Abstract
In the randomized, placebo-controlled FREEDOM study of women aged 60 to 90 years with postmenopausal osteoporosis, treatment with denosumab once every 6 months for 36 months significantly reduced hip and new vertebral fracture risk by 40% and 68%, respectively. To gain further insight into this efficacy, we performed a nonlinear finite element analysis (FEA) of hip and spine quantitative computed tomography (QCT) scans to estimate hip and spine strength in a subset of FREEDOM subjects (n = 48 placebo; n = 51 denosumab) at baseline, 12, 24, and 36 months. We found that, compared with baseline, the finite element estimates of hip strength increased from 12 months (5.3%; p < 0.0001) and through 36 months (8.6%; p < 0.0001) in the denosumab group. For the placebo group, hip strength did not change at 12 months and decreased at 36 months (–5.6%; p < 0.0001). Similar changes were observed at the spine: strength increased by 18.2% at 36 months for the denosumab group (p < 0.0001) and decreased by –4.2% for the placebo group (p = 0.002). At 36 months, hip and spine strength increased for the denosumab group compared with the placebo group by 14.3% (p < 0.0001) and 22.4% (p < 0.0001), respectively. Further analysis of the finite element models indicated that strength associated with the trabecular bone was lost at the hip and spine in the placebo group, whereas strength associated with both the trabecular and cortical bone improved in the denosumab group. In conclusion, treatment with denosumab increased hip and spine strength as estimated by FEA of QCT scans compared with both baseline and placebo owing to positive treatment effects in both the trabecular and cortical bone compartments. These findings provide insight into the mechanism by which denosumab reduces fracture risk for postmenopausal women with osteoporosis.
Keywords: DENOSUMAB, HIP STRENGTH, SPINE STRENGTH, FINITE ELEMENT ANALYSIS, OSTEOPOROSIS
Introduction
Denosumab (Prolia®, Amgen Inc., Thousand Oaks, CA, USA) is a fully human monoclonal antibody that inhibits receptor activator of NF-κB ligand (RANKL), a key modulator of osteoclast formation, function, and survival.1–4 In the phase 3 Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) study of 7808 postmenopausal women with osteoporosis, aged between 60 and 90 years, denosumab treatment reduced the risk of hip and new vertebral fractures over 36 months compared with placebo by 40% and 68%, respectively.5 The fracture efficacy in the overall FREEDOM population was associated with significant differences from baseline to month 36 between denosumab and placebo in areal bone mineral density (BMD) at the total hip and lumbar spine of 6.4% and 8.8%, respectively,6 and rapid and marked reductions in markers of bone resorption with an attenuation of the reduction at the end of each 6-month dosing interval.5,7 Despite the insight gained from the changes in BMD and bone turnover markers, it remains unclear to what extent denosumab altered hip or spine strength compared with baseline and placebo, and the extent that denosumab modified bone strength in the trabecular and cortical compartments, both of which are relevant to understanding mechanisms of fracture protection.
Because directly measuring the strength of any bone in a living human subject is not feasible, an alternative and FDA-approved approach to monitor treatment effects is to noninvasively estimate changes in strength using finite element analysis (FEA) of quantitative computed tomography (QCT) scans. Consistently validated for assessment of whole-bone strength in various cadaver studies,8–16 FEA of hip and spine QCT scans has been shown to predict new incident hip17–19 and spine16 fractures and to differentiate those with or without prevalent spine20–23 and general fractures.24 FEA has also been used to study aging effects in hip strength,25 estimate changes in hip26–29 and spine28,30–33 strength in response to various treatments as well as space flight,34 and estimate changes in strength that are directly associated with changes in the bone compartments.35,36
To gain further insight into the observed clinical efficacy of denosumab in reducing the risk of hip and spine fractures, in a subset of subjects in the FREEDOM study, we applied FEA to the hip and spine QCT scans to noninvasively assess changes in hip and spine strength associated with denosumab treatment over 36 months.
Materials and Methods
Study design and participants
FREEDOM was an international, randomized, placebo-controlled study in postmenopausal women with osteoporosis.5 Participants received placebo or denosumab 60 mg subcutaneously every 6 months for 36 months, with daily supplements of calcium (≥1000 mg) and vitamin D (≥400 IU). Postmenopausal women aged between 60 and 90 years with a BMD T-score of <−2.5 at the lumbar spine and/or total hip, and not <–4.0 at either site, were included in the study. Women were excluded if they had any severe or >2 moderate vertebral fractures, conditions that affected bone metabolism, or had taken oral bisphosphonates for ≥36 months. Women were eligible if they had taken oral bisphosphonates for ≤36 months but none during the 12 months preceding enrollment into the study. Study centers in the FREEDOM study, with expertise and access to a qualified scanner, invited subjects to participate in a substudy of the hip and lumbar spine QCT measurements. Calibration issues and unavailability of analyzable scans at different time points limited the analyses to 99 subjects (n = 48 placebo; n = 51 denosumab).
The protocol was approved by an independent ethics committee or institutional review board at each study site. Details of the methods and main results of the FREEDOM study have been reported previously.5
Assessments
The QCT methodology used to assess BMD and geometric parameters of the total hip and lumbar spine using whole-body scanners at selected study centers has been described previously, as has the main BMD analysis.37 Scans were performed at 120 kV with a pitch of 1 using 170 mAs in the hip and 100 mAs in the spine, and reconstructed using a medium kernel and a field of view of 400 mm in the hip and 360 mm in the spine. The reconstructed slice thickness was ≤1.25 mm. Scans were obtained at baseline, 12, 24, and 36 months, and covered 1 cm above the femoral head to 2 cm below the lesser trochanter for the hip assessment, or both L1 and L2 vertebrae for the spine assessment. Scanner calibration and stability were assessed and documented by Synarc (Portland, OR, USA, and Hamburg, Germany) circulating a European Spine Phantom (ESP).
All image processing and image analysis for FEA assessments were performed blinded to treatment, by O.N. Diagnostics (Berkeley, CA, USA), as described elsewhere.19,25,26,28 Briefly, the QCT images were calibrated, segmented, and converted into finite element models (∼40,000 elements per model), using cube-shaped, eight-noded brick elements (1.5 mm-sided for the hip and 1.0 mm-sided for the spine). Because delineating the true cortical envelope is difficult from clinical-resolution CT scans, particularly where the cortex is thin, we defined a “cortical” compartment to include all obvious cortical bone (defined as bone having an apparent BMD of >1.0 g/cm3) plus any other bone within a fixed distance of the periosteal surface (3 mm for the hip; 2 mm for the spine); the trabecular compartment was defined as all remaining trabecular bone (Fig. 1). Element-specific elastic properties (isotropic for the hip; anisotropic for the spine) and elastic-plastic failure properties were all derived from the calibrated volumetric BMD values, using validated empirical relations, and (for the hip) using higher strengths in compression than tension.38–40 After registering each bone to its baseline configuration, boundary conditions were applied to simulate a severe, unprotected fall to the side of the hip, with the diaphysis angled at 15° with respect to the ground and 15° of internal rotation. Femoral strength was defined from the resulting nonlinear force-strain curve of the whole femur as the force at 4.0% strain. For the spine, a similar approach was taken in which a thin layer of plastic was applied over each endplate through which the vertebral body was loaded to simulated failure in uniform compression. Spine strength was defined from the resulting nonlinear force-strain curve of the whole vertebral body as the force at 1.9% strain. For both hip and spine, these implementations have been shown to provide excellent correlations with measured strength from biomechanical cadaver experiments with a Y = X type of absolute agreement between model and experiment.16,19,41
Figure 1.
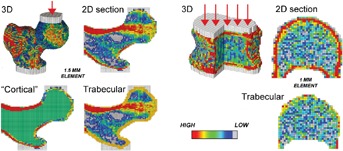
Example of finite element models for one subject at baseline. The full three-dimensional model is shown, with a schematic of how the loads are applied (through center of head at hip; distributed evenly for spine). The models for estimating strength changes associated with changes in only the trabecular and “cortical” compartments are also shown (thin 2D sections only). Our definition of the “cortical” compartment included all obvious cortical bone (defined as bone having an apparent BMD of >1.0 g/cm3) plus any other bone within a fixed distance of the periosteal surface (3 mm for the hip; 2 mm for the spine); the trabecular compartment was defined as all of the remaining trabecular bone.
We also performed controlled variations of these models to provide additional strength outcomes, including trabecular and “cortical” strengths (Fig. 1). Hip trabecular strength was computed by turning all the bone in the “cortical” compartment at each time point into plastic (a stiffer plastic for the compact cortical bone and a less stiff plastic for the adjacent trabecular bone) and then virtually reloading the resulting model to failure as described above for the intact femur. Likewise, hip “cortical” strength was computed by turning all the bone in the trabecular compartment into (a low-stiffness) plastic and then virtually reloading to failure,26 and spine trabecular strength was computed by virtually removing the outer 2 mm of bone and then virtually reloading to failure.32 Because a spine model comprised only of a thin shell of cortical bone would be prone to buckling if virtually loaded to failure, which would have questionable relevance, spine “cortical” strength was calculated as the overall spine strength minus the spine trabecular strength. We note that these trabecular and “cortical” strengths were additive for the spine but not for the hip; this is because of the complex nonparallel load transfer paths in the hip between the trabecular and “cortical” compartments, both of which were present in all hip models.
Statistical analysis
Subjects having both a baseline and ≥1 post-baseline FEA result were included in the analysis. There was no imputation of missing data. The percentage and absolute changes from baseline in hip and spine strength were analyzed using an analysis of covariance (ANCOVA) model including treatment and adjusting for baseline value and age strata (stratification factor). Least-squares means and 95% confidence intervals (CIs) for each treatment and for the treatment difference (denosumab – placebo) at each time point were generated. All analyses were exploratory and post-hoc. The p values and CIs were not adjusted for multiplicity.
Results
The baseline characteristics in this subset of women from the FREEDOM study (Table 1) were similar to those reported for the overall study population.5 Mean age was 74.1 years in the placebo group and 73.3 years in the denosumab group. Mean total hip dual-energy X-ray absorptiometry (DXA) T-score was –2.0 and –1.7 in the placebo and denosumab groups, respectively, and –2.8 at the lumbar spine in both treatment groups.
Table 1.
Baseline Characteristics
| Placebo n = 48 | Denosumab n = 51 | |
|---|---|---|
| Age (years) | 74.1 (6.0) | 73.3 (4.2) |
| BMI (kg/m2) | 25.7 (4.6) | 26.3 (4.5) |
| Total hip QCT BMD (mg/cm3) | 217 (31) | 227 (34) |
| Lumbar spine (trabecular) QCT BMD (mg/cm3) | 64.5 (17.2) | 69.6 (18.8) |
| Total hip BMD DXA T-score | –2.0 (0.8) | –1.7 (0.9) |
| Lumbar spine BMD DXA T-score | –2.8 (0.7) | –2.8 (0.5) |
| Total hip FEA strength (Newtons) | 2273 (570) | 2512 (686) |
| Total spine FEA strength (Newtons) | 2841 (628) | 2879 (583) |
Values are means (standard deviations).
BMI = body mass index; QCT = quantitative computed tomography; BMD = bone mineral density; DXA = dual-energy X-ray absorptiometry; FEA = finite element analysis.
For the women treated with denosumab, hip strength as estimated by FEA increased significantly compared with baseline by 5.3% at 12 months (p < 0.0001) and progressively over time, reaching 8.6% at 36 months (p < 0.0001 compared with baseline and 12 months) (Fig. 2A). By contrast, for the women treated with placebo, hip strength did not change at 12 months and decreased thereafter to −5.6% by 36 months compared with baseline (p < 0.0001). At 36 months, hip strength for the denosumab group increased by 14.3% compared with placebo (p < 0.0001). Similar changes occurred at the spine, but the increases in strength were larger. From baseline, spine strength increased by 18.2% (p < 0.0001) for the denosumab group and decreased by –4.2% (p = 0.002) for the placebo group at 36 months (Fig. 2B), corresponding to a relative increase in spine strength for the denosumab group of 22.4% compared with placebo (p < 0.0001). The corresponding absolute changes in strength at the hip and spine were also significantly higher for the denosumab group compared with baseline and the placebo group (p ≤ 0.0001 for all). At 36 months, mean absolute change from baseline in estimated hip strength was +196 Newtons (N) in the denosumab group compared with –123 N in the placebo group (p < 0.0001). At the spine, strength increased by +513 N in the denosumab group compared with a decrease of –127 N in the placebo group.
Figure 2.
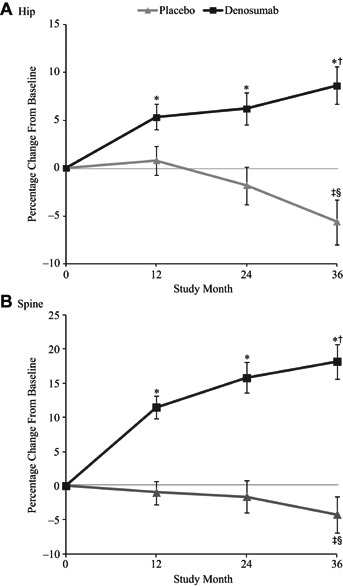
Mean percentage change from baseline in strength for the hip (A) and spine (B), as estimated by the finite element analysis (FEA). *p < 0.0001 versus both baseline and placebo; †p < 0.0001 versus 12 months; ‡p < 0.005 versus baseline; §p < 0.05 versus 12 months. Least-squares means ± 95% confidence intervals.
Further analysis of the finite element models revealed increases in strength associated with changes in both the trabecular and “cortical” bone compartments in the denosumab group for both the hip and spine (Fig. 3). By contrast, in the placebo group, the hip and spine strength associated with the trabecular compartment preferentially decreased from baseline through 36 months, whereas the strength associated with the “cortical” compartment was preserved.
Figure 3.
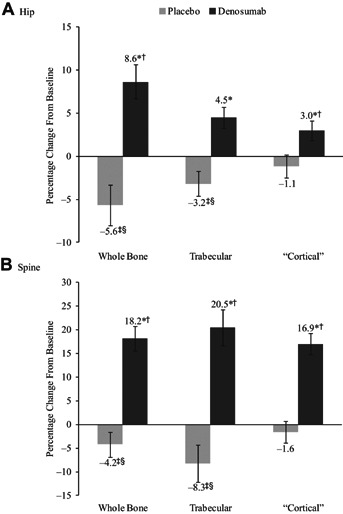
Mean percentage change in whole bone, trabecular, and “cortical” compartment strength for the hip (A) and spine (B), as estimated by the finite element analysis (FEA), at 36 months. *p < 0.0001 versus both baseline and placebo; †p < 0.01 versus 12 months; ‡p < 0.005 versus baseline; §p < 0.05 versus 12 months. Least-squares means ± 95% confidence intervals.
For individual subjects at 36 months, changes in hip strength were significantly, but weakly, correlated with changes in spine strength for the denosumab group (r = 0.38; p = 0.02) but not for the placebo group (r = 0.18; p = 0.39) (Fig. 4). At 36 months, spine strength increased in all denosumab-treated subjects and hip strength increased in all but two subjects, whereas both the hip and spine strength decreased for the majority of placebo-treated subjects.
Figure 4.
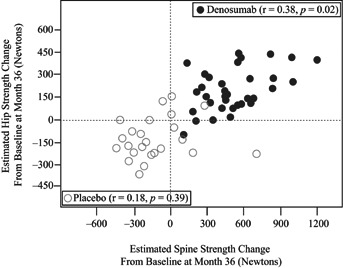
Relationship between changes from baseline in hip and spine strength (in Newtons), as estimated by the finite element analysis (FEA), in denosumab (closed circles) and placebo (open circles) subjects.
Discussion
In providing estimates of how denosumab alters both hip and spine strength compared with baseline and placebo, these results offer insight into the mechanisms by which denosumab reduces hip and spine fracture risk. The overall strength improvements observed here—which were all derived from FEA of the QCT scans and thus represent estimates of actual changes in strength—were relatively large at both the hip and spine, and substantiate the clinical relevance of the observed and previously described gains in DXA BMD with denosumab therapy, including the gains observed in the overall FREEDOM study.5,6,42 Indeed, the progressive increases in estimated strength in response to denosumab treatment reported here suggest that the continued gains in BMD from denosumab treatment, previously reported in the phase 2 study for up to 8 years and up to 6 years in the FREEDOM extension study, are of clinical consequence.43,44 This finding is consistent with the strong relationship reported by Austin and colleagues45 between DXA BMD gains and the documented fracture risk reductions with denosumab treatment.
The improvements in estimated strength were associated with positive changes in hip and spine strength of both the trabecular and “cortical” compartments for those women treated with denosumab. In our models, these changes in compartmental strength can be influenced by changes in overall density and/or volume of the compartments, although we did not assess changes in volume. A recently published analysis of this QCT cohort, using the Mindways software,46 reported a significant treatment effect on the volumes of both the trabecular (decrease) and cortical (increase) compartments, but no volume change related to the external geometry, suggesting an endosteal shift of what was defined as trabecular to cortical bone. This could be real or may be associated with assumptions made in the trabecular–cortical segmentation by the Mindways software. In general, it is impossible to explicitly differentiate trabecular from cortical bone from the resolution of the clinical QCT scans used in this study. Thus, a more definitive assessment of possible endosteal changes from trabecular to or from cortical bone remains an ongoing topic of active research. However, the finite element assessment in our study used a single continuous volume-averaged material property relation for the bone that does not depend on any classification into trabecular or cortical bone, and thus our finite element strength estimates are less influenced by the spatial voxel classification. The changes in trabecular and “cortical” strength, as computed for the hip, involve highly idealized models in which either the trabecular or “cortical” compartment, respectively, is homogenized across subjects and over time. Results from those calculations, while insightful, need to be interpreted carefully because those homogenized compartments only reflect generic measures of bone density.
The positive effect of denosumab on both the trabecular and “cortical” compartments, reported in other studies of denosumab versus placebo and versus alendronate,47,48 may be the result of denosumab’s ability to robustly diminish bone resorption throughout the skeleton.49 The substantial reduction in bone resorption biomarkers,7 which occurs to a greater degree for denosumab than bisphosphonates,50,51 reflects a combination of rapidly arresting bone resorption and inhibiting osteoclastic differentiation throughout the skeleton.7,52 The data in the current study confirm that these changes appear robust across patients because improvements in strength from baseline were observed in nearly all subjects treated with denosumab. Further, the large effect of denosumab treatment on the trabecular compartment directly reversed the significant trabecular loss that occurred in the placebo group. These findings suggest that for patients treated with calcium and vitamin D only (placebo group), the trabecular bone is continually being lost, and therefore, overall femoral strength may eventually come to depend more on the remaining cortical bone as it takes up more load from the weakened trabecular compartment. If so, then the cortical bone might assume an added biomechanical role in those individuals having the greatest risk for hip fracture, as suggested by others,53,54 and improvements in the cortical compartment might therefore be particularly beneficial under those conditions.
Our results may help explain the hip fracture efficacy observed in FREEDOM.5 In the present study, treatment with denosumab produced a 14.3% strength effect at 36 months relative to the effect in subjects treated with just calcium and vitamin D (placebo group). Other analyses of the FREEDOM study have reported an age-related efficacy effect: Hip fracture reduction increased from 40% in the overall cohort to 62% in subjects 75 years and older.48,55 In general, the degree of fracture efficacy of any treatment on fracture risk reduction in a clinical trial depends on both the fracture risk of the population at baseline as well as the effect of the treatment on the change in overall bone strength. Biomechanically, for any individual patient, there should be a threshold effect related to the critical level of loading required to actually fracture a bone—external forces below the threshold do not fracture the bone, whereas forces above the threshold do, with little gray area between (although that threshold force may vary across patients and with fall conditions and is difficult to predict).56
Therefore, changes in strength in response to therapies might need not only to be compared with placebo (for which strength is expected to decline over time), but also and perhaps more importantly, compared with baseline, with any possible threshold effect potentially acting as an interaction effect between these cohort and baseline factors. For example, the further an individual subject is from their own fracture threshold at the start of the study, the greater the strength improvement needed to take that individual above the fracture threshold force needed to prevent a fracture. These considerations imply that, in a clinical drug trial, increases in strength from baseline should improve fracture efficacy, and the larger the increase, the more robust the efficacy—but the efficacy itself may depend on the baseline level of strength, being greatest if the baseline strength, on average, is close to this putative fracture threshold in the majority of subjects. This mechanism could potentially explain the age-related hip efficacy observed in the FREEDOM trial. In this way, interventions that merely maintain strength are expected to be better than no intervention at all and may be sufficient for people in whom the risk for fracture is not in an elevated category because such treatments would maintain strength at a sufficient level to avoid a fracture with a fall or equivalent trauma. However, actually improving strength may be necessary to reduce fracture rates in people at elevated fracture risk, particularly if their strength is low to start.56
There are limitations in our study. First, the small sample size prevented assessment of the relationship between changes in estimated strength and fracture outcomes at the subject level. However, the larger FREEDOM trial, from which our sample was derived, randomly assigned participants to denosumab treatment or placebo, and the baseline characteristics were similar between our sample and the overall FREEDOM cohort5 and were similar between treatment groups. Thus, it is reasonable to assume that the general trends observed in the original FREEDOM cohort would also apply to this substudy. Other limitations relate to the methods used to assess changes in strength. Although it is not possible to directly assess changes in whole-bone strength in living humans, FEA of clinical QCT scans is arguably the best currently available noninvasive technique that can be used in vivo in patients at both hip and spine locations.36 This technique has been validated by multiple independent research groups for the hip and spine in both cadaver bone strength8–16 and clinical fracture outcome studies,16–24 and it is an FDA-approved approach that provides an integrative outcome of whole-bone strength that is intuitively associated with the etiology of osteoporotic fractures. The models have limited spatial resolution (∼1 mm imaging voxels); therefore, they cannot capture the bone microstructure, including the trabecular microarchitecture, Haversian porosity, any microstructure of the endosteal bone at the interface between well-defined cortical and trabecular bone, or the shell-type microstructure of the thin cortex around the femoral neck. The limited spatial resolution also precludes accounting for potential microscale effects of denosumab. For example, it has been proposed that reducing bone turnover and inhibiting “stress risers” may be an additional mechanism by which antiresorptive therapies reduce fracture risk,57,58 an effect that would serve to further increase strength for the denosumab group. Lastly, no adjustment was made for any possible changes in the tissue-level material properties resulting from treatment, such as increased secondary mineralization. However, biomechanical studies in adult ovariectomized cynomolgus monkeys treated with denosumab for 16 months have shown no detectable biomechanical negative effects on tissue-level material properties.59 Even so, results should be interpreted with these limitations in mind.
In summary, denosumab administered subcutaneously at a dose of 60 mg every 6 months substantially improved FEA estimates of hip and spine strength, both compared with baseline and the administration of calcium and vitamin D only over 36 months. These improvements were progressive over time and were influenced by parallel positive estimated strength changes in both the trabecular and “cortical” compartments. These observations highlight the potential benefit of denosumab to improve and reverse the negative consequence of postmenopausal- and aging-induced skeletal decay on strength, and to markedly reduce fracture incidence in subjects at increased or high risk for hip and spine fractures.
Acknowledgments
Amgen Inc. (Thousand Oaks, CA, USA) sponsored this study. The authors thank Mandy Suggitt and Erica Rockabrand, PhD, of Amgen Inc. for their medical writing assistance in the preparation of this manuscript.
Authors’ roles: TMK, HR, and CL contributed to the conception and design of the study, and analysis and interpretation of the data. MRM, HKG, DK, JRZ, JPB, SG, CR, MLB, RE, DLK, KE, and TF contributed to the acquisition of data and analysis and interpretation of the data. All authors participated in drafting or revising the manuscript and approved the final version of the manuscript for submission. TK and CL take responsibility for the integrity of the data analysis.
Disclosures
TMK is a consultant for Amgen Inc. and Merck; has received research grant support from Amgen Inc., Eli Lilly, Merck, and Novartis, and is a shareholder of O.N. Diagnostics.
MRM has received research grant support from Amgen Inc. and was an advisory board member for Amgen Inc.
HKG is a consultant and/or speaker for Amgen Inc., BMS, Genentech, Eli Lilly, GSK Janssen, Merck, Novartis, Pfizer, ONO, Roche, and Servier, and is a shareholder of Synarc.
JRZ has received research grant support from Amgen Inc. and is a consultant and speaker for Amgen Inc.
DK is a consultant and/or speaker for Amgen Inc., Eli Lilly, Merck, Novartis, and Servier, and has received grant support from Amgen Inc., Biosante, Eli Lilly, GSK, Merck, Novartis, Pfizer, Roche, and Servier.
JPB is a consultant, speaker, and an advisory board member for Amgen Inc., Eli Lilly, and Merck, and has received research grant support and honoraria from Abbott, Amgen Inc., BMS, Eli Lilly, Merck, Novartis, Pfizer, Roche, Sanofi-Aventis, Servier, Takeda, and Warner Chilcott.
SG has received research grant support from Amgen Inc. and is a consultant for Amgen Inc.
CR has received research grant support from Medi, consulting and advisory fees from Amgen Inc., Eli Lilly, and Novartis, lecture fees from Novartis and Warner Chilcott, and is a shareholder in Ion Med Systems.
MLB has received research grant support and honoraria from Amgen Inc.
RE has received consulting fees from Amgen Inc., GSK, Novartis, ONO, Pfizer, Servier, and Warner Chilcott, research grant support from Amgen Inc., AstraZeneca, Novartis, and Warner Chilcott, and is a speaker for Eli Lilly.
DLK is an employee of and has equity interest in O.N. Diagnostics.
KE is an employee and shareholder of Synarc, and is an advisory board member for Amgen Inc.
TF is an employee and shareholder of Synarc.
HR and CL are employees of and own stock or stock options in Amgen Inc.
References
- Lacey DL, Tan HL, Lu J, Kaufman S, Van G, Qiu W, Rattan A, Scully S, Fletcher F, Juan T, Kelley M, Burgess TL, Boyle WJ, Polverino AJ. Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol. 2000;157:435–48. doi: 10.1016/S0002-9440(10)64556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Udagawa N, Takahashi N, Yasuda H, Mizuno A, Itoh K, Ueno Y, Shinki T, Gillespie MT, Martin TJ, Higashio K, Suda T. Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology. 2000;141:3478–84. doi: 10.1210/endo.141.9.7634. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–65. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- Bolognese MA, Teglbjaerg CS, Zanchetta JR, Lippuner K, McClung MR, Brandi ML, Hoiseth A, Lakatos P, Moffett AH, Lorenc RS, Wang A, Libanati C. Denosumab significantly increases DXA BMD at both trabecular and cortical sites: results from the FREEDOM study. J Clin Densitom. 2012;16:147–53. doi: 10.1016/j.jocd.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Eastell R, Christiansen C, Grauer A, Kutilek S, Libanati C, McClung MR, Reid IR, Resch H, Siris E, Uebelhart D, Wang A, Weryha G, Cummings SR. Effects of denosumab on bone turnover markers in postmenopausal osteoporosis. J Bone Miner Res. 2011;26:530–7. doi: 10.1002/jbmr.251. [DOI] [PubMed] [Google Scholar]
- Bessho M, Ohnishi I, Matsuyama J, Matsumoto T, Imai K, Nakamura K. Prediction of strength and strain of the proximal femur by a CT-based finite element method. J Biomech. 2007;40:1745–53. doi: 10.1016/j.jbiomech.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Buckley JM, Loo K, Motherway J. Comparison of quantitative computed tomography-based measures in predicting vertebral compressive strength. Bone. 2007;40:767–74. doi: 10.1016/j.bone.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody DD, Gross GJ, Hou FJ, Spencer HJ, Goldstein SA, Fyhrie DP. Femoral strength is better predicted by finite element models than QCT and DXA. J Biomech. 1999;32:1013–20. doi: 10.1016/s0021-9290(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Crawford RP, Cann CE, Keaveny TM. Finite element models predict in vitro vertebral body compressive strength better than quantitative computed tomography. Bone. 2003;33:744–50. doi: 10.1016/s8756-3282(03)00210-2. [DOI] [PubMed] [Google Scholar]
- Imai K, Ohnishi I, Bessho M, Nakamura K. Nonlinear finite element model predicts vertebral bone strength and fracture site. Spine. 2006;31:1789–94. doi: 10.1097/01.brs.0000225993.57349.df. [DOI] [PubMed] [Google Scholar]
- Keyak JH, Falkinstein Y. Comparison of in situ and in vitro CT scan-based finite element model predictions of proximal femoral fracture load. Med Eng Phys. 2003;25:781–7. doi: 10.1016/s1350-4533(03)00081-x. [DOI] [PubMed] [Google Scholar]
- Lengsfeld M, Schmitt J, Alter P, Kaminsky J, Leppek R. Comparison of geometry-based and CT voxel-based finite element modelling and experimental validation. Med Eng Phys. 1998;20:515–22. doi: 10.1016/s1350-4533(98)00054-x. [DOI] [PubMed] [Google Scholar]
- Martin H, Werner J, Andresen R, Schober HC, Schmitz KP. Noninvasive assessment of stiffness and failure load of human vertebrae from CT-data. Biomed Tech (Berl) 1998;43:82–8. doi: 10.1515/bmte.1998.43.4.82. [DOI] [PubMed] [Google Scholar]
- Wang X, Sanyal A, Cawthon PM, Palermo L, Jekir M, Christensen J, Ensrud KE, Cummings SR, Orwoll E, Black DM, Keaveny TM. Prediction of new clinical vertebral fractures in elderly men using finite element analysis of CT scans. J Bone Miner Res. 2012;27:808–16. doi: 10.1002/jbmr.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyak JH, Sigurdsson S, Karlsdottir G, Oskarsdottir D, Sigmarsdottir A, Zhao S, Kornak J, Harris TB, Sigurdsson G, Jonsson BY, Siggeirsdottir K, Eiriksdottir G, Gudnason V, Lang TF. Male-female differences in the association between incident hip fracture and proximal femoral strength: a finite element analysis study. Bone. 2011;48:1239–45. doi: 10.1016/j.bone.2011.03.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopperdahl DL, Hoffmann P, Sigurdsson S, Aspelund T, Siggeirsdottir K, Eiriksdottir G, Harris T, Gudnason VG, Keaveny TM. Enhancement of hip fracture prediction using finite element analysis of CT scans. J Bone Miner Res. 2010:S114. doi: 10.1002/jbmr.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwoll ES, Marshall LM, Nielson CM, Cummings SR, Lapidus J, Cauley JA, Ensrud K, Lane N, Hoffmann PF, Kopperdahl DL, Keaveny TM. Finite element analysis of the proximal femur and hip fracture risk in older men. J Bone Miner Res. 2009;24:475–83. doi: 10.1359/JBMR.081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner KG, Cann CE, Hasegawa BH. Effect of bone distribution on vertebral strength: assessment with patient-specific nonlinear finite element analysis. Radiology. 1991;179:669–74. doi: 10.1148/radiology.179.3.2027972. [DOI] [PubMed] [Google Scholar]
- Imai K, Ohnishi I, Yamamoto S, Nakamura K. In vivo assessment of lumbar vertebral strength in elderly women using computed tomography-based nonlinear finite element model. Spine. 2008;33:27–32. doi: 10.1097/BRS.0b013e31815e3993. [DOI] [PubMed] [Google Scholar]
- Melton LJ, 3rd, Riggs BL, Keaveny TM, Achenbach SJ, Hoffmann PF, Camp JJ, Rouleau PA, Bouxsein ML, Amin S, Atkinson EJ, Robb RA, Khosla S. Structural determinants of vertebral fracture risk. J Bone Miner Res. 2007;22:1885–92. doi: 10.1359/jbmr.070728. [DOI] [PubMed] [Google Scholar]
- Melton LJ, 3rd, Riggs BL, Keaveny TM, Achenbach SJ, Kopperdahl D, Camp JJ, Rouleau PA, Amin S, Atkinson EJ, Robb RA, Therneau TM, Khosla S. Relation of vertebral deformities to bone density, structure, and strength. J Bone Miner Res. 2010;25:1922–30. doi: 10.1002/jbmr.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin S, Kopperdhal DL. Melton LJ 3rd, Achenbach SJ, Therneau TM, Riggs BL, Keaveny TM, Khosla S. Association of hip strength estimates by finite-element analysis with fractures in women and men. J Bone Miner Res. 2011;26:1593–600. doi: 10.1002/jbmr.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keaveny TM, Kopperdahl DL, Melton LJ, 3rd, Hoffmann PF, Amin S, Riggs BL, Khosla S. Age-dependence of femoral strength in white women and men. J Bone Miner Res. 2010;25:994–1001. doi: 10.1359/jbmr.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keaveny TM, Hoffmann PF, Singh M, Palermo L, Bilezikian JP, Greenspan SL, Black DM. Femoral bone strength and its relation to cortical and trabecular changes after treatment with PTH, alendronate, and their combination as assessed by finite element analysis of quantitative CT scans. J Bone Miner Res. 2008;23:1974–82. doi: 10.1359/JBMR.080805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keaveny TM, McClung MR, Wan X, Kopperdahl DL, Mitlak BH, Krohn K. Femoral strength in osteoporotic women treated with teriparatide or alendronate. Bone. 2012;50:165–70. doi: 10.1016/j.bone.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Lewiecki EM, Keaveny TM, Kopperdahl DL, Genant HK, Engelke K, Fuerst T, Kivitz A, Davies RY, Fitzpatrick LA. Once-monthly oral ibandronate improves biomechanical determinants of bone strength in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2009;94:171–80. doi: 10.1210/jc.2008-1807. [DOI] [PubMed] [Google Scholar]
- Lian KC, Lang TF, Keyak JH, Modin GW, Rehman Q, Do L, Lane NE. Differences in hip quantitative computed tomography (QCT) measurements of bone mineral density and bone strength between glucocorticoid-treated and glucocorticoid-naive postmenopausal women. Osteoporos Int. 2005;16:642–50. doi: 10.1007/s00198-004-1736-9. [DOI] [PubMed] [Google Scholar]
- Chevalier Y, Quek E, Borah B, Gross G, Stewart J, Lang T, Zysset P. Biomechanical effects of teriparatide in women with osteoporosis treated previously with alendronate and risedronate: results from quantitative computed tomography-based finite element analysis of the vertebral body. Bone. 2010;46:41–8. doi: 10.1016/j.bone.2009.09.032. [DOI] [PubMed] [Google Scholar]
- Graeff C, Chevalier Y, Charlebois M, Varga P, Pahr D, Nickelsen TN, Morlock MM, Gluer CC, Zysset PK. Improvements in vertebral body strength under teriparatide treatment assessed in vivo by finite element analysis: results from the EUROFORS study. J Bone Miner Res. 2009;24:1672–80. doi: 10.1359/jbmr.090416. [DOI] [PubMed] [Google Scholar]
- Keaveny TM, Donley DW, Hoffmann PF, Mitlak BH, Glass EV, San Martin JA. Effects of teriparatide and alendronate on vertebral strength as assessed by finite element modeling of QCT scans in women with osteoporosis. J Bone Miner Res. 2007;22:149–57. doi: 10.1359/jbmr.061011. [DOI] [PubMed] [Google Scholar]
- Mawatari T, Miura H, Hamai S, Shuto T, Nakashima Y, Okazaki K, Kinukawa N, Sakai S, Hoffmann PF, Iwamoto Y, Keaveny TM. Vertebral strength changes in rheumatoid arthritis patients treated with alendronate, as assessed by finite element analysis of clinical computed tomography scans: a prospective randomized clinical trial. Arthritis Rheum. 2008;58:3340–9. doi: 10.1002/art.23988. [DOI] [PubMed] [Google Scholar]
- Keyak JH, Koyama AK, LeBlanc A, Lu Y, Lang TF. Reduction in proximal femoral strength due to long-duration spaceflight. Bone. 2009;44:449–53. doi: 10.1016/j.bone.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Bonnick SL. Noninvasive assessments of bone strength. Curr Opin Endocrinol Diabetes Obes. 2007;14:451–7. doi: 10.1097/MED.0b013e3282f154a7. [DOI] [PubMed] [Google Scholar]
- Keaveny TM. Biomechanical computed tomography-noninvasive bone strength analysis using clinical computed tomography scans. Ann NY Acad Sci. 2010;1192:57–65. doi: 10.1111/j.1749-6632.2009.05348.x. [DOI] [PubMed] [Google Scholar]
- McClung MR, Zanchetta JR, Høiseth A, Kendler DL, Yuen CK, Brown JP, Stonkus S, Goemaere S, Recknor C, Woodson GC, Bolognese MA, Franek E, Brandi ML, Wang A, Libanati C. Denosumab densitometric changes assessed by quantitative computed tomgraphy at the spine and hip in postmenopausal women with osteoporosis. J Clin Densitom. 2013;16:250–6. doi: 10.1016/j.jocd.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Morgan EF, Keaveny TM. Dependence of yield strain of human trabecular bone on anatomic site. J Biomech. 2001;34:569–77. doi: 10.1016/s0021-9290(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Morgan EF, Bayraktar HH, Keaveny TM. Trabecular bone modulus-density relationships depend on anatomic site. J Biomech. 2003;36:897–04. doi: 10.1016/s0021-9290(03)00071-x. [DOI] [PubMed] [Google Scholar]
- Bayraktar HH, Morgan EF, Niebur GL, Morris GE, Wong EK, Keaveny TM. Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. J Biomech. 2004;37:27–35. doi: 10.1016/s0021-9290(03)00257-4. [DOI] [PubMed] [Google Scholar]
- Roberts BJ, Kopperdahl DL, Thrall E, Muller JA, Keaveny TM, Bouxsein ML. Prediction of femoral strength in a sideways fall configuration using QCT-based finite element analysis. Bone. 2009;44:S72. [Google Scholar]
- Lewiecki EM, Miller PD, McClung MR, Cohen SB, Bolognese MA, Liu Y, Wang A, Siddhanti S, Fitzpatrick LA. Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J Bone Miner Res. 2007;22:1832–41. doi: 10.1359/jbmr.070809. [DOI] [PubMed] [Google Scholar]
- Bone HG, Brown JP, Chapurlat R, Franchimont N, Brandi ML, Czerwiński E, Krieg M-A, Man Z, Mellström D, Radominski SC, Reginster JY, Resch H, Román JA, Daizadeh NS, Wang A, Geller ML, Wagman RB, Papapoulos S. The effect of six years of denosumab treatment on new vertebral and nonvertebral fractures in postmenopausal women with osteoporosis: results from the FREEDOM extension trial. Endocr Rev. 2012;33:S18–22. [Google Scholar]
- McClung MR, Lewiecki EM, Geller ML, Bolognese MA, Peacock M, Weinstein RL, Ding B, Rockabrand E, Wagman RB, Miller PD. Effect of denosumab on bone mineral density and biochemical markers of bone turnover: 8-year results of a phase 2 clinical trial. Osteoporos Int. 2013;24:227–35. doi: 10.1007/s00198-012-2052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin M, Yang YC, Vittinghoff E, Adami S, Boonen S, Bauer DC, Bianchi G, Bolognese MA, Christiansen C, Eastell R, Grauer A, Hawkins F, Kendler DL, Oliveri B, McClung MR, Reid IR, Siris ES, Zanchetta J, Zerbini CA, Libanati C, Cummings SR. Relationship between bone mineral density changes with denosumab treatment and risk reduction for vertebral and nonvertebral fractures. J Bone Miner Res. 2012;27:687–93. doi: 10.1002/jbmr.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung MR, Zanchetta JR, Høiseth A, Kendler DL, Yuen CK, Brown JP, Stonkus S, Goemaere S, Recknor C, Woodson GC, Bolognese MA, Franek E, Brandi ML, Wang A, Libanati C. Denosumab densitometric changes assessed by QCT at the spine and hip in postmenopausal women with osteoporosis. J Clin Densitom. 2013;16:250–6. doi: 10.1016/j.jocd.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Seeman E, Delmas PD, Hanley DA, Sellmeyer D, Cheung AM, Shane E, Kearns A, Thomas T, Boyd SK, Boutroy S, Bogado C, Majumdar S, Fan M, Libanati C, Zanchetta J. Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res. 2010;25:1886–94. doi: 10.1002/jbmr.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JA, Recknor C, Moffet AH, Adachi JD, Franek E, Lewiecki EM, McClung MR, Mautalen CA, Ragi Eis S, Nicholson GC, Muschitz C, Nuti R, Törring O, Wang A, Libanati C. Impact of denosumab on the peripheral skeleton of postmenopausal women with osteoporosis: bone density, mass, and strength of the radius, and wrist fracture. Menopause. 2013;20:130–7. doi: 10.1097/gme.0b013e318267f909. [DOI] [PubMed] [Google Scholar]
- Kostenuik PJ, Smith SY, Jolette J, Schroeder J, Pyrah I, Ominsky MS. Decreased bone remodeling and porosity are associated with improved bone strength in ovariectomized cynomolgus monkeys treated with denosumab, a fully human RANKL antibody. Bone. 2011;49:151–61. doi: 10.1016/j.bone.2011.03.769. [DOI] [PubMed] [Google Scholar]
- Brown JP, Prince RL, Deal C, Recker RR, Kiel DP, de Gregorio LH, Hadji P, Hofbauer LC, Alvaro-Gracia JM, Wang H, Austin M, Wagman RB, Newmark R, Libanati C, San Martin J, Bone HG. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2009;24:153–61. doi: 10.1359/jbmr.0809010. [DOI] [PubMed] [Google Scholar]
- Kendler DL, Roux C, Benhamou CL, Brown JP, Lillestol M, Siddhanti S, Man HS, San Martin J, Bone HG. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res. 2010;25:72–81. doi: 10.1359/jbmr.090716. [DOI] [PubMed] [Google Scholar]
- Kostenuik PJ, Nguyen HQ, McCabe J, Warmington KS, Kurahara C, Sun N, Chen C, Li L, Cattley RC, Van G, Scully S, Elliott R, Grisanti M, Morony S, Tan HL, Asuncion F, Li X, Ominsky MS, Stolina M, Dwyer D, Dougall WC, Hawkins N, Boyle WJ, Simonet WS, Sullivan JK. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J Bone Miner Res. 2009;24:182–95. doi: 10.1359/jbmr.081112. [DOI] [PubMed] [Google Scholar]
- Black DM, Bouxsein ML, Marshall LM, Cummings SR, Lang TF, Cauley JA, Ensrud KE, Nielson CM, Orwoll ES. Osteoporotic Fractures in Men Research G. Proximal femoral structure and the prediction of hip fracture in men: a large prospective study using QCT. J Bone Miner Res. 2008;23:1326–33. doi: 10.1359/JBMR.080316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousson V, Le Bras A, Roqueplan F, Kang Y, Mitton D, Kolta S, Bergot C, Skalli W, Vicaut E, Kalender W, Engelke K, Laredo JD. Volumetric quantitative computed tomography of the proximal femur: relationships linking geometric and densitometric variables to bone strength. Role for compact bone. Osteoporos Int. 2006;17:855–64. doi: 10.1007/s00198-006-0074-5. [DOI] [PubMed] [Google Scholar]
- Boonen S, Adachi JD, Man Z, Cummings SR, Lippuner K, Torring O, Gallagher JC, Farrerons J, Wang A, Franchimont N, San Martin J, Grauer A, McClung M. Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab. 2011;96:1727–36. doi: 10.1210/jc.2010-2784. [DOI] [PubMed] [Google Scholar]
- Keaveny TM, Bouxsein ML. Theoretical implications of the biomechanical fracture threshold. J Bone Miner Res. 2008;23:1541–7. doi: 10.1359/JBMR.080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ., 3rd Bone turnover matters: the raloxifene treatment paradox of dramatic decreases in vertebral fractures without commensurate increases in bone density. J Bone Miner Res. 2002;17:11–4. doi: 10.1359/jbmr.2002.17.1.11. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. High bone turnover is intrinsically harmful: two paths to a similar conclusion. The Parfitt view. J Bone Miner Res. 2002;17:1558–9. doi: 10.1359/jbmr.2002.17.8.1558. [DOI] [PubMed] [Google Scholar]
- Ominsky MS, Stouch B, Schroeder J, Pyrah I, Stolina M, Smith SY, Kostenuik PJ. Denosumab, a fully human RANKL antibody, reduced bone turnover markers and increased trabecular and cortical bone mass, density, and strength in ovariectomized cynomolgus monkeys. Bone. 2011;49:162–73. doi: 10.1016/j.bone.2011.04.001. [DOI] [PubMed] [Google Scholar]


