Abstract
Background
Vatreptacog alfa, a recombinant factor VIIa (rFVIIa) analog with three amino acid substitutions and 99% identity to native FVIIa, was developed to improve the treatment of hemophilic patients with inhibitors.
Objectives
To confirm the safety and assess the efficacy of vatreptacog alfa in treating bleeding episodes in hemophilic patients with inhibitors.
Patients and methods
In this international, multicenter, randomized, double-blind, active-controlled, crossover, confirmatory phase III trial (adept™2) in patients with hemophilia A or B and inhibitors, bleeds were randomized 3 : 2 to treatment with vatreptacog alfa (one to three doses at 80 μg kg−1) or rFVIIa (one to three doses at 90 μg kg−1). Treatment failures after three doses of trial product (TP) were managed according to the local standard of care.
Results
In the 72 patients enrolled, 567 bleeds were treated with TP. Both vatreptacog alfa and rFVIIa gave 93% effective bleeding control at 12 h. Vatreptacog alfa was superior to rFVIIa in secondary efficacy outcomes, including the number of doses used to treat a bleed and sustained bleeding control 24–48 h after the first dose. Eight patients (11%) developed antibodies against vatreptacog alfa, including four with cross-reactivity against rFVIIa and one with an in vitro neutralizing effect to vatreptacog alfa.
Conclusions
This large randomized controlled trial confirmed the well-established efficacy and safety profile of rFVIIa, and showed that vatreptacog alfa had similar or better efficacy than rFVIIa. However, because of the development of anti-drug antibodies, a positive benefit–risk profile is unlikely to be achieved with vatreptacog alfa.
Keywords: antibodies; clinical trial, phase III; hemophilia; inhibitors; recombinant factor VIIa
Introduction
The development of neutralizing antibodies (inhibitors) against replacement coagulation factor VIII or FIX remains an important clinical challenge in hemophilic patients, with limited treatment options. Only two FVIII/FIX bypassing agents, recombinant human activated FVII (rFVIIa) (NovoSeven; Novo Nordisk A/S, Bagsværd, Denmark) and plasma-derived activated prothrombin complex concentrate (FEIBA VH; Baxter AG, Vienna, Austria), are currently available. Despite their well-established efficacy and safety profiles 1–5, the hemostatic efficacy of bypassing agents is inferior to that of FVIII or FIX replacement therapy in patients without inhibitors.
Vatreptacog alfa is an activated recombinant human FVII analog produced in a CHO cell line cultured in serum-free media. It has 99% amino acid identity to native FVIIa, with the only difference being three amino acid substitutions (V158D/E296V/M298Q) in the protease domain. These substitutions were introduced to increase the tissue factor-independent enzymatic activity of vatreptacog alfa when bound to activated platelets 6–9. Vatreptacog alfa was developed to improve treatment by offering rapid and sustained resolution of bleeding episodes in hemophilic patients with inhibitors. A previous phase II dose-escalation trial (adept™1) demonstrated safety and preliminary efficacy for vatreptacog alfa in hemophilic patients with inhibitors, with no anti-drug antibodies being detected 10.
The aim of the adept™2 trial was to assess the efficacy and confirm the safety of vatreptacog alfa for treatment of bleeds in hemophilic patients with inhibitors. Unexpectedly, this phase III trial, representing the largest of its kind ever conducted in patients with inhibitors, demonstrated the development of anti-vatreptacog alfa antibodies, indicating that small sequence changes can significantly alter the immunogenicity of rFVIIa compounds.
Methods and patients
The study protocol and all amendments were reviewed and approved by the independent ethics committee or institutional review board of each participating study site. Subjects or their legally authorized representatives provided written informed consent for any study-related procedure. The trial was registered at ClinicalTrials.gov (Registration Number: NCT 01392547).
Patients
Male patients, ≥ 12 years of age with congenital hemophilia A or B and inhibitors of FVIII or FIX, and who had experienced at least five bleeds requiring hemostatic drug treatment within the 12 months prior to trial entry, were eligible for inclusion. Key exclusion criteria were as follows: HIV positivity with a CD4+ count of < 200 × 106 cells mL−1, a low platelet count (< 50 000 μL−1), severe liver (alanine transaminase level of > 3 times the upper limit of normal) or known renal disease, coagulation disorders other than congenital hemophilia, or any clinical signs or known history of arterial thrombotic events or previous deep vein thrombosis or pulmonary embolism.
Trial design and treatment
This was an international, multicenter, randomized, double-blind, active-controlled, crossover, confirmatory phase III trial conducted from July 2011 to August 2012. The objective of the trial was to confirm the safety and to assess the efficacy of vatreptacog alfa as compared with rFVIIa in the treatment of acute bleeds in hemophilic patients with inhibitors. Scheduled visits were performed prior to initiation of home treatment, at 3-month intervals thereafter, and at the end of the trial. During the scheduled visits, safety was monitored with clinical and laboratory assessments, including screening for anti-drug antibodies and assessment of rFVIIa activity 10 min after a single dose administration of vatreptacog alfa (recovery).
Trial treatment was primarily given in a home setting. All bleeds, irrespective of location and severity, were treated. Each bleeding episode was randomized (3 : 2) to treatment with one to three doses of vatreptacog alfa at 80 μg kg−1 or one to three doses of rFVIIa at 90 μg kg−1 (which is the standard dose approved worldwide). Randomization and assignment of dispensing unit number were handled by the interactive voice/interactive web response system; assignment to treatment was performed at the scheduled visits. The initial dose of trial product (TP) was to be administered as soon as the patient recognized the symptoms of a bleed, and preferably within 2 h of onset. Treatment with a second or third dose of TP was indicated if assessment at 3 h or 6 h, respectively, after the first TP administration revealed no improvement or a worsening of bleeding symptoms. Alternative hemostatic agents (other than TP) could be given according to the local standard of care if the bleed could not be controlled with up to three doses of TP. Any use of alternative hemostatic medication within 12 h after the first TP administration was considered to be a treatment failure.
A total of 500 bleeds were planned to be treated with TP, including 300 bleeds treated with vatreptacog alfa and 200 treated with rFVIIa. In order to sufficiently evaluate the potential risk of anti-drug antibody development, it was estimated that ≥ 15 patients had to have ≥ 10 days of exposure to vatreptacog alfa.
Vatreptacog alfa and rFVIIa were manufactured by Novo Nordisk (Bagsværd, Denmark), and were provided as a sterile freeze-dried powder in single-use vials of 2.0 mg and 2.3 mg, respectively, to be reconstituted with 2.1 mL of histidine solvent for intravenous injection.
Outcome measures
Patients used an electronic diary (eDiary) to report details of the bleeding episodes and treatment outcome.
Efficacy assessments
Efficacy assessments were performed at predose and up to 48 h after the initial dose of TP, including evaluation of bleeding symptoms prior to each administration of TP, at 1, 3, 6, and 9 h after the first dose of TP, and prior to administration of additional hemostatic medications (other than TP). Administration of additional hemostatic medication was assessed at 1, 3, 6, 9, 12, 24, and 48 h after the initial dose. Pain was assessed with a visual analog scale integrated in the eDiary.
The primary efficacy endpoint was effective bleeding control, defined as no additional hemostatic medication (other than TP) given within 12 h after the first dose of TP. Secondary efficacy endpoints included: evaluation of effective and sustained bleeding control 24 h and 48 h after the initial dose (defined as no use of additional hemostatic medication to treat the same bleed); number of doses of TP given for each bleed; and change in pain assessments over time (baseline pain immediately prior to the initial dose and 1, 3, 6, and 9 h after the first dose of TP).
Safety assessments
Continuous surveillance of safety data was performed during the trial by the Novo Nordisk safety committee. The main safety endpoint was immunogenicity, i.e. the development of antibodies against vatreptacog alfa and/or rFVIIa. Secondary safety endpoints included adverse events (non-serious and serious), including thromboembolic events, laboratory safety parameters (hematology, coagulation-related parameters [prothrombin time, activated partial thromboplastin time, and clinical chemistry]), physical examination, and vital signs. A central laboratory (Quest Diagnostics, Heston, UK) was responsible for analyzing all standard laboratory safety test results, except for hematology results, which were analyzed at local laboratories. Laboratory test results outside the normal reference range were evaluated for clinical relevance by the investigator. The recovery of FVIIa activity (as a potential indicator of formation of neutralizing antibodies) was assessed 10 min after a single dose administration of 80 μg kg−1 vatreptacog alfa in a non-bleeding state at each scheduled dose visit and at the end-of-trial visit. Samples were analyzed by Novo Nordisk A/S (Måløv, Denmark). Threshold values of FVIIa activity (< 25% of the first measurement 10 min after the first vatreptacog alfa dose in a patient) were predefined and implemented in a decision-tree for binding and neutralizing antibody tests.
Testing for anti-drug antibodies
Samples for anti-drug antibody testing were taken prior to the first drug exposure, at least every 3 months during the trial at each of the scheduled visits (predose), and at least 1 month after the last TP administration at the end of the study.
Assessment of binding antibodies
All study samples were analyzed by a central laboratory (Celerion, Zürich, Switzerland) for antibodies binding to vatreptacog alfa and rFVIIa in a validated radioimmunoassay (RIA), in which samples were incubated with 125I-labeled vatreptacog alfa or rFVIIa. The specificity of the antibodies was analyzed in a competition RIA in which a sample was incubated with an excess of the unlabeled antigen of interest. Cut-points were established on the basis of assay and intraindividual variation, and a 0.1% false-positive rate. The sensitivity of the assays was ∼ 160 ng mL−1 anti-FVIIa and/or anti-vatreptacog alfa antibodies. The antibody titer was defined as the last dilution giving a result above the titration cut-point (2 × assay cut-point). A titer of 1 was assigned to antibody-positive samples with a result above the assay cut-point but below the titration cut-point.
Assessment of neutralizing antibodies
All samples that tested positive for vatreptacog alfa-binding and/or rFVIIa-binding antibodies were further characterized and tested for neutralizing activity in in vitro neutralizing clot assays that measured neutralization of vatreptacog alfa or endogenous human FVIIa with an ACL Future instrument (ILS Laboratories, Allerod, Denmark) in a central laboratory at Novo Nordisk A/S (Måløv, Denmark). For detection of neutralization of vatreptacog alfa activity, the pretreatment and trial samples were mixed with vatreptacog alfa, and the time to clot formation after addition of soluble truncated recombinant tissue factor was measured (STACLOT kit FVIIa-rTF; Diagnostica Stago, Parsippany, NJ, USA). In parallel, the samples were analyzed for neutralizing activity against endogenous human FVIIa by initiating clot formation with full-length tissue factor (thromboplastin), which leads to activation of endogenous FVII to FVIIa. Samples with decreased clot activity in the primary neutralizing assay were confirmed for neutralizing antibodies with this same assay, but including a heat inactivation (30 min at 60 °C) step to minimize intraindividual variation. Cut-points in these confirmatory analyses were established on the basis of intraindividual and interindividual variation, and a 0.1% false-positive rate.
Statistical analysis
Data from all patients exposed to at least one dose of TP were included in the safety analysis dataset. For the efficacy full analysis set, data from all patients with at least one efficacy evaluation postdose were included.
Effective bleeding control was analyzed with a logistic regression model adjusting for treatment, type of bleed (joint or non-joint), and baseline pain score. Patient effect was included as a random variable to account for repeated events via an exchangeable working matrix. The primary test was a non-inferiority test of vatreptacog alfa as compared with rFVIIa using a non-inferiority bound for the log odds ratio corresponding to 15% on an absolute scale. If non-inferiority was established, superiority of vatreptacog alfa over rFVIIa (null hypothesis: the effect of vatreptacog alfa is equal or worse than the effect of rFVIIa) was tested in the same model based on a one-sided test at a 2.5% alpha level. In order to ensure an adequate number of bleeding episodes to address the efficacy endpoints, a total of 500 bleeds were planned to be treated with TP. On the assumption of true success rates of 97% for vatreptacog alfa and 90% for rFVIIa, 300 bleeding episodes treated with vatreptacog alfa and 200 bleeding episodes treated with rFVIIa would give > 99% power to demonstrate non-inferiority with a 15% non-inferiority bound. The power for subsequently demonstrating superiority was 90%, based on simulations with the binomial distribution. The secondary efficacy endpoints were analyzed with a logistic regression model similar to that used for the primary analysis. Safety endpoints were presented using summary/descriptive statistics.
Results
Patient characteristics
Seventy-two hemophilic patients with inhibitors (age, 12–71 years; mean, 30 years) were enrolled in the trial (66 hemophilia A; six hemophilia B), recruited from a total of 46 centers in 18 countries in Africa, Asia, Europe, North America, and South America. Of these, 69 patients received TP for treatment of acute bleeds, and three patients did not have any bleeds but were exposed to vatreptacog alfa at the first scheduled dose visit. Details of the flow of participants through each stage of the trial are shown in Fig. 1.
Figure 1.
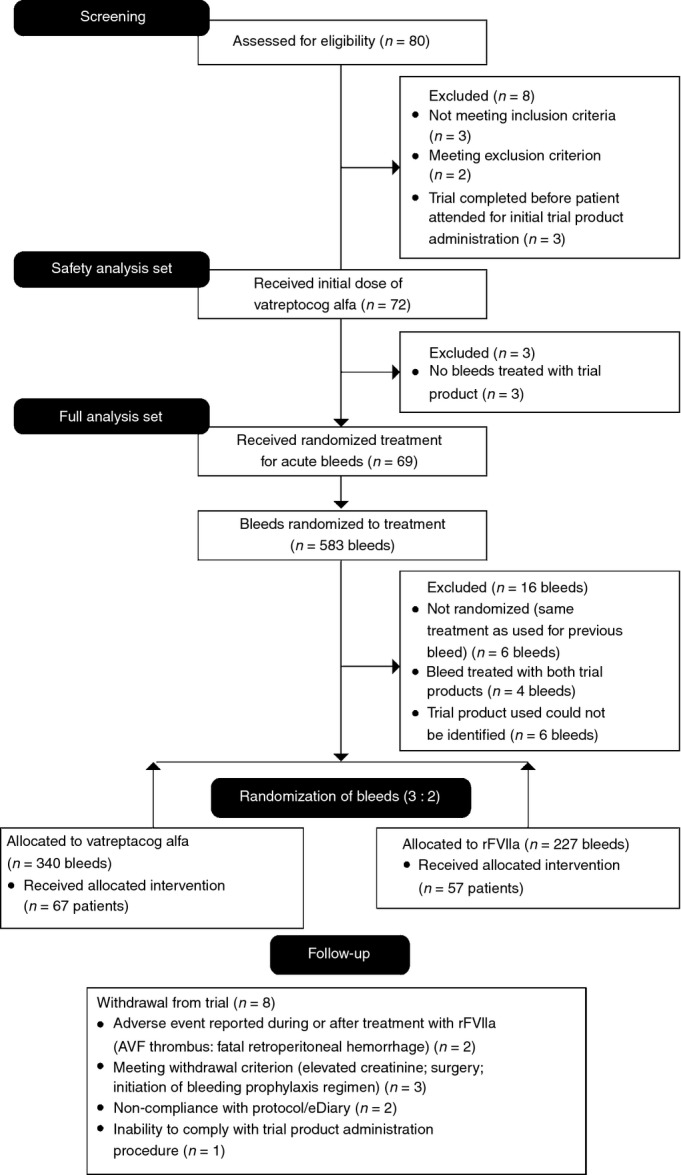
CONSORT diagram showing the flow of participants through each stage of the trial. AVF, arteriovenous fistula; eDiary, electronic diary; rFVIIa, recombinant factor VIIa.
Abnormal clinically significant baseline findings (as judged by the investigator) in the musculoskeletal system were reported in approximately half of the patients (35/72), and reflected the underlying disease and the consequences thereof, including muscle atrophy and arthropathy as related to hemophilia.
Treatment of bleeds
The trial was conducted and completed according to the study protocol.
Overall, 567 bleeds (including bleeds in joints or mucocutaneous, muscle, soft tissue or other sites) were treated with TP in a total of 69 patients, including 340 bleeds treated with vatreptacog alfa, and 227 bleeds treated with rFVIIa. In individual patients, the total number of bleeds treated with TP ranged from one to 42 (Fig. 2A). In total, 57 patients had 1–16 bleeds treated with rFVIIa and 67 patients had 1–26 bleeds treated with vatreptacog alfa.
Figure 2.
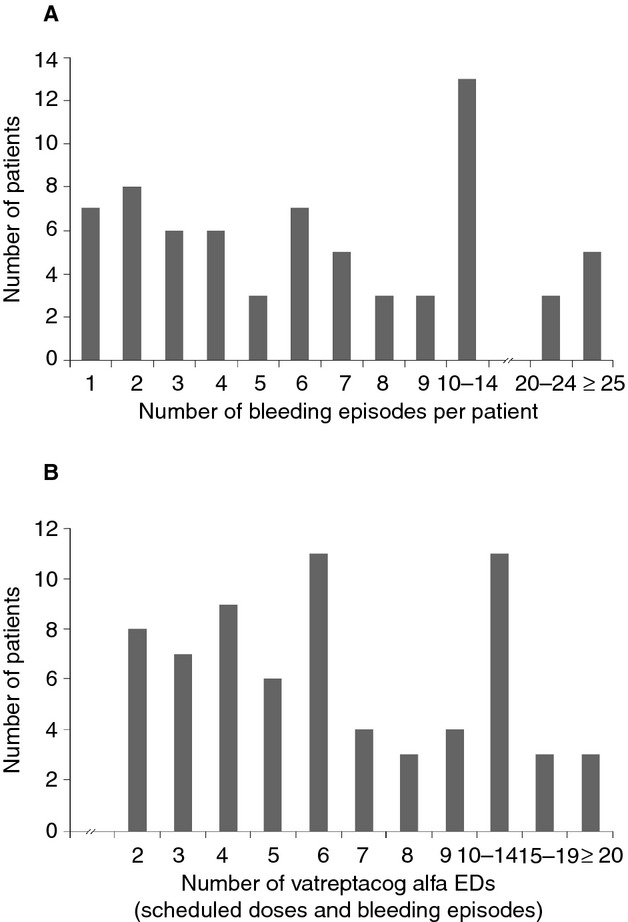
The number of bleeding episodes treated per patient and exposure days to vatreptacog alfa. (A) Distribution of the number of bleeding episodes treated per patient. Of 69 patients with treatment requiring bleeds, 30 patients had ≤ 5 bleeds, 18 patients had 6–9 bleeds, and 21 patients had ≥ 10 bleeds treated with trial product during the trial. (B) Distribution of vatreptacog alfa exposure days (EDs) per patient. Seventeen patients had ≥ 10 vatreptacog alfa EDs.
The proportion of target joint bleeds (defined as joints with three or more bleeds in the past 6 months) was similar among bleeds treated with vatreptacog alfa (112/340; 33%) and those treated with rFVIIa (71/227; 31%). Overall, no clinically relevant differences (i.e. differences judged to have an impact on the results and conclusions of the trial) in bleeding episode characteristics (type, severity, and bleeding site) were apparent between bleeds treated with vatreptacog alfa and those treated with rFVIIa.
The majority of bleeds (94%) were treated at home, and most bleeds were treated within the initial 2 h after onset of bleeding, as recommended in the protocol. The median time from onset of bleeding to the initial dose was 0.4 h (mean, 1.3 h) for bleeds treated with vatreptacog alfa and rFVIIa. Late-treated bleeds (> 2 h) responded as well to treatment with vatreptacog alfa or rFVIIa as early-treated bleeds.
Evaluation of efficacy
Effective bleeding control
For the majority of bleeds (529/567; 93%), effective bleeding control was achieved with one to three doses of TP, and 56 of 69 treated patients (81.2%) had no treatment failures (Table 1). The ability of vatreptacog alfa to induce effective bleeding control (primary efficacy endpoint) was non-inferior to that of rFVIIa. In subsequent testing for superiority of the ability of vatreptacog alfa to induce effective bleeding control, vatreptacog alfa was found to be not superior to rFVIIa (P = 0.9703).
Table 1.
Main efficacy results*
| Number of bleeding episodes (%) | ||
|---|---|---|
| Vatreptacog alfa 80 μg kg−1 | rFVIIa 90 μg kg−1 | |
| Primary endpoint – effective bleeding control | ||
| Additional hemostatic agents given within 12 h (P = 0.9703) | ||
| Bleeding episodes | 340 (100.0) | 227 (100.0) |
| Yes | 22 (6.5) | 16 (7.0) |
| No | 318 (93.5) | 211 (93.0) |
| Secondary endpoint – sustained bleeding control* | ||
| Additional hemostatic agents given within 24 h (P = 0.0223) | ||
| Bleeding episodes* | 337 (100.0) | 225 (100.0) |
| Yes | 31 (9.2) | 31 (13.8) |
| No | 306 (90.8) | 194 (86.2) |
| Additional hemostatic agents given within 48 h (P = 0.0102) | ||
| Bleeding episodes* | 321 (100.0) | 214 (100.0) |
| Yes | 53 (16.5) | 51 (23.8) |
| No | 268 (83.5) | 163 (76.2) |
| Secondary endpoint – number of doses of trial product given per bleed | ||
| Bleeding episodes | 340 (100.0) | 227 (100.0) |
| Bleeds treated with one dose | 51 (15.0) | 23 (10.1) |
| Bleeds treated with two doses | 94 (27.6) | 62 (27.3) |
| Bleeds treated with three doses | 195 (57.4) | 142 (62.6) |
| Mean (SD), P = 0.0304 | 2.42 (0.74) | 2.52 (0.67) |
rFVIIa, recombinant factor VIIa; SD, standard deviation.
The P-values provided are for statistical tests performed for superiority of vatreptacog alfa over rFVIIa. †If a patient was treated for a new bleed in another location within 24–48 h, it was not possible to evaluate whether the effect of the original treatment was sustained over 24–48 h, as the new treatment would help to ensure that the effect was sustained. Secondary endpoints covering sustained bleeding control were therefore analyzed and summarized only for bleeds that were not followed by a new bleed at a different location within 24–48 h after the first trial product administration.
Effective and sustained bleeding control
For the majority of bleeds, effective and sustained bleeding control with no need for alternative hemostatic medication 24–48 h after initiation of treatment with TP was achieved with per-protocol treatment, including 90.8% and 83.5% of bleeds treated with vatreptacog alfa, and 86.2% and 76.2% of bleeds treated with rFVIIa, respectively (Table 1). Vatreptacog alfa was superior to rFVIIa in obtaining sustained bleeding control at 24 h and 48 h after the first TP administration (P = 0.0223 and P = 0.0102, respectively).
Number of doses of TP administered per bleeding episode
The mean number of doses administered to control bleeding within 9 h after the first TP administration was statistically significantly lower for vatreptacog alfa (2.42 doses) than for rFVIIa (2.52 doses; P = 0.0304; Table 1).
Change in pain assessments over time
Analysis of the subgroup of bleeding episodes for which baseline pain was recorded showed a significantly greater difference (reduction) in pain assessment over time for vatreptacog alfa at 6 h (P = 0.0042) and 9 h (P = 0.0399) after the initial dose than for rFVIIa, whereas no significant differences were seen at 1 h (P = 0.9759) or 3 h (P = 0.1397).
All primary and secondary efficacy evaluations were further analyzed according to the following subgroups: location of bleed (joint bleed [subdivided further into target joint bleed and non-target joint bleed], mucocutaneous, muscle, soft tissue and other); cause of bleed (traumatic or spontaneous); early (< 2 h from onset to treatment) vs. late (> 2 h from onset to treatment) treatment of bleeds; home treatment vs. hospital treatment; baseline pain assessment; and age group (adolescent, adult). This analysis did not indicate any relevant differences in primary and secondary efficacy outcome measures for any subgroup, and comparisons of vatreptacog alfa to rFVIIa in terms of efficacy was consistent across all subgroups analyzed.
Evaluation of safety
Overall, both vatreptacog alfa and rFVIIa were well tolerated, with a low frequency of adverse events (AEs). Most AEs were rated as mild non-serious events, and were judged by the investigator as unlikely to be related to TP. Except for immunogenicity, no safety signals were revealed by any of the general safety assessments, and no clinically relevant changes (i.e. changes judged to have an impact on the results and conclusions of the trial) were apparent in the safety parameters tested.
Among the 340 bleeds treated with vatreptacog alfa, a total of 40 AEs were reported during 31 bleeds (9.0%), including three serious AEs (SAEs) (dental caries, telangiectasia, and hematoma) evaluated as being not related to TP administration. Among the 227 bleeds treated with rFVIIa, 11 AEs were reported in association with 11 bleeds (4.8%), including four SAEs (fatal retroperitoneal hemorrhage, small intestinal obstruction, pyelonephritis, and rectal abscess) evaluated as not being related to TP administration, and one SAE evaluated as being possibly related to TP administration. This latter event occurred in a 53-year-old male with several comorbidities and an arteriovenous fistula (AVF) implanted as a venous access several years prior to enrollment in the trial. The patient suffered from a bleed in the palm of his hand, and treated himself, via the AVF, with three doses of TP. Approximately 20 h after the initial TP administration, the patient continued treatment with commercial rFVIIa and tranexamic acid via the same AVF. Shortly thereafter, a thrombus of the AVF was diagnosed, and the patient was withdrawn from the trial. No other signs or symptoms indicating thromboembolic events were reported in any patient during the trial.
Immunogenicity
A main secondary objective of the trial was to evaluate the immunogenicity of vatreptacog alfa (formation of anti-drug antibodies). Among 72 patients exposed, the number of vatreptacog alfa exposure days (EDs) ranged from 1 to 28, with a total of 17 patients who had ≥ 10 EDs (Fig. 2B). The total number of vatreptacog alfa doses administered in individual patients ranged from one to 80.
Binding antibodies specific for vatreptacog alfa were detected in eight patients (11%; age range, 14–72 years). On the basis of medical history, including evaluation of concomitant diseases and comedications, no medical condition judged to possibly predispose to the development of an anti-vatreptacog alfa immune response could be identified in any of the anti-drug antibody-positive patients. Baseline demographics, medical history, and baseline safety assessments did not reveal any differences between patients with or without anti-drug antibodies.
In seven of eight patients, the anti-drug antibodies developed after < 10 vatreptacog alfa EDs, and in one patient between visit sampling performed at 14 and 28 EDs. In four of eight patients, anti-vatreptacog alfa antibodies developed low-titer cross-reactivity against rFVIIa (Table 2). In one of the patients with cross-reacting binding antibodies, in vitro neutralizing activity against vatreptacog alfa was observed in a single blood sample at day 250 after the first vatreptacog alfa exposure, and after the patient had completed treatment with TP. No indication of reduced recovery of FVIIa activity 10 min postdose was seen in any of the patients with anti-drug antibodies. Tests for neutralizing activity against endogenous FVIIa gave negative results for all patients at all visits.
Table 2.
Overview of anti-vatreptacog alfa antibody development
| Patient | Vatreptacog alfa-binding antibody | Peak anti-vatreptacog alfa antibody titer | rFVIIa-binding antibody | Vatreptacog alfa-neutralizing antibody | FVIIa-neutralizing antibody |
|---|---|---|---|---|---|
| A | Positive | 16 | Negative | Negative | Negative |
| B | Positive | 4 | Negative | Negative | Negative |
| C | Positive | 4 | Negative | Negative | Negative |
| D | Positive | 1 | Negative | Negative | Negative |
| E | Positive | 256 | Positive | Negative | Negative |
| F | Positive | 256 | Positive | Negative | Negative |
| G | Positive | 64 | Positive | Negative | Negative |
| H | Positive | 64 | Positive | Positive* | Negative |
A sample was considered to be antibody-positive if it provided a result above the predefined cut-point in the specific assay (binding or neutralizing antibody assay).
An in vitro neutralizing effect was detected in one sample; subsequent samples tested negative.
One patient with negative test results for anti-vatreptacog alfa antibodies had negative results (just below the assay cut-point) for rFVIIa-binding antibodies at the time of inclusion and during the trial, and low-titer positive results (just above the assay cut-point) for anti-rFVIIa binding antibodies in one sample collected at the end of the trial.
No clinical manifestations of the antibodies were seen in any of the anti-drug antibody-positive patients, and all of these patients responded well to treatment with vatreptacog alfa and/or rFVIIa.
Discussion
Several approaches to improve treatment outcomes in hemophilic patients with inhibitors have been explored, including the administration of rFVIIa via alternative routes (e.g. subcutaneous injection) 11,12, and the development of modified rFVIIa molecules with improved therapeutic characteristics. Strategies have focused on extending the rFVIIa plasma half-life by PEGylation or protein fusion technology 13–17, and/or increasing the potency and rate of onset of hemostatic action of rFVIIa through rational and targeted protein modification 6,7,18–20.
Here, we report the results of a large, randomized, controlled phase III trial (adept™2), conducted with the goal of confirming the safety and assessing the efficacy of vatreptacog alfa in the treatment of bleeds in hemophilic patients with inhibitors.
In this randomized controlled trial, both vatreptacog alfa and rFVIIa showed a high efficacy, of 93%, in controlling bleeding at 12 h. Both drugs were highly effective in controlling bleeding episodes of all types (including joint, mucocutaneous, muscle, soft tissue and other bleeds). The observed clinical efficacy of rFVIIa was consistent with data from published clinical trials and experience 1–5,10,21–24, a finding that supports the choice of rFVIIa as an active control in the trial design. Vatreptacog alfa showed significantly better sustained bleeding control (24–48 h after the initial dose) and pain relief (6 h and 9 h after the initial dose) than rFVIIa, and fewer doses were required to control bleeds.
In the current trial, both rFVIIa and vatreptacog alfa were well tolerated, with a relatively low frequency of AEs (4.8% and 9.0%, respectively), and no clinical or laboratory safety signals were revealed by any of the general safety assessments.
The well-established safety profile of rFVIIa was confirmed in the adept™2 trial. rFVIIa has an amino acid sequence identical to that of endogenous FVIIa, and its risk of immunogenicity is very low. Throughout > 18 years of clinical use, no documented case of development of inhibitors (neutralizing antibodies) against rFVIIa has been reported in hemophilic patients with inhibitors. In contrast, in the current study, eight of 72 patients (11%) developed vatreptacog alfa-binding antibodies, revealing a strong immunogenicity safety signal not seen in previous phases of vatreptacog alfa clinical development. In seven of the eight patients, the anti-vatreptacog alfa antibodies developed after < 10 EDs. In four patients, the anti-vatreptacog alfa antibodies had low-titer cross-reactivity with rFVIIa, and one of these patients tested positive for anti-drug antibodies with in vitro neutralizing activity against vatreptacog alfa. This latter patient was not exposed further to vatreptacog alfa after detection of the neutralizing antibody activity, so it is not known whether the titer of the neutralizing activity would have increased further after additional exposures.
The development of anti-drug antibodies is a recognized risk of novel recombinant protein therapeutics 25. Nevertheless, the strong immunogenicity of vatreptacog alfa was unexpected, for several reasons: (i) vatreptacog alfa contains only three amino acid substitutions and has 99% identity with rFVIIa, which has been shown to have very low immunogenicity; (ii) no immunogenic risks were identified during the non-clinical development of vatreptacog alfa 26; and (iii) no anti-drug antibodies were detected in the completed phase I and II clinical trials 10,27. We note that the majority of the 45 patients enrolled in the phase II trial were exposed to vatreptacog alfa only once 10. The overall exposure to vatreptacog alfa was thus markedly higher in the phase III trial, but was still relatively limited, as 52 patients had ≤ 10 EDs. We therefore hypothesize that this difference in exposures led to the different immunogenicity evaluations obtained in the two trials. These findings also suggest that future trials evaluating rFVIIa variants should contain a sufficiently large number of patients and exposures to reliably evaluate clinical immunogenicity profiles.
Given the proven safety and efficacy of rFVIIa, and the anticipated risks associated with anti-drug antibody development following vatreptacog alfa treatment, Novo Nordisk decided to discontinue the vatreptacog alfa development program. This decision was based on the potential risk that continued treatment with vatreptacog alfa might have resulted in the formation of antibodies that would not only prevent further treatment with vatreptacog alfa, but also influence the efficacy of future treatment with rFVIIa, and, in a worst-case scenario, might cross-react with endogenous FVIIa. Interestingly, the clinical development of another sequence-modified rFVIIa, BAY 86-6150, which contains six amino acid changes as compared with native FVIIa 19,20, was recently terminated by the sponsor because of the development of neutralizing anti-drug antibodies 28. The challenges associated with the clinical development of new rFVIIa products, especially sequence-modified analogs, may be substantial. It will be essential to provide strong and direct evidence in sufficiently powered randomized controlled trials that rFVIIa variants are safe – mainly in terms of immunogenic and thrombogenic risks – and are capable of providing better treatment outcomes than the original rFVIIa.
Addendum
S. R. Lentz was the Global Principal Investigator: he enrolled patients in the study, and participated in the planning of the manuscript, data analysis, writing, and critical review and approval of the manuscript. S. Ehrenforth made a substantial contribution to the concept and design of the trial, analysis and interpretation of data, writing, and critical review and approval of the manuscript. K. Nana Weldingh established the validated antibody assay formats, analyzed and interpreted the antibody data, and was involved in the writing and design of the paper. F. Abdul Karim, J. Mahlangu, T. Matsushita, and J. Windyga enrolled patients in the study, and participated in the planning of the manuscript, data analysis and interpretation, and critical review and approval of the manuscript.
Acknowledgments
Editorial assistance to the authors during the preparation of this manuscript was provided by S. Eastwood (medical writer, PAREXEL) and was financially supported by Novo Nordisk in compliance with international guidelines for good publication practice. We thank the adept™2 investigators for their excellent participation in this trial. We also thank H. Friis Andersen (employed by Novo Nordisk A/S) for performing the statistical analyses.
Appendix
Participating principal investigators and sites
Austria: A. Weltermann. Brazil: E. de Paula and M. Cerqueira. Croatia: S. Zupancic-Salek. Greece: O. Katsarou and M. Economou. Hungary: L. Nemes and Z. Boda. Italy: E. Santagostino and G. Tagariello. Japan: H. Hanabusa, K. Fukutake, M. Taki, M. Shima, and T. Matsushita. Malaysia: F. Abdul Karim. Poland: M. Gorska-Kosicka and J. Windyga. Romania: M. Serban. Russian Federation: T. Andreeva. Serbia: A. Savic and I. Elezovic. South Africa: J. Mahlangu. Taiwan: W. Tsay and M. Shen. Thailand: A. Chuansumrit and P. Angchaisuksiri. Turkey: K. Kavakli, A. Kupesiz, and I. Sasmaz. UK: B. Madan, S. Rangarajan, and P. Giangrande. USA: K. Saxena, S. Lentz, M. Recht, C. Kempton, J. Barrett, G. Young, D. Quon, A. Stopeck, J. Lin, A. Ameri, S. Kaicker, P. Kuriakose, D. Obzut, M. Wang, I. Ortiz, and A. Sori.
Disclosure of Conflict of Interests
S. R. Lentz reports grants from Novo Nordisk during the conduct of the study, and personal fees from Novo Nordisk unrelated to the submitted work. S. Ehrenforth and K. N. Weldingh report personal fees from Novo Nordisk unrelated to the submitted work. T. Matsushita reports personal fees from Novo Nordisk during the conduct of the study; grants and personal fees from Baxter, Bayer and Kaketsuken; personal fees from Biogen/Idec, Novo Nordisk and GlaxoSmithKline; trial support from Chugai; and grants from Kyowa/Kirin unrelated to the submitted work. J. Windyga reports grants and non-financial support from Novo Nordisk during the conduct of the study, as well as grants from Baxter, Bayer, Biogen, LFB, Novo Nordisk, and Octapharma unrelated to the submitted work. J. N. Mahlangu has acted as an adviser to Amgen, Bayer, and Novo Nordisk, and has received research support from Bayer, Novo Nordisk, and Biogen.
References
- 1.Abshire T, Kenet G. Recombinant factor VIIa: review of efficacy, dosing regimens and safety in patients with congenital and acquired factor VIII or IX inhibitors. J Thromb Haemost. 2004;2:899–909. doi: 10.1111/j.1538-7836.2004.00759.x. [DOI] [PubMed] [Google Scholar]
- 2.Kavakli K, Makris M, Zulfikar B, Erhardtsen E, Abrams ZS, Kenet G NovoSeven trial (F7HAEM-1510) investigators. Home treatment of haemarthroses using a single dose regimen of recombinant activated factor VII in patients with haemophilia and inhibitors. A multi-centre, randomised, double-blind, cross-over trial. Thromb Haemost. 2006;95:600–5. [PubMed] [Google Scholar]
- 3.Young G, Shafer FE, Rojas P, Seremetis S. Single 270 microg kg(−1)-dose rFVIIa vs. standard 90 microg kg(−1)-dose rFVIIa and APCC for home treatment of joint bleeds in haemophilia patients with inhibitors: a randomized comparison. Haemophilia. 2008;14:287–94. doi: 10.1111/j.1365-2516.2007.01601.x. [DOI] [PubMed] [Google Scholar]
- 4.Astermark J, Donfield SM, DiMichele DM, Gringeri A, Gilbert SA, Waters J, Berntorp E. A randomized comparison of bypassing agents in hemophilia complicated by an inhibitor: the FEIBA NovoSeven Comparative (FENOC) Study. Blood. 2007;109:546–51. doi: 10.1182/blood-2006-04-017988. [DOI] [PubMed] [Google Scholar]
- 5.Parameswaran R, Shapiro AD, Gill JC, Kessler CM HTRS Registry Investigators. Dose effect and efficacy of rFVIIa in the treatment of haemophilia patients with inhibitors: analysis from the Hemophilia and Thrombosis Research Society Registry. Haemophilia. 2005;11:100–6. doi: 10.1111/j.1365-2516.2005.01075.x. [DOI] [PubMed] [Google Scholar]
- 6.Persson E, Kjalke M, Olsen OH. Rational design of coagulation factor VIIa variants with substantially increased intrinsic activity. Proc Natl Acad Sci USA. 2001;98:13583–8. doi: 10.1073/pnas.241339498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persson E, Olsen OH, Bjørn SE, Ezban M. Vatreptacog alfa from conception to clinical proof of concept. Semin Thromb Hemost. 2012;38:274–81. doi: 10.1055/s-0032-1302442. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh S, Ezban M, Persson E, Pendurthi U, Hedner U, Rao LV. Activity and regulation of factor VIIa analogs with increased potency at the endothelial cell surface. J Thromb Haemost. 2007;5:336–46. doi: 10.1111/j.1538-7836.2007.02308.x. [DOI] [PubMed] [Google Scholar]
- 9.Brophy DF, Martin EJ, Nolte ME, Kuhn JG, Barrett J, Ezban M. Factor VIIa analog has marked effects on platelet function and clot kinetics in blood from patients with hemophilia A. Blood Coagul Fibrinolysis. 2010;21:539–46. doi: 10.1097/MBC.0b013e32833b63e9. [DOI] [PubMed] [Google Scholar]
- 10.De Paula EV, Kavakli K, Mahlangu J, Ayob Y, Lentz SR, Morfini M, Nemes L, Šalek SZ, Shima M, Windyga J, Ehrenforth S, Chuansumrit A, 1804 (adept™1) investigators Recombinant factor VIIa analog (vatreptacog alfa [activated]) for treatment of joint bleeds in hemophilia patients with inhibitors: a randomized controlled trial. J Thromb Haemost. 2012;10:81–9. doi: 10.1111/j.1538-7836.2011.04549.x. [DOI] [PubMed] [Google Scholar]
- 11.Tiede A, Friedrich U, Stenmo C, Allen G, Giangrande P, Goudemand J, Hay C, Holmström M, Klamroth R, Lethagen S, McKenzie S, Miesbach W, Negrier C, Yuste VJ, Berntorp E. Safety and pharmacokinetics of subcutaneously administered recombinant activated factor VII (rFVIIa) J Thromb Haemost. 2011;9:1191–9. doi: 10.1111/j.1538-7836.2011.04293.x. [DOI] [PubMed] [Google Scholar]
- 12.Ludlam CA, Smith MP, Morfini M, Gringeri A, Santagostino E, Savidge GF. A prospective study of recombinant activated factor VII administered by continuous infusion to inhibitor patients undergoing elective major orthopaedic surgery: a pharmacokinetic and efficacy evaluation. Br J Haematol. 2003;120:808–13. doi: 10.1046/j.1365-2141.2003.04173.x. [DOI] [PubMed] [Google Scholar]
- 13.Yatuv R, Dayan I, Carmel-Goren L, Robinson M, Aviv I, Goldenberg-Furmanov M, Baru M. Enhancement of factor VIIa haemostatic efficacy by formulation with PEGylated liposomes. Haemophilia. 2008;14:476–83. doi: 10.1111/j.1365-2516.2008.01741.x. [DOI] [PubMed] [Google Scholar]
- 14.Moss J, Rosholm A, Lauren A. Safety and pharmacokinetics of a glycoPEGylated recombinant activated factor VII derivative: a randomized first human dose trial in healthy subjects. J Thromb Haemost. 2011;9:1368–74. doi: 10.1111/j.1538-7836.2011.04344.x. [DOI] [PubMed] [Google Scholar]
- 15.Ljung R, Karim FA, Saxena K, Suzuki T, Arkhammar P, Rosholm A, Giangrande P, for The Pioneer™1 Investigators 40K glycoPEGylated, recombinant FVIIa: 3-month, double-blind, randomized trial of safety, pharmacokinetics and preliminary efficacy in hemophilia patients with inhibitors. J Thromb Haemost. 2013;11:1260–8. doi: 10.1111/jth.12237. [DOI] [PubMed] [Google Scholar]
- 16.Dickneite G, Zollner S, Weimer T, Schürmann D, Schmidbauer S, Raquet E, Mueller-Cohrs J, Pragst I, Schulte S. Prolonged serum half-life of a recombinant fusion protein linking activated coagulation factor VII with albumin (FVIIa-FP) in different preclinical species. J Thromb Haemost. 2011;9(Suppl. 2):385. [Google Scholar]
- 17.Golor G, Bensen-Kennedy D, Haffner S, Easton R, Jung K, Moises T, Lawo J-P, Joch C, Veldman A. Safety and pharmacokinetics of a recombinant fusion protein linking coagulation factor VIIa with albumin in healthy volunteers. J Thromb Haemost. 2013;11:1977–85. doi: 10.1111/jth.12409. [DOI] [PubMed] [Google Scholar]
- 18.Toso R, Bernardi F, Tidd T, Pinotti M, Camire RM, Marchetti G, High KA, Pollak ES. Factor VII mutant V154G models a zymogen-like form of factor VIIa. Biochem J. 2003;369:563–71. doi: 10.1042/BJ20020888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sim DS, Koellnberger M, Gu JM, Bornaes C, Kragh M, Clausen SK, Murphy JE, Haaning J. BAY 86-6150, a novel recombinant factor VIIa variant, has a significantly higher therapeutic index than eptacog alfa (activated) in rabbit bleeding and thrombosis models. J Thromb Haemost. 2011;9(Suppl. 2):297. [Google Scholar]
- 20.Mahlangu JN, Coetzee MJ, Laffan M, Windyga J, Yee TT, Schroeder J, Haaning J, Siegel JE, Lemm G. Phase I, randomized, double-blind, placebo-controlled, single-dose escalation study of the recombinant factor VIIa variant BAY 86-6150 in hemophilia. J Thromb Haemost. 2012;10:773–80. doi: 10.1111/j.1538-7836.2012.04667.x. [DOI] [PubMed] [Google Scholar]
- 21.Schneiderman J, Rubin E, Nugent DJ, Young G. Sequential therapy with activated prothrombin complex concentrates and recombinant FVIIa in patients with severe haemophilia and inhibitors: update of our previous experience. Haemophilia. 2007;13:244–8. doi: 10.1111/j.1365-2516.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 22.Abshire T, Kenet G. Safety update on the use of recombinant factor VIIa and the treatment of congenital and acquired deficiency of factor VIII or IX with inhibitors. Haemophilia. 2008;14:898–902. doi: 10.1111/j.1365-2516.2008.01829.x. [DOI] [PubMed] [Google Scholar]
- 23.Salaj P, Ovesna P, Penka M, Hedner U. Analyses of recombinant activated factor VII treatments from clinical practice for rapid bleeding and acute pain control in haemophilia patients with inhibitors. Haemophilia. 2012;18:e409–11. doi: 10.1111/j.1365-2516.2012.02920.x. [DOI] [PubMed] [Google Scholar]
- 24.Chambost H, Santagostino E, Laffan M, Kavakli K ONE Registry Steering Committee on behalf of the investigators. Real-world outcomes with recombinant factor VIIa treatment of acute bleeds in haemophilia patients with inhibitors: results from the international ONE registry. Haemophilia. 2013;19:571–7. doi: 10.1111/hae.12140. [DOI] [PubMed] [Google Scholar]
- 25.Tamilvanan S, Raja NL, Sa B, Basu SK. Clinical concerns of immunogenicity produced at the cellular level by biopharmaceuticals following their parenteral administration in human body. J Drug Target. 2010;18:489–98. doi: 10.3109/10611861003649746. [DOI] [PubMed] [Google Scholar]
- 26.Sommer C, Norbert Jørgensen P, Salanti Z, Clausen JT, Jensen LB. Immunogenicity of novel recombinant human activated factor VII analogues on factor VII neonatally tolerized rats. Thromb Haemost. 2007;98:721–5. [PubMed] [Google Scholar]
- 27.Møss J, Scharling B, Ezban M, Møller Sørensen T. Evaluation of the safety and pharmacokinetics of a fast-acting recombinant FVIIa analogue, NN1731, in healthy male subjects. J Thromb Haemost. 2009;7:299–305. doi: 10.1111/j.1538-7836.2008.03253.x. [DOI] [PubMed] [Google Scholar]
- 28. Bayer Investor News: Bayer Provides Update on Phase II/III Trial of BAY 86-6150 BayerNews-20130503_0256. http://press.healthcare.bayer.com/en/press/news-details-page.php/15013/2013-0256. Accessed 25 June 2014.


