Abstract
Background
Previous studies indicate that many different aspects of attention are impaired in children diagnosed with developmental dyslexia (DD). The objective of the present study was to identify cognitive profiles of DD on the basis of attentional test performance.
Material/Methods
78 children with DD (30 girls, 48 boys, mean age of 12 years ±8 months) and 32 age- and sex-matched non-dyslexic children (14 girls, 18 boys) were examined using a battery of standardized tests of reading, phonological and attentional processes (alertness, covert shift of attention, divided attention, inhibition, flexibility, vigilance, and visual search). Cluster analysis was used to identify subtypes of DD.
Results
Dyslexic children showed deficits in alertness, covert shift of attention, divided attention, flexibility, and visual search. Three different subtypes of DD were identified, each characterized by poorer performance on the reading, phonological awareness, and visual search tasks. Additionally, children in cluster no. 1 displayed deficits in flexibility and divided attention. In contrast to non-dyslexic children, cluster no. 2 performed poorer in tasks involving alertness, covert shift of attention, divided attention, and vigilance. Cluster no. 3 showed impaired covert shift of attention.
Conclusions
These results indicate different patterns of attentional impairments in dyslexic children. Remediation programs should address the individual child’s deficit profile.
MeSH Keywords: Attention, Cluster Analysis, Dyslexia
Background
Developmental dyslexia (DD) is a language disorder defined as the specific reading impairments not caused by lowered intellectual abilities, lack of motivation, sensory acuity deficits, or inadequate education. Dyslexia may affect written text comprehension, recognition of written words, and ability to read aloud [1].
The cognitive causes of DD have not yet been elucidated. One of the most common theories postulates that dyslexia stems from a phonological deficit manifested by difficulties in manipulation, integration, or segregation of the speech sounds that form a word [2]. DD was also conceptualized as an auditory temporal processing deficit [3], impaired automatization of basic articulatory and auditory abilities due to cerebellar dysfunction [4], or an impairment of the magnocellular system resulting in blurred representation of visual stimuli such as letters [5,6]. The coexistence of 2 major, relatively independent dysfunctions in DD – phonological and cerebellar deficits – was also postulated [7,8].
The role of attentional deficit in DD has been a subject of debate. Hari and Renvall proposed a theory of pathophysiological sluggish attention shifting (SAS) in DD [9]. Accordingly, dyslexic readers display sluggish attentional capture and prolonged time of attentional focus that results from a right parietal lobe dysfunction [10–12]. Another theory proposed a limited visual attention span as a core cognitive deficit in DD [13]. It postulates a reduced number of discrete visual elements that can be processed in parallel, which interferes with the encoding of letter sequences, leading to reading difficulties. Furthermore, visual attention span disorder can predict reading abilities independently from phonological deficits [13].
It is not clear whether dyslexic readers have an overall attention impairment or are affected by more specific deficits. Studies show that children with DD perform poorly in some aspects of attention, whereas others do relatively well.
In keeping with the multidimensional nature of attention, several different aspects of attention have been identified. Posner has proposed 3 independent components of visual attention: alerting, orienting, and executive [14].
Alerting is defined as a preparatory phase to detect a target, and can be assessed using warning signals before the target presentation. Orienting attention is an ability to increase the general level of attention in anticipation of a known stimulus and it may be evaluated by a cue indicating a locus of a target. Executive attention refers to an ability to suppress irrelevant or conflicting information. Research shows that dyslexic readers perform poorly on the executive and orienting systems, but not on the alerting system [15–17]. The executive deficit in DD is manifested by an inability to suppress interfering information and difficulties in inhibition of inappropriate reactions [16,18–20]. The orienting component of attention is often investigated using the spatial cueing facilitation task, in which normal readers show a significant cueing effect (e.g., a peripheral visual cue) improves the accuracy when target and cue are presented at the same location. In contrast, this effect is not typically observed in dyslexic readers [17,21].
DD individuals also demonstrate a visuospatial deficit [22–33], often investigated using a visual search task in which a subject has to identify elements, like a letter or shape, in a background of similar elements [26,27,31,32]. Visual search deficit has often been considered a result of dysfunction of the magnocellular part of the visual system, which plays an important role in guiding visual attention [11,32,34]. There is, however, evidence for independence of an ability to visual search on the magnocellular functions. In one of the studies [31], children with DD responded as quickly as possible to the target (a black circle) presented among distractors (a black circle with a gap). In general, the DD group performed more slowly than controls, but when dyslexic children were divided into groups with or without a magnocellular deficit, none of the groups differed significantly from children with normal reading ability. The authors provided an alternative to the magnocellular system dysfunction explanation for poor performance on visual search tasks in DD. Accordingly, a target (e.g., a letter) is degraded or masked by non-targets in the surroundings and this could be due to difficulty inhibiting stimuli that are not the focus of attention.
Visual scanning in DD was also investigated using alphabet characters as search stimuli. In a series of experiments conducted by Casco et al. [32], children with DD who showed the most profound deficits in the cancellation task also had significantly slower reading rate and committed more errors during reading than children who performed best in the task. The authors concluded that a related difficulty in visual search and reading might be due to a deficit in the visual selective attention mechanism.
There is also evidence of impaired divided attention in DD [35]. In this study [35] young adults with DD performed 2 tasks concurrently: Serial Reaction Time (SRT) and tone counting tasks. The SRT test was conducted over 1 practice session corresponded to an acquisition phase of learning and a second session, 24 h later, which was used to investigate the consolidation process. In contrast to normal readers, the DD group showed a delayed acquisition of the motor skill in SRT and no effect of enhanced consolidation. The results indicate less effective sequence learning under the condition of divided attention in dyslexic readers.
Considering the variety of attentional problems in dyslexic readers, it seems reasonable to search for subtypes of DD characterized by distinguishable impairments. Therefore, the main purpose of this study was to identify distinct patterns of attentional deficits in children with DD. This approach might provide more information on the nature of attentional dysfunction in DD and have important implications for development-specific, and thus more effective, therapies.
In the present study we used well-established tests to assess the following attention functions: alertness, covert shift of attention, divided attention, flexibility, inhibition, vigilance, and visual search. These attentional processes were derived from the neuropsychological models proposed by Posner and Petersen [14] and Manly et al. [36]. A cluster analysis was performed to determine different profiles of attentional deficits in DD.
Material and Methods
Participants
The study sample consisted of 78 Polish primary school children diagnosed with dyslexia (30 girls, 48 boys, mean age of 12 years ±8 months, age range: 9 years 10 months to 12 years 6 months), and 32 children without reading difficulties (14 girls and 18 boys, mean age of 11 years ±9 months, age range: 10 years to 12 years 6 months). Dyslexic and control groups were not significantly different in chronological age (z=.22, p>.05), total IQ (z=1.50, p>.05), verbal and nonverbal IQ (z=1.93, p>.05 and z=.45, p>.05, respectively), Digit Span Forward (z=1.50, p>.05), and Digit Span Backward (z=1.68, p>.05). There were also no significant gender differences between DD and control children (z=.15, p>.05).
Children with DD were recruited by an experienced multidisciplinary team in 4 Psychological and Educational Clinics in Warsaw. They were diagnosed using the Polish normalized test battery for diagnosis of dyslexia in either third [37] or fifth grades [38] and the Wechsler Intelligence Scale for Children – Revised (WISC-R) [39]. Children without reading difficulties were recruited from 4 primary schools in Warsaw. All participants had a similar social and educational background, were right-handed [40], attended school regularly, had normal IQ (>90), and had no history of neuropsychiatric disorders or head trauma. None of the children had diagnosis of ASD, ADD/ADHD, or SLI, and none displayed any symptoms of these disorders. Each participant had hearing in both ears ≤20 dB HL for each of the following frequencies: 125, 250, 500, 750, 1000, 1500, 2000, 4000, and 8000 Hz (standard tonal audiometry) and normal or corrected-to-normal vision.
Parents of each child provided written informed consent for participation in this study. The study was approved by the ethics committee at the Institute of Physiology and Pathology of Hearing and conformed to the Declaration of Helsinki for research on humans.
Materials and procedure
Children with DD performed the tests in the Institute of Physiology and Pathology of Hearing and control children at their schools or in the Institute of Physiology and Pathology of Hearing. Children were tested individually, in a quiet room. The tasks were performed in 2 sessions of approximately 2 hours each, with a break of a few days between sessions. The order of tests was counterbalanced over participants.
Reading
Reading abilities were assessed reading both real words and artificial words. In the Real Word Reading, the child should read aloud a list of 89 real Polish words as quickly as possible. The number of correctly read words in 60 s is recorded. In the Artificial Word Reading, the child is asked to read aloud a list of 71 artificial words as quickly and accurately as possible. The number of correctly read words in 60 s is calculated. Both these tests are included in the Polish normalized test battery for diagnosis of dyslexia in the third grade [38].
Phonological skills
Phonological skills were assessed using the Phonological Awareness Test [41], which includes 4 tasks. The first is to split a single word into syllables and add the syllable “ka” before each syllable of the word (e.g., a word /rano/ (morning) is pronounced by an experimenter and the child should say: /ka-ra/ – /ka-no/). The second task is to decipher coded words (e.g., a response to the word: /ka-pa/ – /ka-sek/ should be: /pasek/ (a belt)/). During the last 2 parts of the test, the word coding using “ka” was done at the sentence level. Specifically, the child was asked to add “ka” before each syllable of each word in a sentence. For example, after hearing a sentence: /Pada deszcz/ (It’s raining) the child should say: /kapa/ – /kada/ – /kadeszcz/. Finally, the fourth task was to decipher coded words in a sentence, for example, to say: /Mama gotuje obiad/ (Mother is cooking the dinner) in response to the coded sentence provided by an experimenter: /Kama/ – /kama/ – /kago/ – /katu/ – /kaje/ – /kao/ – /kabiad/. The percentages of correctly coded syllables and correctly recognized deciphered words are recorded.
Attention
The Alertness, Covert Shift of Attention, Divided Attention, Flexibility, Go/NoGo, and Vigilance tests from the computerized Test of Attentional Performance (TAP) battery [42], as well as D2 Attention Assessment Test [43], were administered to evaluate different aspects of attention. Each test was preceded by a training session to familiarize the child with the procedure.
Alertness is examined under 2 conditions. In the first condition, the child is asked to press a key as quickly as possible to a white cross appearing at the center of the screen at randomly varying intervals. The task measures tonic arousal, which refers to the general state of wakefulness. In a second condition, reaction time is recorded in response to a white cross preceded by a warning tone. This test evaluates phasic alertness, defined as transient focusing of attention on an anticipated event. The mean reaction time and the total number of errors (omissions and anticipations) are calculated separately for each condition.
The Covert Shift of Attention refers to ability to focus attention on part of the surrounding space. The task is to press the key as quickly as possible when the target cross appears on the left or on the right side of the screen. Before each cross, a cue (an arrow pointing either to the right or to the left) appears, which indicates the expected side of the target,. The cue is correct in 80% of the trials and incorrect in 20%. Following an incorrect cue, attentional focus is initially shifted to the cued side, after which there is a new shift of focus to the actual occurrence of the target stimulus. The mean reaction times and the number of errors in trials with valid and invalid cues are recorded.
In the Divided attention test, a visual and an auditory task are performed in parallel. In the visual task, a quadratic field of dots (4×4) appears at the center of the screen, indicating 16 positions at which 6–8 crosses appear. The child is asked to press the response key as quickly as possible when 4 crosses form the corners of a small square. In the auditory task, a high and a low tone are presented alternately in sequence. The child presses the key as quickly as possible when the same tone occurs twice in a row. The total number of missed signals and average reaction time on the visual and auditory tasks are recorded.
The Flexibility test measures ability to change perceptual set easily. A letter and a number are simultaneously presented on the right and left of the center of the screen. The task is to press the left or right key according to whether the letter appears on the left or the right of the center. The test comprises 50 trails. The total number of errors and mean reaction time are recorded.
The Go/NoGo test measures an ability to perform a certain task under time pressure and to inhibit inappropriate behavioral reactions. Two types of stimuli – 20 plus symbols (“+”) and 20 crosses (“×”) – are presented sequentially in the center of the screen. The child is asked to press the key as quickly as possible whenever the cross appears. No reaction is required in response to the plus symbol. The total number of errors and mean reaction time are calculated.
The Vigilance test examines ability to focus and maintain attention over a long period of time. Two squares are presented one above the other in the center of the screen, between which a grate pattern moves up and down. The task is to press the key as quickly as possible whenever the “grate” appears twice in a row in the same square. The number of errors and mean reaction time are calculated.
The D2 Attention Assessment Test measures ability to explore the visual environment in search of the target stimuli. The task consists of the letters “d” and “p” with 1–4 dashes, arranged either individually or in pairs above and below the letter. The task is to scan across each line in order to identify and cross-out each “d” with 2 dashes. The test comprises 14 lines (47 letters per a line). The child has 20 s for searching a line. The total number of errors and cancelled letters (both correctly and incorrectly) are recorded. The number of errors refers to the correctness level and cancelled letters are a measure of general processing speed.
Data analyses
Since some variables did not have a normal distribution and there was a considerable difference in the number of participants between the dyslexic and the control group, a non-parametric test (Mann-Whitney U test) was used for between-group comparisons.
To reduce the data set before exploring the subtypes of DD, a principal component analysis (PCA) with a varimax rotation was conducted in dyslexic children on the data obtained in attentional tests. Only variables with normal distribution were included. Normality was tested for each variable using the Kolmogorov-Smirnov test. Because the analyzed variables were measured on different scales (e.g., percentages and milliseconds) prior to the PCA, the data were standardized. All factor loadings greater than ±.50 were used for interpretation.
The existence of subtypes of dyslexia was tested with a 2- step cluster analysis performed on factors identified in the PCA. The analysis was run for a maximum of 15 clusters, log-likelihood distance estimation, Akaike’s information criterion, no noise-handling for outlier treatment, initial distance change threshold of 0, a maximum of 8 branches per leaf node, and a maximum of 3 tree depth levels. The cluster analysis was conducted on the factors previously extracted in the PCA.
One-way ANOVA and tests of contrasts were used to compare the variables between dyslexic clusters and the control group. All statistical analyses were conducted with SPSS 20 for Windows.
Results
Between-group comparisons
Not surprisingly, children with DD achieved significantly lower scores than the control group in the Real Word Reading, Artificial Word Reading, and Phonological Awareness tests (Table 1).
Table 1.
Neuropsychological test performance (median, minimum and maximum values) in dyslexic and control group as well as z values for between-groups comparisons (Mann-Whitney’s test). The statistically significant differences are marked with asterisks.
| Variable | Dyslexia (n=78) | Control group (n=32) | z value |
|---|---|---|---|
| Me (min–max) | Me (min–max) | ||
| Total IQ | 99.5 (90–122) | 101 (91–119) | 1.50 |
| Verbal IQ | 98.5 (80–126) | 100.5 (84–120) | 1.93 |
| Non-verbal IQ | 100 (79–125) | 102 (80–134) | .45 |
| Real Word Reading | 51 (13–78) | 77 (46–89) | 6.35*** |
| Artificial Word Reading | 27 (5–57) | 51.5 (28–70) | 6.53*** |
| Digit Span Forward | 5 (2–8) | 5.5 (2–9) | 1.50 |
| Digit Span Backward | 4 (1–6) | 4 (2–8) | 1.85 |
| Phonological Awareness Test | 64 (15–89) | 77.5 (60–95) | 4.80*** |
| Alertness without warning signal (errors) | 2 (0–7) | 2 (0–8) | .16 |
| Alertness without warning signal (MRT) | 271.5 (199–479) | 248.5 (184–326) | 3.24*** |
| Alertness with warning signal (errors) | 5 (0–46) | 4 (1–21) | 1.09 |
| Alertness with warning signal (MRT) | 247.5 (183–411) | 238 (177–411) | 3.04** |
| Covert Shift of Attention – valid cues (errors) | 2 (0–26) | 1 (0–4) | 2.88** |
| Covert Shift of Attention – valid cues (MRT) | 513.5 (323–908) | 478.5 (255–713) | 2.20* |
| Covert Shift of Attention – invalid cues (errors) | 3 (0–30) | 3 (0–13) | 1.94 |
| Covert Shift of Attention – invalid cues (MRT) | 581 (323–964) | 502.5 (305–799) | 2.48* |
| D2 Attention Assessment Test (errors) | 29 (8–70) | 8 (1–40) | 6.51*** |
| D2 Attention Assessment Test (letters) | 343 (224–539) | 416 (331–653) | 5.25*** |
| Divided Attention (errors) | 7 (2–22) | 7 (0–12) | .82 |
| Divided Attention (MRT) | 904 (624.5–1645.5) | 613 (454–1503) | 4.75*** |
| Flexibility (errors) | 3 (0–20) | 3 (0–5) | 1.01 |
| Flexibility (MRT) | 589.5 (406–1075) | 546.5 (314–900) | 2.04* |
| Go/NoGo (errors) | 4 (0–14) | 3 (0–11) | 1.72 |
| Go/NoGo (MRT) | 489 (293–790) | 449 (342–714) | 1.03 |
| Vigilance (errors) | 2 (0–14) | 1 (0–8) | 1.71 |
| Vigilance (MRT) | 767 (450–1487) | 704.5 (431–1083) | 1.86 |
p<0.05;
p<0.01;
p<0.001;
MRT – mean reaction time.
Considering the attentional functions, dyslexic children performed poorly on the Alertness, Divided Attention, and Flexibility tests (longer reaction times), as well as on the Covert Shift of Attention task (longer reaction times in trials with both valid and invalid cues, more errors in trials with valid cues). They also had significantly more errors and less cancelled letters than the control children in the D2 Attention Assessment test. There were no significant between-group differences in performance on the Go/NoGo and Vigilance tests. Detailed results are shown in Table 1.
Principal component analysis (PCA)
The PCA was calculated only on the variables with a normal distribution – mean reaction times in the Alertness (trials with and without warning signal), Covert Shift of Attention (trials with valid and invalid cues), Divided Attention, Flexibility, Go/NoGo, and Vigilance tasks, as well as the number of errors and cancelled letters in the D2 Attention Assessment test.
This PCA revealed a 5-factor solution. The first factor – “Alertness” – accounted for 19.78% of the variance (eigenvalue=1.98) and received high loading from the Alertness test. The second factor – “Covert shift of attention” – accounted for 19.13% of the variance (eigenvalue=1.91) and received input from the Covert Shift of Attention task. The third factor – “Attentional shifting” – explained 18.85% of the variance (eigenvalue=1.89) and obtained high loadings from the Flexibility, Go/NoGo, and Divided Attention tests. The fourth factor – “Visual search”—accounted for 13.41% of the variance (eigenvalue=1.34) and received input from the D2 Attention Assessment test. The fifth factor – “Vigilance” – explained 10.84% of the variance (eigenvalue=1.08) and received high loading from the Vigilance task. All these factors cumulatively accounted for 82.03% of the variance. The rotated factor loadings are presented in Table 2.
Table 2.
Principal component analysis showing rotated factor loadings on different aspects of attention. The factor loadings greater than .50 are written in bold font.
| Factor loadings | |||||
|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | |
| Alertness with signal (MRT) | .904 | .184 | .100 | .024 | .011 |
| Alertness without signal (MRT) | .890 | .147 | .080 | .074 | .217 |
| Covert Shift of Attention – valid cues (MRT) | .105 | .957 | −.005 | .060 | .044 |
| Covert Shift of Attention – invalid cues (MRT) | .196 | .935 | .083 | .080 | .002 |
| Flexibility (MRT) | .045 | −.070 | .901 | .063 | −.037 |
| Go/NoGo (MRT) | .171 | .143 | .833 | .054 | −.117 |
| Divided Attention (MRT) | −.432 | .066 | .531 | .061 | .509 |
| D2 Attention Assessment Test (errors) | −.090 | .186 | −.064 | .833 | −.049 |
| D2 Attention Assessment Test (letters) | −.179 | .056 | −.199 | −.787 | −.083 |
| Vigilance (MRT) | .250 | .022 | −.171 | .010 | .867 |
MRT – mean reaction time.
Clusters of children with DD
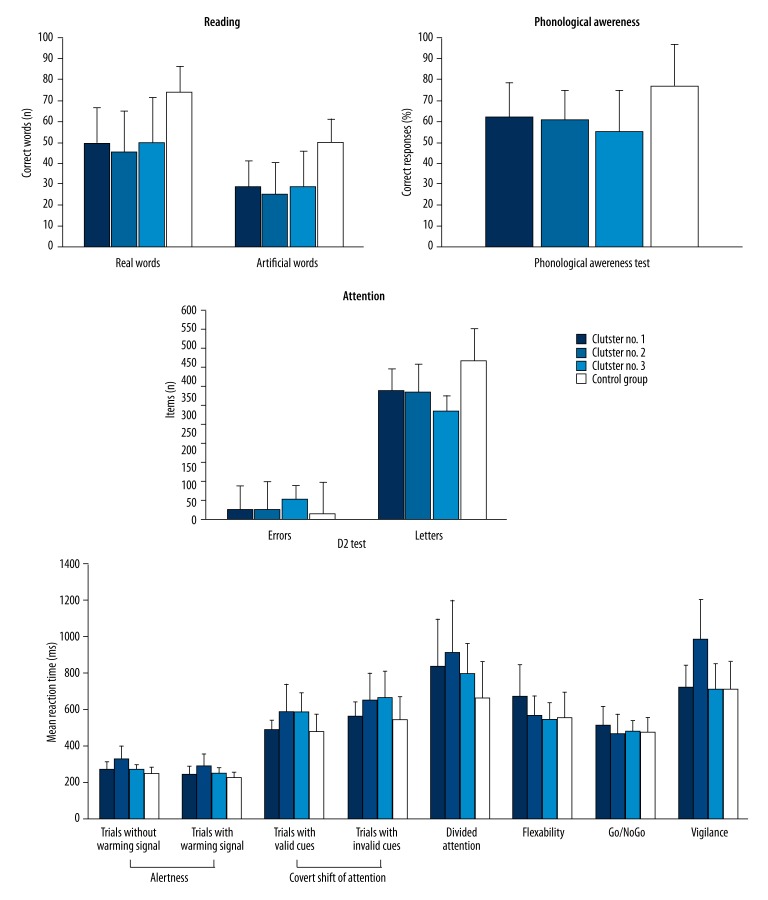
Two-step cluster analysis revealed 3 clusters (Figure 1). Cluster 1 included 43 children, cluster 2 included 20 children, and cluster 3 included 15 children. There were no significant differences between the clusters and the control group in gender (z=.94, p>.05), chronological age (F(3,109)=2.60, p>.05), total IQ (F(3,109)=2.15, p>.05), and verbal and nonverbal IQs (F(3,109)=1.76, p>.05 and F(3,109)=1.59, p>.05, respectively), or Digit Span Forward (F(3,109)=1.03, p>.05) and Digit Span Backward tests (F(3,109)=1.95, p>.05).
Figure 1.
Reading, phonological, and attentional tests performance (mean and standard deviation values) in dyslexic clusters and the control group.
The clusters significantly differed from the control group in the number of scores achieved in the Real Word Reading (F(3,109)=17.30, p<.001) and Artificial Word Reading (F(3,109)=22.90, p<.001), as well as in the Phonological Awareness test (F(3,109)=10.16, p<.001). There were also significant differences between dyslexic clusters and the control group in mean reaction time in the Alertness (F(3,109)=11.52, p<.001 for trials without warning signal and F(3,109)=9.89, p <.001 for trials with warning signal), Divided Attention (F(3,109)=5.35, p<.01), Flexibility (F(3,109)=5.52, p<.01), Covert Shift of Attention in trials with both valid (F(3,109)=8.94, p<.001) and invalid cues F(3,109)=5.92, p<.001), as well as in the Vigilance tests (F(3,109)=16.63, p<.001). Significant differences in the number of errors were found in the Vigilance (F(3,109)=4.22, p<.01) and in trials with valid cue in the Covert Shift of Attention tasks (F(3,109)=3.16, p<.05). In the D2 Attention Assessment test, dyslexic clusters and the control group were significantly different in the number of errors and cancelled letters (F(3,109)=61.15, p<.001 and F(3,109)=15.47, p<.001, respectively).
There were no significant differences between the clusters and control group in the Go/NoGo test (F(3,109)=1.57, p>.05 for errors and F(3,109)=1.61, p>.05 for mean reaction time).
Characteristics of the clusters
The detailed characteristics of particular clusters of DD and the control group are shown in Figure 1 and Table 3.
Table 3.
Neuropsychological test performance (means and standard deviations) of dyslexic clusters and the control group and t statistic values for between – group comparisons. The statistically significant differences are marked with asterisks.
| Variable | Mean (standard deviation) | t value | |||||
|---|---|---|---|---|---|---|---|
| Cluster no. 1 | Cluster no. 2 | Cluster no. 3 | Control group | Cluster no.1 vs. control group | Cluster no. 2 vs. control group | Cluster no. 3 vs. control group | |
| Total IQ | 101.37 (7.88) | 101 (7.88) | 97.13 (5.30) | 103.25 (8.27) | – | – | – |
| Verbal IQ | 98.88 (10.46) | 98.15 (10.76) | 97.87 (8.90) | 103.47 (10.89) | – | – | – |
| Nonverbal IQ | 102.72 (11.61) | 103.80 (7.98) | 96.60 (8.22) | 103.69 (13.36) | – | – | – |
| Digit Span Forward | 4.95 (1.38) | 4.95 (1.57) | 4.73 (1.67) | 5.47 (1.78) | – | – | – |
| Digit Span Backward | 3.88 (1.05) | 3.65 (1.27) | 3.40 (1.18) | 4.22 (1.29) | – | – | – |
| Real Word Reading | 49.49 (17.34) | 45.80 (19.21) | 49.93 (21.38) | 74.06 (11.91) | 6.21*** | 5.85*** | 4.55*** |
| Artificial Word Reading | 29.19 (12.01) | 25.75 (14.24) | 29.20 (16.45) | 50.28 (10.59) | 7.10*** | 6.86*** | 5.29*** |
| Phonological Awareness Test | 62.40 (16.38) | 61.45 (13.39) | 54.73 (20.16) | 76.94 (9.54) | 4.21*** | 3.67*** | 4.80*** |
| Attention | |||||||
| Alertness without warning signal (errors) | 1.74 (1.18) | 1.75 (1.02) | 2.07 (.88) | 1.94 (1.52) | – | – | – |
| Alertness without warning signal (MRT) | 269.58 (45.26) | 325.5 (70.41) | 271.73 (27.91) | 248.25 (35.63) | 1.96 | 5.81*** | 1.61 |
| Alertness with warning signal (errors) | 7.3 (7.89) | 7.9 (5.34) | 6.87 (3.94) | 6.22 (5.34) | – | – | – |
| Alertness with warning signal (MRT) | 245.51 (37.78) | 289.35 (62.18) | 249.87 (32.32) | 228.69 (23.72) | 1.82 | 5.39** | 1.71 |
| Covert shift of attention – valid cues (errors) | 2.47 (2.68) | 1.85 (1.18) | 4.07 (6.24) | 1.34 (1.0) | 1.64 | .61 | 2.98** |
| Covert shift of attention – valid cues (MRT) | 488.26 (50.77) | 583.15 (151.88) | 586.53 (103.66) | 478.41 (92.21) | .44 | 3.86*** | 3.63*** |
| Covert shift of attention – invalid cues (errors) | 5.16 (5.9) | 4.35 (2.89) | 5.33 (7.06) | 3 (2.63) | – | – | – |
| Covert shift of attention – invalid cues (MRT) | 562.47 (78.35) | 645.3 (147.29) | 661.6 (143.29) | 540.63 (128.18) | .80 | 3.12** | 3.29*** |
| D2 Attention Assessment Test (errors) | 26.21 (11.38) | 27.9 (10.49) | 56.07 (11.61) | 11.19 (9.01) | 6.06*** | 5.53*** | 13.52*** |
| D2 Attention Assessment Test (letters) | 363.58 (66.57) | 361.55 (76.41) | 310.27 (36.93) | 450.44 (90.64) | 5.07*** | 4.25*** | 6.1*** |
| Divided Attention (errors) | 7.21 (3.92) | 8.95 (4.88) | 7.13 (2.77) | 6.59 (3.09) | – | – | – |
| Divided Attention (MRT) | 838.21 (255.38) | 908.15 (281.73) | 794.6 (164.7) | 665.19 (192.75) | 3.17** | 3.65*** | 1.77 |
| Flexibility(errors) | 3.02 (2.99) | 4.05 (2.95) | 2.67 (1.5) | 2.56 (1.19) | – | – | – |
| Flexibility(MRT) | 669.14 (173.24) | 569.6 (104.44) | 546.07 (86.86) | 553.78 (136.34) | 3.47*** | .39 | .17 |
| Go/NoGo (errors) | 4 (2.94) | 4.95 (2.37) | 5.13 (3.72) | 3.53 (2.76) | – | – | – |
| Go/NoGo (MRT) | 512.93 (103.99) | 469.2 (103.13) | 481.47 (55.94) | 473.53 (79.71) | – | – | – |
| Vigilance (errors) | 2.4 (2.28) | 4.5 (3.86) | 2.53 (2.26) | 2.00 (2.11) | .65 | 3.39*** | .66 |
| Vigilance (MRT) | 717.49 (120.88) | 979.95 (218.66) | 706.4 (138.35) | 709.84 (149.56) | .21 | 6.19*** | .08 |
p <.05;
p <.01;
p <.001;
MRT – mean reaction time.
Children in cluster 1 had significantly more errors and less cancelled letters in the D2 Attention Assessment test, and had longer reaction times compared to the control group in the Divided Attention and Flexibility tests.
In comparison to non-dyslexic children, cluster 2 had more errors and less cancelled letters in the D2 Attention Assessment test, longer reaction times in the Alertness, Divided Attention, and Covert Shift of Attention tests, and longer reaction time and more errors in the Vigilance task.
Cluster 3 performed worse than the control group in the D2 Attention Assessment test (more errors and less cancelled letters) and in the Covert Shift of Attention task (longer reaction times in trials with valid and invalid cues accompanied by more errors in trials with valid cues).
Discussion
The main issue addressed to the study concerns the nature of attentional deficits associated with DD. Compared to age- and gender-matched controls, dyslexic children showed impairments in tasks requiring visual search, alertness, covert shift of attention, divided attention, and flexibility but not in those involving inhibition or vigilance (Table 1). Since there were no significant differences between dyslexic and control groups in chronological age, gender, intellectual abilities, and short-term memory span, the impaired performance on attentional tests in DD cannot be attributed to these factors.
In the present study, attentional problems in DD coexisted with reduced phonological awareness, which is postulated as one of the main cognitive causes of dyslexia [2,44]. Our results might also support the cerebellar deficit hypothesis, which argues that cerebellar abnormalities cause the impaired automatization of articulatory skills and auditory processes leading to the reading problems [4,45–47]. Nicolson and Fawcett proposed 2 mechanisms by which the cerebellum might be involved in DD [47]. One mechanism assumes that cerebellar dysfunction during infancy causes mild motor problems that lead to articulation difficulties, impaired sensitivity to the phonemic structure of language, and to the phonological deficit. The other mechanism assumes that a cerebellar dysfunction leads to reduced processing speed, which would affect a broad spectrum of cognitive functions, including attention.
In the present study, children with DD had longer reaction times than their normal-reading peers in tests measuring different aspects of attention (Table 1). Thus, a slowing of processing speed as a consequence of the cerebellar dysfunction seems to provide a reasonable explanation for reduced performance on the attentional tasks in dyslexia.
Since prefrontal and posterior parietal cortices are considered brain structures crucial for attention [48–50], the close anatomical connections to the cerebellum indicate a cerebellar involvement in this function. A dysfunction of the cerebro-cerebellar circuit has been previously found in ADHD [51], and patients with cerebellar lesions have attentional problems [52]. The cerebellum is considered a brain structure that “prepares internal conditions […] by repositioning sensory receptors; by altering cerebral blood flow levels; by enhancing neural signal to noise; by enhancing neural responsiveness in hippocampus, thalamus, and superior colliculus; by modulating motor control systems” [53 p. 2]. Therefore, sensory processing and motor and attentional performance are facilitated by these preparations.
General or specific deficits in DD?
The present study indicates specific difficulties rather than an overall attentional impairment in DD. The interpretation of the results is, however, not straightforward.
Previous studies indicate specific, not global, attentional impairments in DD [15,16,54]. Marzocchi et al. [54] found that dyslexic children performed poorly on sustained and executive attention but not in selective and orienting attention tasks. Bednarek et al. [16] provided evidence of an impaired executive component of attention and preserved alerting and orienting systems. Buchholz and Davies [15] found deficits in the orienting and executive components of attention in dyslexic adults, and the alerting system appeared to be intact. Within this framework, our study showing impaired alertness, attentional shifting, divided attention, and visual search – but not vigilance and inhibition – processes, provide further evidence of specific attentional deficits in dyslexic children.
We found that DD readers had longer reaction times than the control group in tests measuring tonic and phasic alertness. Impaired alertness, which is general wakefulness that enables a person to respond quickly and appropriately to a given demand [42], might influence every attention performance. The visual search task is timed and thus is affected by processing speed. The Covert Shift of Attention, Divided Attention, and Flexibility tests also require quick reactions to presented stimuli. Because in our study dyslexic children experienced difficulties not only in tasks involved a more complex cognitive process, where a selective response to relevant stimuli is required, but also in a simple reaction time task (Alertness), the most likely explanation for these findings could be a general motor slowness in DD.
Petersen and Posner [55] proposed a right hemispheric noradrenergic alerting network in the locus coeruleus in the brainstem as the origin of the norepinephrine system [56], as well as the frontal and parietal areas. Further, research indicates right lateralization of the processes involved in tonic alertness and left hemisphere mechanisms of phasic alertness [57]. Because in our study children with DD performed worse than controls in both types of alertness tests, we might consider such deficits as resulting from dysfunction of the right and left hemispheric attentional network in DD.
The present study provides evidence of flexibility and divided attention dysfunctions in dyslexic children. Theoretically, these deficits might affect the reading process. Flexibility of attention is necessary for shifting focus from word to word during reading, and ability to pay attention to several things at once (divided attention) is necessary for the reading process because in DD it is not automatic and must be consciously controlled.
In our study, children with DD differed significantly from controls in the computerized Flexibility task, but no differences between groups were observed in the Go/NoGo task. These results are in accordance with those provided by Reiter et al. [18], who used exactly the same computerized tests as we did. The Go/NoGo task appeared to be relatively easy for dyslexic children and, therefore, might not be an adequate measure of inhibition deficits in DD, particularly since results from the Flexibility task might indicate a reduced ability to suppress irrelevant responses in the dyslexic group.
Although children with DD made a number of errors comparable to non-dyslexic children in the Flexibility task, the mean reaction times they achieved were significantly longer. Thus, the interpretation of the results in terms of impaired ability to shift attention or suppress irrelevant reactions is not obvious. Considering the scores on other attentional tasks obtained in our study by dyslexic children, it is more likely that poorer performance on the Flexibility test results from a general motor slowness in this group.
Similarly, in the present study, children with DD had significantly longer reaction times in the Divided Attention test than their normal reading peers, but the groups were not significantly different in the number of errors in this task. Again, when interpreting the results of the test, one should consider a slowed processing speed as affecting the performance on the Divided Attention task.
In the present study, children with DD also showed deficits in covert attentional shifting. Difficulties in changing perceptual set may result in ineffective integration of letters in words alone and words in sentences, leading to reading difficulties. Specifically, the dyslexic group had longer reaction times compared to normal-reading controls in trials with valid and invalid cues. In general, in the valid cue condition, reaction times are faster than in the invalid cue condition [22], as was found in our study in the dyslexic and control groups. Attention directed to a visual field by the valid cue facilities selection of information in that region, whereas the invalid cue condition corresponds to attentional inhibition (suppressed processing at an unselected location). The present study shows that both these functions – attentional facilitation and inhibition – were impaired in children with DD.
According to Posner and Petersen [14], the covert shift of attention depends on the magnocellular system, which is responsible for timing visual events and projects to the occipital and parietal cortex. Therefore, our results also support the existence of magnocellular deficit in DD [6].
We found impairments in visual search in dyslexic children. A possible explanation of these results comes from SAS theory [9], according to which the attention-related deficit in processing of rapid sequences is caused by parietal lobe damage. SAS postulates an abnormally long attentional dwell time in persons with DD, which might lead to difficulties with fast disengaging from one visual stimulus to another. Therefore, such a deficit may result in less accurate performance on the visual search tasks.
In the present study, children with DD were asked to perform visual scanning of letters. Thus, interpretation of poorer performance on the visual search task might be rather complicated, considering an impaired reading ability and familiarity with letters in dyslexic readers. Visual search performance might be also influenced by working memory, which is necessary to keep representation of the target stimulus active while performing the search task. This may affect search strategies and result in slower search time.
DD readers were not significantly different from the control group in the Vigilance test in our study (Table 1). Dyslexic children were previously found to be impaired in continuous performance tasks, which are used as a measure of sustained attention [58]. However, a lack of deficits in maintaining attentional focus over a longer period of time in dyslexic readers was also reported [59]. This discrepancy in the results might be due to the fact that impairments in vigilance become more apparent with an increasing level of task difficulty (e.g., when multiple objects must be attended) [60]. Nicolson and Fawcett [61] showed that dyslexic children performed as well as the controls in simple reaction time to tones but not to choice reaction time to 2 stimuli (tone and flash). Thus, the increased task complexity could account for the discrepancy in the vigilance task performance of dyslexic subjects.
There were no significant differences between dyslexic and the control group in the Go/NoGo and Vigilance tests in the present study. These tasks are often used to discriminate ADHD from control groups [62,63]. Therefore, our results are less likely to be due to possible selection biases, such as inclusion of DD children with co-occurring ADHD.
To summarize, results of the present study points to specific difficulties in DD and not an overall attentional impairment. Dyslexic children performed poorly on the alerting and orienting components of attention, flexibility, divided attention, and visual search, but not on inhibition and vigilance tests. Since many different factors might affect the attentional test performance, they should be taken into account when interpreting the results.
Subgroups of DD
To further explore the nature of attentional processes in DD, first the PCA was conducted and, then, the cluster analysis was calculated to distinguish different patterns of attentional deficits in dyslexic children. As shown in Table 2, the PCA revealed 5 different factors: “Alertness”, “Covert shift of attention”, “Attentional shifting”, “Visual search”, and “Vigilance”. Unfortunately, due to requirements restricting the variables that can be included in the PCA, the analysis was predominantly based on the mean reaction times achieved in the tasks. Otherwise, the number of errors, which is an important measure of the correctness level, could have significantly influenced the PCA results.
The cluster analysis showed 3 subtypes of DD (Figure 1, Table 3). Cluster 1 displayed deficits in visual search, flexibility, and divided attention. Cluster 2 poorly performed on the tasks measuring alertness, covert shift of attention, divided attention, visual search, and vigilance. Cluster 3 showed impairments in covert shift of attention and visual search tests.
Other authors also showed that children with DD might be classified into groups. King et al. [64] distinguished 4 dyslexic groups characterized by deficit in either phonological awareness or rapid naming, both these tasks, or none. Heim et al. [65] identified 3 clusters of dyslexic children impaired in either phonological awareness, or attention or phonological skills, in combination with auditory and magnocellular functions. We believe that the present study is the first in which different attentional processes were explored to identify cognitive patterns in DD.
We found that each cluster performed poorly on visual search, which might indicate that it is a general cognitive problem in DD. However, a visual search task is rather complex (see the previous section for details) and poorer performance on this test might be due to different factors. Accordingly, in cluster 1, which also displayed deficits in the Flexibility and Divided Attention tasks, impaired attentional shifting or executive function could be a major dysfunction. Cluster 2 had longer reaction times compared to the control group in all tests used in the study except for the Flexibility and Go/NoGo, and might have slowed information processing speed. Finally, co-occurring deficits in visual search and covert shift of attention in Cluster 3 indicates an impaired ability to focus attention on part of the visual field as a major attentional problem in DD. Therefore, the cluster analytic approach might help to classify attentional deficits in DD.
Conclusions
The present study provides evidence of heterogeneity of attentional deficits in DD. The cluster analysis appeared to be a useful method for subtyping DD children based on attentional test performance. Three distinct patterns of cognitive functioning in DD were identified, corresponding either to the executive dysfunction or slowed processing speed or visuospatial deficit. As a consequence for diagnosis and therapy, a view of multiple cognitive impairments in DD might promote use of intervention programs focused on the individual child’s deficit profile.
Future studies might use the cluster analytic approach to include additional cognitive functions in accordance with recent theories in DD, such as phonological skills, auditory processing, or rapid naming. These analyses could improve classification of multiple cognitive deficits in reading disorders.
Footnotes
Source of support: This study was supported by the Polish Ministry for Science and Higher Education Grant No. N N403 214939
References
- 1.ICD-10: The ICD-10 Classification of Mental and Behavioural Disorders. Clinical Description and Diagnostic Guidelines. Warsaw: Vesalius & IPN; 2000. [Google Scholar]
- 2.Liberman IY. Segmentation of the Spoken Word and Reading Acquisition. Bulletin of the Orton Society. 1973;XXIII:65–77. [Google Scholar]
- 3.Tallal P. Auditory temporal perception, phonics, and reading disability in children. Brain Lang. 1980;9:182–98. doi: 10.1016/0093-934x(80)90139-x. [DOI] [PubMed] [Google Scholar]
- 4.Nicolson RI, Fawcett AJ, Berry EL, et al. Association of abnormal cerebellar activation with motor learning difficulties in dyslexic adults. Lancet. 1999;353(9165):1662–67. doi: 10.1016/S0140-6736(98)09165-X. [DOI] [PubMed] [Google Scholar]
- 5.Stein J, Walsh V. To see but not to read: the magnocellular theory of dyslexia. Trends Neurosci. 1997;20:147–52. doi: 10.1016/s0166-2236(96)01005-3. [DOI] [PubMed] [Google Scholar]
- 6.Stein J. The magnocellular theory of developmental dyslexia. Dyslexia. 2001;7(1):12–36. doi: 10.1002/dys.186. [DOI] [PubMed] [Google Scholar]
- 7.Wolf M, Bowers PG. Naming-speed processes and developmental reading disabilities: an introduction to the special issue on the double-deficit hypothesis. J Learn Disabil. 2000;33(4):322–24. doi: 10.1177/002221940003300404. [DOI] [PubMed] [Google Scholar]
- 8.Krasowicz-Kupis G, Borkowska AR, Pietras I. Rapid automatized naming, phonology and dyslexia in Polish children. Med Sci Monit. 2009;5(9):460–69. [PubMed] [Google Scholar]
- 9.Hari R, Renvall H. Impaired processing of rapid stimulus sequences in dyslexia. Trends Cogn Sci. 2001;5(12):525–32. doi: 10.1016/s1364-6613(00)01801-5. [DOI] [PubMed] [Google Scholar]
- 10.Hari R, Valta M, Uutela K. Prolonged attentional dwell time in dyslexic adults. Neurosci Lett. 1999;271(3):202–4. doi: 10.1016/s0304-3940(99)00547-9. [DOI] [PubMed] [Google Scholar]
- 11.Facoetti A, Trussardi AN, Ruffino M, et al. Multisensory spatial attention deficits are predictive of phonological decoding skills in developmental dyslexia. J Cogn Neurosci. 2010;22(5):1011–25. doi: 10.1162/jocn.2009.21232. [DOI] [PubMed] [Google Scholar]
- 12.Facoetti A, Lorusso ML, Paganoni P, et al. The role of visuospatial attention in developmental dyslexia: evidence from a rehabilitation study. Cogn Brain Res. 2003;15(2):154–64. doi: 10.1016/s0926-6410(02)00148-9. [DOI] [PubMed] [Google Scholar]
- 13.Bosse ML, Tainturier MJ, Valdois S. Developmental dyslexia: The visual attention span deficit hypothesis. Cognition. 2007;104(2):198–230. doi: 10.1016/j.cognition.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 15.Buchholz J, Aimola Davies A. Adults with dyslexia demonstrate attentional orienting deficits. Dyslexia. 2008;14(4):247–70. doi: 10.1002/dys.356. [DOI] [PubMed] [Google Scholar]
- 16.Bednarek DB, Saldaña D, Quintero-Gallego E, et al. Attentional deficit in dyslexia: a general or specific impairment? Neuroreport. 2004;15(11):1787–90. doi: 10.1097/01.wnr.0000134843.33260.bf. [DOI] [PubMed] [Google Scholar]
- 17.Heiervang E, Hugdahl K. Impaired visual attention in children with dyslexia. J Learn Disabil. 2003;36(1):68–73. doi: 10.1177/00222194030360010801. [DOI] [PubMed] [Google Scholar]
- 18.Reiter A, Tucha O, Lange KW. Executive functions in children with dyslexia. Dyslexia. 2005;11(2):116–31. doi: 10.1002/dys.289. [DOI] [PubMed] [Google Scholar]
- 19.Helland T, Asbjørnsen A. Executive functions in dyslexia. Child Neuropsychol. 2000;6(1):37–48. doi: 10.1076/0929-7049(200003)6:1;1-B;FT037. [DOI] [PubMed] [Google Scholar]
- 20.Willcutt EG, Pennington BF, Olson RK, et al. Neuropsychological analyses of comorbidity between reading disability and attention deficit hyperactivity disorder: in search of the common deficit. Dev Neuropsychol. 2005;27(1):35–78. doi: 10.1207/s15326942dn2701_3. [DOI] [PubMed] [Google Scholar]
- 21.Facoetti A, Lorusso ML, Paganoni P, et al. The time course of attentional focusing in dyslexic and normally reading children. Brain Cogn. 2003;53(2):181–84. doi: 10.1016/s0278-2626(03)00105-2. [DOI] [PubMed] [Google Scholar]
- 22.Facoetti A, Zorzi M, Cestnick L, et al. The relationship between visuo-spatial attention and nonword reading in developmental dyslexia. Cogn Neuropsychol. 2006;23(6):841–55. doi: 10.1080/02643290500483090. [DOI] [PubMed] [Google Scholar]
- 23.Ferretti G, Mazzotti S, Brizzolara D. Visual scanning and reading ability in normal and dyslexic children. Behav Neurol. 2008;19(1):87–92. doi: 10.1155/2008/564561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franceschini S, Gori S, Ruffino M, et al. A Causal Link between Visual Spatial Attention and Reading Acquisition. Curr Biol. 2012;22(9):814–19. doi: 10.1016/j.cub.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Gabay Y, Gabay S, Schiff R, et al. Visuospatial Attention Deficits in Developmental Dyslexia: Evidence from Visual and Mental Number Line Bisection Tasks. Arch Clin Neuropsychol. 2013;28(8):829–36. doi: 10.1093/arclin/act076. [DOI] [PubMed] [Google Scholar]
- 26.Huang HC, Wang TY. Stimulus effects on cancellation task performance in children with and without dyslexia. Behav Res Methods. 2009;41(2):539–45. doi: 10.3758/BRM.41.2.539. [DOI] [PubMed] [Google Scholar]
- 27.Iles J, Walsh V, Richardson A. Visual search performance in dyslexia. Dyslexia. 2000;6(3):163–77. doi: 10.1002/1099-0909(200007/09)6:3<163::AID-DYS150>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 28.Lallier M, Donnadieu S, Valdois S. Investigating the role of visual and auditory search in reading and developmental dyslexia. Front Hum Neurosci. 2013;7:597. doi: 10.3389/fnhum.2013.00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sireteanu R, Goebel C, Goertz R, et al. Impaired Serial Visual Search in Children with Developmental Dyslexia. Ann NY Acad Sci. 2008;1145(1):199–211. doi: 10.1196/annals.1416.021. [DOI] [PubMed] [Google Scholar]
- 30.Vidyasagar TR, Pammer K. Dyslexia: a deficit in visuo-spatial attention, not in phonological processing. Trends Cogn Sci. 2010;14(2):57–63. doi: 10.1016/j.tics.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Wright CM, Conlon EG, Dyck M. Visual search deficits are independent of magnocellular deficits in dyslexia. Ann Dyslexia. 2011;62(1):53–69. doi: 10.1007/s11881-011-0061-1. [DOI] [PubMed] [Google Scholar]
- 32.Casco C, Tressoldi PE, Dellantonio A. Visual selective attention and reading efficiency are related in children. Cortex. 1998;34(4):531–46. doi: 10.1016/s0010-9452(08)70512-4. [DOI] [PubMed] [Google Scholar]
- 33.Lipowska M, Czaplewska E, Wysocka A. Visuospatial deficits of dyslexic children. Med Sci Monit. 2011;17(4):CR216–21. doi: 10.12659/MSM.881718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vidyasagar TR, Pammer K. Impaired visual search in dyslexia relates to the role of the magnocellular pathway in attention. Neuroreport. 1999;10(6):1283–87. doi: 10.1097/00001756-199904260-00024. [DOI] [PubMed] [Google Scholar]
- 35.Gabay Y, Schiff R, Vakil E. Attentional requirements during acquisition and consolidation of a skill in normal readers and developmental dyslexics. Neuropsychology. 2012;26(6):744–57. doi: 10.1037/a0030235. [DOI] [PubMed] [Google Scholar]
- 36.Manly T, Anderson V, Nimmo-Smith I, et al. The differential assessment of children’s attention: The Test of Everyday Attention for Children (TEA-Ch), normative sample and ADHD performance. J Child Psychol Psychiatry. 2001;42(8):1065–81. doi: 10.1111/1469-7610.00806. [DOI] [PubMed] [Google Scholar]
- 37.Bogdanowicz M, Jaworowska A, Krasowicz-Kupis G, et al. Dyslexia 3 – Diagnosis of dyslexia in third grades. Warsaw: PTP; 2008. [Google Scholar]
- 38.Jaworowska A, Matczak A, Stańczak J. Dyslexia 5 – Diagnosis of dyslexia in fifth grades. Warsaw: PTP; 2010. [Google Scholar]
- 39.Matczak A, Piotrowska A, Ciarkowska W. WISC – R – Wechsler’s Intelligence Scale for Children – Revised. Warsaw: PTP; 2008. [Google Scholar]
- 40.Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 41.Kaja B, Nair R. Phonological Awareness Scale. In: Kaja B, editor. Diagnosis of dyslexia. Bydgoszcz: Kazimierz Wielki University in Bydgoszcz Press; 2003. pp. 70–94. [Google Scholar]
- 42.Zimmermann P, Fimm B. Test of Attentional Performance Version 2.2. Herzogenrath: Psytest; 2007. [Google Scholar]
- 43.Brickenkamp R. D2 Attention Assessment Test. Warsaw: PTP; 2003. [Google Scholar]
- 44.Łockiewicz M, Bogdanowicz KM, Bogdanowicz M, et al. Memory impairments in adults with dyslexia. Acta Neuropsychologica. 2012;10(2):215–29. [Google Scholar]
- 45.Fawcett AJ, Nicolson RI. Dyslexia: the role of the cerebellum. Electronic Journal of Research in Educational Psychology. 2001;2(2):35–58. [Google Scholar]
- 46.Fawcett AJ, Nicolson RI. Performance of Dyslexic Children on Cerebellar and Cognitive Tests. J Mot Behav. 1999;31(1):68–78. doi: 10.1080/00222899909601892. [DOI] [PubMed] [Google Scholar]
- 47.Nicolson RI, Fawcett AJ, Dean P. Developmental dyslexia: The cerebellar deficit hypothesis. Trends Neurosci. 2001;24:508–11. doi: 10.1016/s0166-2236(00)01896-8. [DOI] [PubMed] [Google Scholar]
- 48.Van Zomeren AH, Brouwer WH. Clinical neuropsychology of attention. New York: Oxford University Press; 1994. [Google Scholar]
- 49.Pachalska M, Mańko G, Kropotov ID, et al. Evaluation of neurotherapy for a patient with chronic impaired self-awareness and secondary ADHD after severe TBI and long term coma using event-related potentials. Acta Neuropsychologica. 2012;10(3):399–417. [Google Scholar]
- 50.Pąchalska M, Kropotov ID, Mańko G, et al. Evaluation of a neurotherapy program for a child with ADHD with Benign Partial Epilepsy with Rolandic Spikes (BPERS) using event-related potentials. Med Sci Monit. 2012;18(11):CS94–104. doi: 10.12659/MSM.883531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashtari M, Kumra S, Bhaskar SL, et al. Attention-deficit/hyperactivity disorder: a preliminary diffusion tensor imaging study. Biol Psychiatry. 2005;57:448–55. doi: 10.1016/j.biopsych.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 52.Gottwald B, Mihajlovic Z, Wilde B, Mehdorn HM. Does the cerebellum contribute to specific aspects of attention? Neuropsychologia. 2003;41(11):1452–60. doi: 10.1016/s0028-3932(03)00090-3. [DOI] [PubMed] [Google Scholar]
- 53.Courchesne E, Allen G. Prediction and preparation, fundamental functions of the cerebellum. Learn Mem. 1997;4:1–35. doi: 10.1101/lm.4.1.1. [DOI] [PubMed] [Google Scholar]
- 54.Marzocchi GM, Ornaghi S, Barboglio S. What are the Causes of the Attention Deficits Observed in Children with Dyslexia? Child Neuropsychol. 2009;15(6):567–81. doi: 10.1080/09297040902740660. [DOI] [PubMed] [Google Scholar]
- 55.Petersen SE, Posner MI. The Attention System of the Human Brain: 20 Years After. Annu Rev Neurosci. 2012;35(1):73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus – norepinephrine system in optimal performance. J Comp Neurol. 2005;493(1):99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- 57.Fan J, Mccandliss B, Fossella J, et al. The activation of attentional networks. Neuroimage. 2005;26(2):471–79. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Tarnowski KJ, Prinz RJ, Nay SM. Comparative analysis of attentional deficits in hyperactive and learning-disabled children. J Abnorm Psychol. 1986;95(4):341–45. doi: 10.1037/0021-843X.95.4.341. [DOI] [PubMed] [Google Scholar]
- 59.Taroyan NA, Nicolson RI, Fawcett AJ. Behavioural and neurophysiological correlates of dyslexia in the continuous performance task. Clin Neurophysiol. 2007;118(4):845–55. doi: 10.1016/j.clinph.2006.11.273. [DOI] [PubMed] [Google Scholar]
- 60.Visser TAW, Boden C, Giaschi DE. Children with dyslexia: evidence for visual attention deficits in perception of rapid sequences of objects. Vision Res. 2004;44(21):2521–35. doi: 10.1016/j.visres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 61.Nicolson RI, Fawcett AJ. Reaction times and dyslexia. Q J Exp Psychol A. 1994;47(1):29–48. doi: 10.1080/14640749408401142. [DOI] [PubMed] [Google Scholar]
- 62.Kenemans JL, Bekker EM, Lijffijt M, et al. Attention deficit and impulsivity: Selecting, shifting, and stopping. Int J Psychophysiol. 2005;58(1):59–70. doi: 10.1016/j.ijpsycho.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 63.Huang-Pollock CL, Karalunas SL, Tam H, Moore AN. Evaluating vigilance deficits in ADHD: A meta-analysis of CPT performance. J Abnorm Psychol. 2012;121(2):360–71. doi: 10.1037/a0027205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.King WM, Giess SA, Lombardino LJ. Subtyping of children with developmental dyslexia via bootstrap aggregated clustering and the gap statistic: comparison with the double-deficit hypothesis. Int J Lang Commun Disord. 2007;42(1):77–95. doi: 10.1080/13682820600806680. [DOI] [PubMed] [Google Scholar]
- 65.Heim S, Tschierse J, Amunts K, et al. Cognitive subtypes of dyslexia. Acta Neurobiol Exp (Warsz) 2008;68(1):73–82. doi: 10.55782/ane-2008-1674. [DOI] [PubMed] [Google Scholar]