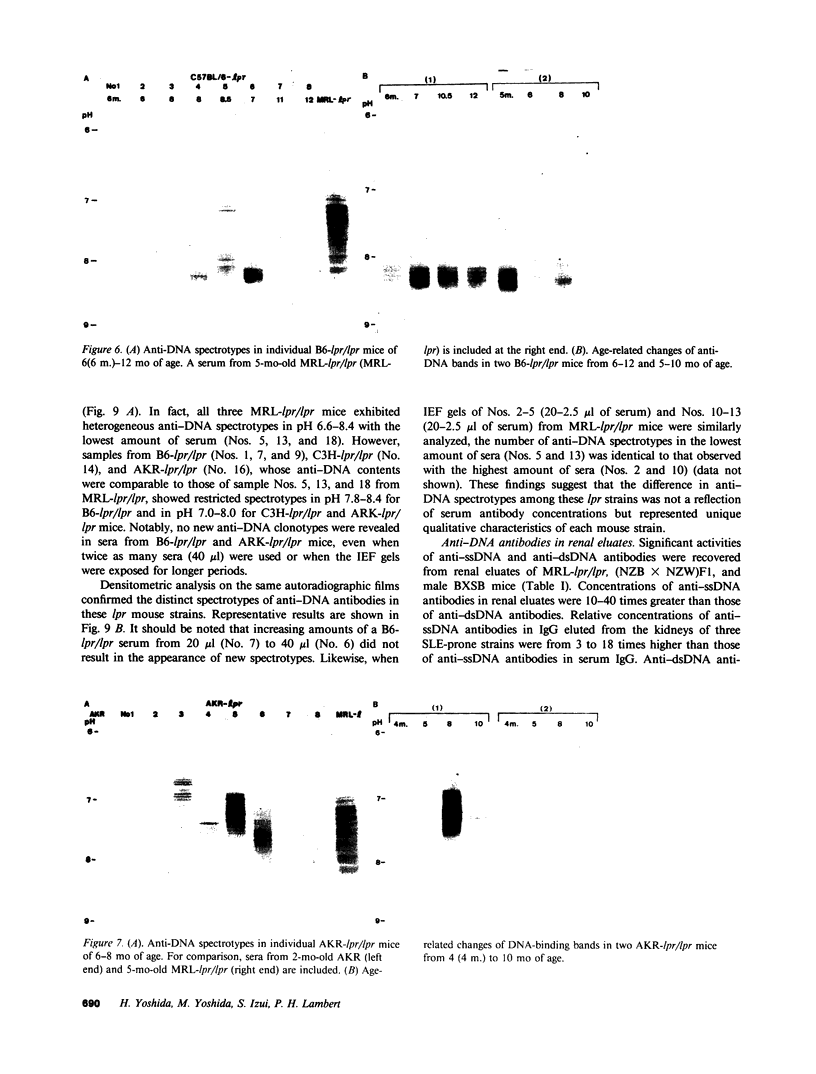
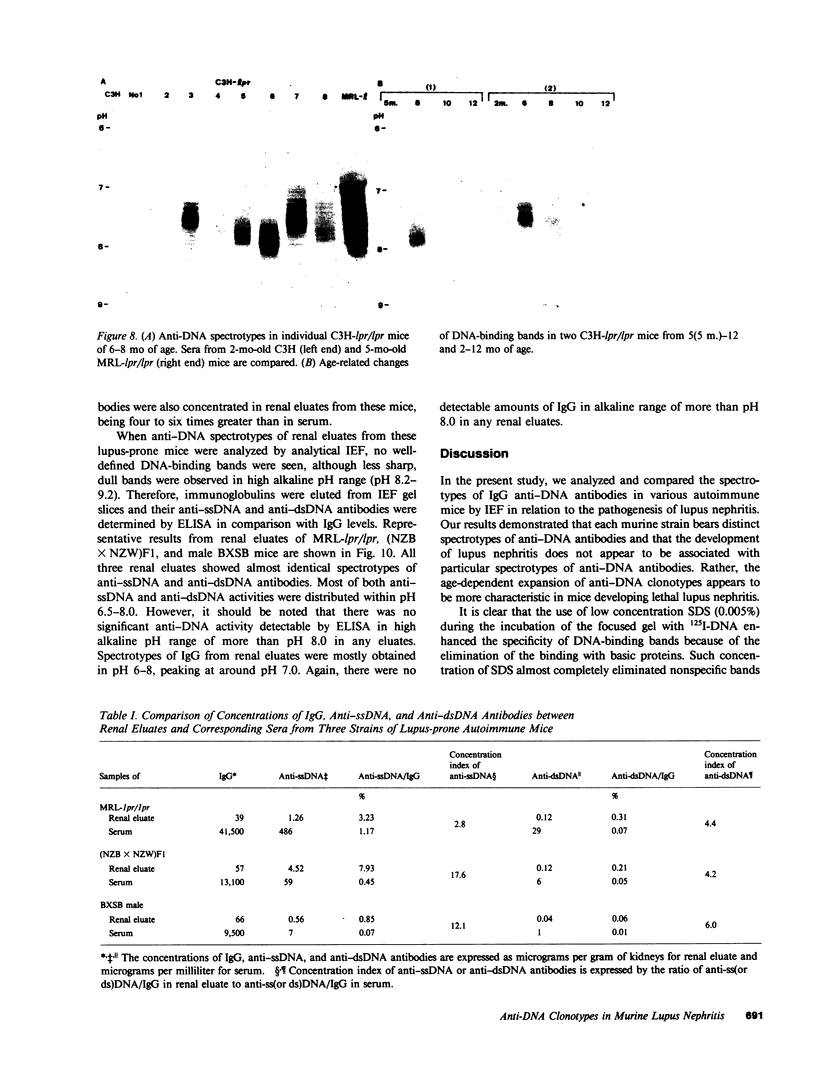
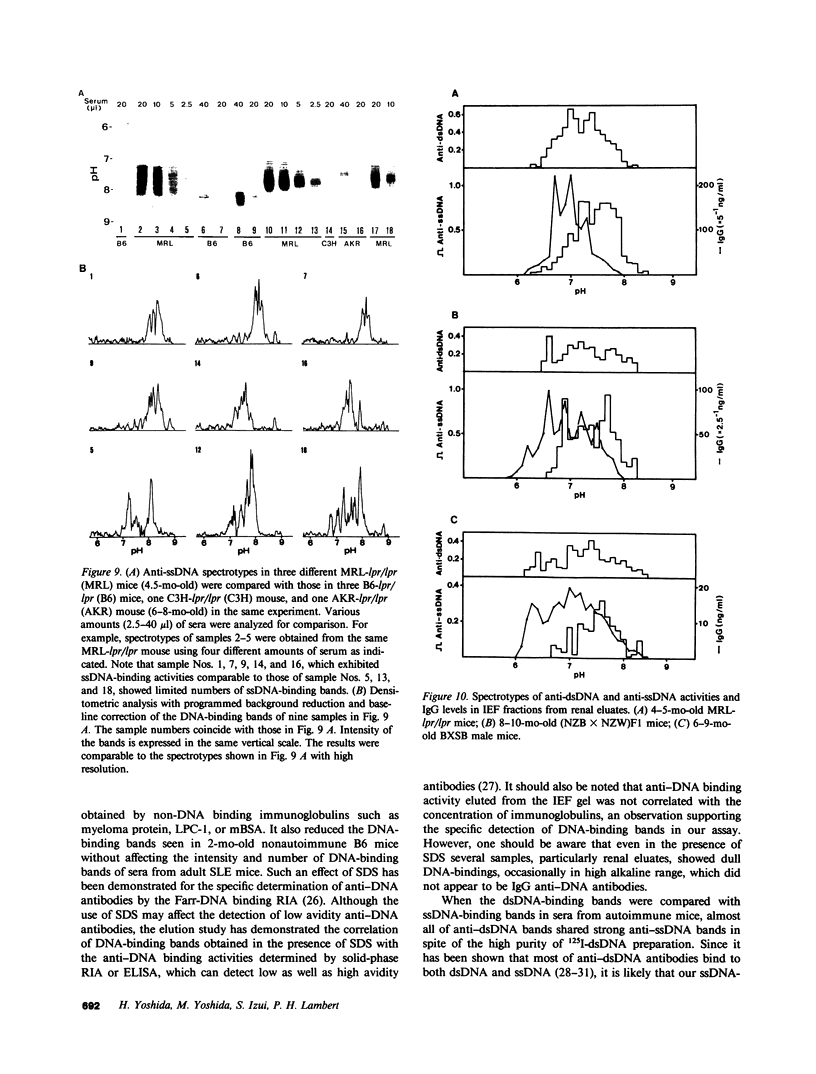
Clonotypes of IgG anti-DNA antibodies were studied by isoelectric focusing in various autoimmune mice with or without lethal lupus nephritis. MRL/MpJ-lpr/lpr mice exhibited the most heterogeneous spectrotypes of anti-DNA antibodies in the pH range from 6.5 to 8.5, with marked variation in individual mice. Female (NZB X NZW)F1 mice expressed rather uniform DNA-binding bands composed of at least five to six distinct subgroups, having isoelectric points from 6.5 to 8.0. Male BXSB mice showed major characteristic bands confined to alkaline pH range from 7.8 to 8.5, similar to C57BL/6J-lpr/lpr mice, which showed markedly restricted bands in this region. Both AKR/J-lpr/lpr and C3H/HeJ-lpr/lpr mice expressed DNA-binding bands mostly focused between pH 6.5 and 8.2. The aging study indicated that three autoimmune mice (MRL/MpJ-lpr/lpr, [NZB X NZW]F1, and male BXSB) that developed fatal glomerulonephritis showed clonal expansion of anti-DNA antibodies throughout their life. In contrast, such age-dependent expansion of anti-DNA clonotypes was not evident in three lpr cogenic mice (C57BL/6J-lpr/lpr, AKR/J-lpr/lpr, and C3H/HeJ-lpr/lpr) that developed only mild glomerulonephritis; rather, their expression of anti-DNA spectrotypes diminished as they aged. Anti-DNA activities in renal eluates from nephritic autoimmune mice were mostly distributed in the pH range from 6.5 to 8.0, without significant concentrations in the high alkaline range of more than pH 8.0. These results suggest that there exist distinct anti-DNA clonotypes in each mouse strain and that the development of lupus nephritis does not appear to be associated with particular spectrotypes of anti-DNA antibodies. Rather, the age-dependent expansion of anti-DNA clonotypes may be a feature more characteristic of mice developing lethal lupus nephritis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews B. S., Eisenberg R. A., Theofilopoulos A. N., Izui S., Wilson C. B., McConahey P. J., Murphy E. D., Roths J. B., Dixon F. J. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978 Nov 1;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann G., Chrambach A. A highly crosslinked, transparent polyacrylamide gel with improved mechanical stability for use in isoelectric focusing and isotachophoresis. Anal Biochem. 1976 Jan;70(1):32–38. doi: 10.1016/s0003-2697(76)80044-9. [DOI] [PubMed] [Google Scholar]
- Bionda A., De Baets M. H., Tzartos S. J., Lindstrom J. M., Weigle W. O., Theophilopoulos A. N. Spectrotypic analysis of antibodies to acetylcholine receptors in experimental autoimmune myasthenia gravis. Clin Exp Immunol. 1984 Jul;57(1):41–50. [PMC free article] [PubMed] [Google Scholar]
- Borel Y., Lewis R. M., Stollar B. D. Prevention of murine lupus nephritis by carrier-dependent induction of immunologic tolerance to denatured DNA. Science. 1973 Oct 5;182(4107):76–78. doi: 10.1126/science.182.4107.76. [DOI] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Dang H., Harbeck R. J. A comparison of anti-DNA antibodies from serum and kidney eluates of NZB x NZW F1 mice. J Clin Lab Immunol. 1982 Nov;9(2):139–145. [PubMed] [Google Scholar]
- Dang H., Harbeck R. J. The in vivo and in vitro glomerular deposition of isolated anti-double-stranded-DNA antibodies in NZB/W mice. Clin Immunol Immunopathol. 1984 Feb;30(2):265–278. doi: 10.1016/0090-1229(84)90061-8. [DOI] [PubMed] [Google Scholar]
- Ebling F., Hahn B. H. Restricted subpopulations of DNA antibodies in kidneys of mice with systemic lupus. Comparison of antibodies in serum and renal eluates. Arthritis Rheum. 1980 Apr;23(4):392–403. doi: 10.1002/art.1780230402. [DOI] [PubMed] [Google Scholar]
- Fischbach M., Rabbie J., Talal N. Comparison of different antinucleic acid antibody spectrotypes in spontaneous, induced, and murine lupus. J Clin Invest. 1981 Oct;68(4):1036–1043. doi: 10.1172/JCI110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G. R., Caulin-Glaser T., Lamm M. E. Charge of circulating immune complexes as a factor in glomerular basement membrane localization in mice. J Clin Invest. 1981 May;67(5):1305–1313. doi: 10.1172/JCI110159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B. H., Ebling F., Freeman S., Clevinger B., Davie J. Production of monoclonal murine antibodies to DNA by somatic cell hybrids. Arthritis Rheum. 1980 Aug;23(8):942–945. doi: 10.1002/art.1780230811. [DOI] [PubMed] [Google Scholar]
- Izui S., Eisenberg R. A. Circulating anti-DNA-rheumatoid factor complexes in MRL/1 mice. Clin Immunol Immunopathol. 1980 Mar;15(3):536–551. doi: 10.1016/0090-1229(80)90065-3. [DOI] [PubMed] [Google Scholar]
- Izui S., Eisenberg R. A., Dixon F. J. IgM rheumatoid factors in mice injected with bacterial lipopolysaccharides. J Immunol. 1979 May;122(5):2096–2102. [PubMed] [Google Scholar]
- Izui S., Kelley V. E., Masuda K., Yoshida H., Roths J. B., Murphy E. D. Induction of various autoantibodies by mutant gene lpr in several strains of mice. J Immunol. 1984 Jul;133(1):227–233. [PubMed] [Google Scholar]
- Izui S., Lambert P. H., Miescher P. A. Determination of anti-DNA antibodies by a modified 125I-labelled DNA-binding test. Elimination of non-specific binding of DNA to non-immunoglobulin basic proteins by using an anionic detergent. Clin Exp Immunol. 1976 Dec;26(3):425–430. [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Anionic sites in the glomerular basement membrane. In vivo and in vitro localization to the laminae rarae by cationic probes. J Cell Biol. 1979 Apr;81(1):137–153. doi: 10.1083/jcb.81.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J. L., Minami R. M., Teplitz R. L. An enzyme-linked immunosorbent assay for antibodies to native and denatured DNA. J Immunol Methods. 1979;29(2):155–165. doi: 10.1016/0022-1759(79)90065-6. [DOI] [PubMed] [Google Scholar]
- Koffler D., Carr R., Agnello V., Thoburn R., Kunkel H. G. Antibodies to polynucleotides in human sera: antigenic specificity and relation to disease. J Exp Med. 1971 Jul 1;134(1):294–312. doi: 10.1084/jem.134.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffler D., Schur P. H., Kunkel H. G. Immunological studies concerning the nephritis of systemic lupus erythematosus. J Exp Med. 1967 Oct 1;126(4):607–624. doi: 10.1084/jem.126.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno A., Yoshida H., Sekita K., Maruyama N., Ozaki S., Hirose S., Shirai T. Genetic regulation of the class conversion of dsDNA-specific antibodies in (NZB X NZW)F1 hybrid. Immunogenetics. 1983;18(5):513–524. doi: 10.1007/BF00364392. [DOI] [PubMed] [Google Scholar]
- Lambert P. H., Dixon F. J. Pathogenesis of the glomerulonephritis of NZB/W mice. J Exp Med. 1968 Mar 1;127(3):507–522. doi: 10.1084/jem.127.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker J. D., Medof M. E., Bennett R. M., Sukhupunyaraksa S. Characterization of DNA used to assay sera for anti-DNA antibodies; determination of the specificities of anti-DNA antibodies in SLE and non-SLE rheumatic disease states. J Immunol. 1977 Feb;118(2):694–701. [PubMed] [Google Scholar]
- Marion T. N., Lawton A. R., 3rd, Kearney J. F., Briles D. E. Anti-DNA autoantibodies in (NZB X NZW)F1 mice are clonally heterogeneous, but the majority share a common idiotype. J Immunol. 1982 Feb;128(2):668–674. [PubMed] [Google Scholar]
- Oite T., Batsford S. R., Mihatsch M. J., Takamiya H., Vogt A. Quantitative studies of in situ immune complex glomerulonephritis in the rat induced by planted, cationized antigen. J Exp Med. 1982 Feb 1;155(2):460–474. doi: 10.1084/jem.155.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoian R., Pillarisetty R., Talal N. Immunological regulation of spontaneous antibodies to DNA and RNA. II. Sequential switch from IgM to IgG in NZB/NZW F1 mice. Immunology. 1977 Jan;32(1):75–79. [PMC free article] [PubMed] [Google Scholar]
- Parker L. P., Hahn B. H., Osterland C. K. Modification of NZB-NZW F1 autoimmune disease by development of tolerance to DNA. J Immunol. 1974 Jul;113(1):292–297. [PubMed] [Google Scholar]
- Rennke H. G., Cotran R. S., Venkatachalam M. A. Role of molecular charge in glomerular permeability. Tracer studies with cationized ferritins. J Cell Biol. 1975 Dec;67(3):638–646. doi: 10.1083/jcb.67.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishido K., Ando T. Estimation of the double-helical content in various single-stranded nucleic acids by treatment with a single strand-specific nuclease. Biochim Biophys Acta. 1972 Dec 22;287(3):477–484. doi: 10.1016/0005-2787(72)90292-4. [DOI] [PubMed] [Google Scholar]
- Steward M. W., Katz F. E., West N. J. The role of low affinity antibody in immune complex disease. The quantity of anti-DNA antibodies in NZB/W F1 hybrid mice. Clin Exp Immunol. 1975 Jul;21(1):121–130. [PMC free article] [PubMed] [Google Scholar]
- Tron F., Charron D., Bach J. F., Talal N. Establishment and characterization of a murine hybridoma secreting monoclonal anti-DNA autoantibody. J Immunol. 1980 Dec;125(6):2805–2809. [PubMed] [Google Scholar]
- Warren R. W., Caster S. A., Roths J. B., Murphy E. D., Pisetsky D. S. The influence of the lpr gene on B cell activation: differential antibody expression in lpr congenic mouse strains. Clin Immunol Immunopathol. 1984 Apr;31(1):65–77. doi: 10.1016/0090-1229(84)90190-9. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Kohno A., Ohta K., Hirose S., Maruyama N., Shirai T. Genetic studies of autoimmunity in New Zealand mice. III. Associations among anti-DNA antibodies, NTA, and renal disease in (NZB x NZW)F1 x NZW backcross mice. J Immunol. 1981 Aug;127(2):433–437. [PubMed] [Google Scholar]