Abstract
Objective
To determine the frequency of diagnostic indications among women seeking to terminate pregnancies for reasons of fetal abnormality, spontaneous fetal demise, or a genetic disorder in a private outpatient clinic specializing in late outpatient abortion procedures.
Method
A total of 1005 women requested termination of pregnancy for reasons of genetic disorder, fetal anomaly, or fetal demise over 20 years (1992–2012). Gestational ages ranged from 12 to 39 weeks. In all cases, a documented diagnosis of fetal abnormality or fetal demise was made prior to referral. Records were reviewed to verify fetal diagnosis for all patients seeking termination of pregnancy for reasons of fetal disorder. Major complications included major unintended surgery, hemorrhage requiring transfusion, or pelvic infection.
Results
Preoperative diagnoses included the following: chromosomal abnormalities (n = 378), genetic syndromes and single gene disorders (n = 30), structural anomalies (n = 494), and other conditions (n = 103). These include 26 cases of spontaneous fetal demise and nine selective terminations of one abnormal twin. The major complication rate was 0.5%.
Conclusions
The majority of diagnoses were in the categories of genetic disorder and neurologic abnormality. © 2014 The Authors. Prenatal Diagnosis published by John Wiley & Sons Ltd.
INTRODUCTION
Among the most tragic aspects of obstetrics practice is the discovery that a desired pregnancy carries a fetal diagnosis of serious developmental abnormality or genetic disorder. A cruel dilemma is presented in a twin pregnancy in which one twin is healthy and the other is stricken with a catastrophic diagnosis. In all of these cases, the woman must decide whether to continue the pregnancy to term and have a child with a serious disorder, risk premature delivery or stillbirth, or decide to terminate the pregnancy in the safest manner possible. A twin pregnancy with a single affected fetus presents the choice of selective termination of the fetus with a poor prognosis.
Unfortunately, for a variety of reasons, the diagnosis of a significant abnormality may not be made or accepted until relatively late in pregnancy. In most of the United States, such patients then have no local, legal options for termination of the pregnancy. Although we perform pregnancy terminations for many reasons, we have served as a referral point for patients with fetal abnormalities for 35 years. A wide variety of methods is used for pregnancy termination for fetal abnormalities discovered late in pregnancy. Both labor induction and dilation and evacuation (D & E) have been presented as principal or alternative methods.1–4 Most studies report the experiences in a teaching hospital setting in which numerous physicians, nurses, and other health care practitioners are involved with a single patient′s care. Outcome variables principally include induction-to-delivery times and procedure times. There is limited information on the range of fetal diagnoses in late terminations of pregnancy for fetal abnormalities in teaching hospital settings.5,6 There are some reports on the frequency of pregnancy termination for specific fetal disorders.7–12
METHODS
We review the primary diagnostic abnormality of fetal disorder in a cohort of patients seeking termination of a desired pregnancy over a 20-year period ending in August 2012. This report does not include the previously reported 124 patients who had abortions for fetal disorder or the four cases of late selective termination.13,14
All patients were seen and procedures performed in a single private physician′s office located near a community hospital. The facility has been specially equipped and staffed to provide for women seeking late abortion. Because of the controversial nature of the service, rigorous security measures are taken to protect patients and staff. Women receive individual counseling and support throughout their experience at the clinic. Diagnostic ultrasound to determine fetal age is performed as the initial procedure on all patients as a first step in the preoperative evaluation. Standard tables for biparietal diameter and femur length are used for verification or accurate preoperative determination of fetal age.15–17
Nearly all patients came to the facility through private referral by a physician or genetic counselor with an established preoperative diagnosis of fetal anomaly or other serious indication for late abortion. Some patients were given no support for pregnancy termination by potential referral sources or were discouraged from terminating the pregnancy, but they found our services by informal means or by Internet search. Clinical records provided by referring physician, genetic counselor, or patient were accepted as adequate documentation of fetal or genetic abnormality. No further verification of diagnosis was performed. In 26 patients, spontaneous fetal demise had occurred late in pregnancy and was the reason why the patient sought assistance (induction of labor had been unsuccessful or declined by the patient).
The basic protocol and procedures used for the termination of pregnancy in these patients have been described.13,14,18–24 This protocol includes preoperative induced fetal demise, serial multiple laminaria dilation of the cervix, induction of labor following amniotomy, intrauterine placement of misoprostol, and instrumental evacuation of the uterus. We estimated fetal age according to fetal foot length and fetal weight on the basis of previously established values.25,26
Patients requesting fetal foot prints or hand prints were provided with these when feasible. For patients requesting viewing, the fetus was placed in a basin lined and covered with a pastel baby blanket and taken to the recovery room. Many patients requested private cremation of the fetus with the ashes given or sent to them. Other private and religious bereavement rituals were supported whenever possible including, on more than one occasion, a Native American bereavement ceremony.
All patients were strongly encouraged to return for follow-up examination if possible and were given forms to send in when they could not return in person for an examination. Arrangements were made for follow-up with the referring or other local physician when the patient came from a long distance, and for the follow-up physician to return a brief report. Standard follow-up instructions included a recommendation for examination 4 weeks after the abortion.
Major complications were defined using criteria of the Centers for Disease Control: major unintended surgery, hemorrhage requiring transfusion, or pelvic infection with 2 or more days of fever and a peak of up to or including 40 °C or with hospitalization for 11 or more days.27
Patients requesting a selective termination in a twin pregnancy were generally seen following the 32nd week of pregnancy to permit optimum development and survival probability for the healthy twin in case premature labor or other complication occurred prior to term. Only patients under the care of a qualified and cooperating obstetrician and clearly documented fetal disorder in one twin were accepted for treatment. The same requirements were made for patients with a single fetus in whom a catastrophic diagnosis was made very late in pregnancy and who requested induced fetal demise. Patients presenting earlier in pregnancy for selective termination were referred to centers with extensive experience in the earlier procedures.28–30
Informed consent for operative procedures and study of materials was obtained from all patients. This retrospective research was determined to be IRB-exempt by Western IRB. Standard summary statistics and central tendency calculations were performed with the assistance of the Excel computer program.
RESULTS
During the period of observation, 1005 women requested termination of pregnancy for reasons of fetal disorder. Of them, 989 had a singleton pregnancy, whereas 16 had a twin gestation. The indication for termination of pregnancy in the latter group included one twin affected by structural or chromosomal abnormality (n = 9, fetal demise was induced in the affected twin), severe twin-to-twin transfusion syndrome (n = 5, pregnancies were terminated), and conjoined twins (n = 2, the pregnancies were terminated). Most of these patients were seen during the last 15 years of the observation period. The proportion of all patients seeking pregnancy termination for fetal disorder increased over time from 2.5% to 30%. For example, of the 7587 patients seen at the clinic from 1/4/92 through 31/8/97, 189 (2.5%) sought termination of pregnancy for reasons of fetal abnormality. But from 1/9/2007 to 31/8/2012, 1251 patients were seen for pregnancy termination, of whom 375, or 30%, were requesting termination for reason of fetal abnormality. This increase reflected a gradual change in clinic policy to accept patients with more advanced gestations, more requests for late termination of pregnancy because of fewer options being available elsewhere, and advances in fetal diagnosis.31–46
More than 95% of patients in this series of 1005 patients had uncomplicated pregnancy terminations with complete evacuation of the uterus The 26 patients presenting with a spontaneous fetal demise late in pregnancy were managed by a D & E procedure following serial multiple laminaria dilation of the cervix. For 12 patients with a single live abnormal fetus, fetal demise was induced at the patient′s request followed by management of the delivery by the patient′s own obstetrician.
The median age of all patients was 32 years (range 14–47 years). The median gestational age for all patients was 24 weeks (range 12–39 weeks) (Figure 1), with older women being seen at either end of the spectrum of gestational ages. Patients seeking selective termination or induced fetal demise tended to be older (median ages 34 and 35 years, respectively) and were requesting these procedures later in pregnancy (median gestations 33 and 36 weeks, respectively).
Figure 1.
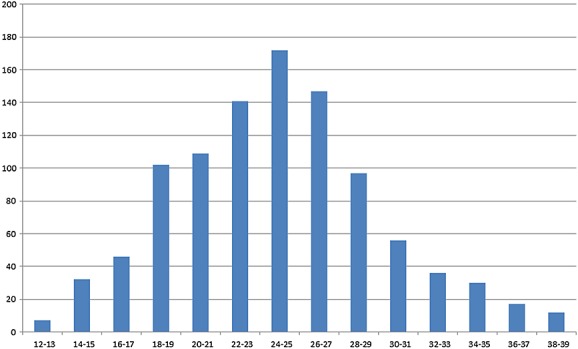
Number of patients by week of gestation
Tables 4 list the general categories of diagnoses according to the most prominent or definite reported fetal preoperative diagnosis. Genetic disorders were seen in over 40% of all patients. Among structural abnormalities, the most common were neural tube defects or neurological abnormalities.
Table 4.
Other conditions (N = 103)
| Teratogens exposure | 4 |
| Congenital infections | 12 |
| Congenital tumors (rhabdomyoma and teratomas) | 9 |
| Fetal growth restriction | 17 |
| Cystic hygroma or hydrops | 17 |
| Severe twin–twin transfusion syndrome | 5 |
| Conjoined twins | 2 |
| Severe oligohydramnios/anhydramnios | 4 |
| Other conditions | 7 |
| Spontaneous fetal death | 26 |
Table 1.
Chromosomal Abnormalities (N = 378)
| Trisomy 21 | 237 |
| Mosaic Trisomy 21 | 2 |
| Trisomy 18 (complete or partial) | 49 |
| Trisomy 13 | 11 |
| Trisomy 13+ 18 + 21, XXY | 2 |
| Trisomy 13 + 18 | 2 |
| Trisomy 21 + 18 | 1 |
| Trisomy 21 + Monosomy 9 | 1 |
| Trisomy 9 (partial or complete) | 2 |
| Mosaic monosomy 21 | 1 |
| Translocations | 9 |
| Deletions (Chromosomes 2, 4, 5, 6, 9, 13, and 18) | 11 |
| Duplications (Chromosomes 9 and 15) | 3 |
| Anomalies involving chromosomes 8, 10, 15, and 22 | 5 |
| Triploidy and polyploidy | 5 |
| Marker chromosomes | 6 |
| i(12)(p10) (Pallister–Killian syndrome) | 2 |
| Turner syndrome | 14 |
| Mosaic Turner syndrome | 1 |
| Other sex chromosome anomalies | 11 |
| Abnormal karyotype not otherwise specified | 3 |
| Total | 378 |
Table 2.
Syndromes and Single gene disorders (N = 30)
| Di George syndrome (22q11 deletion) | 6 |
| Fragile X syndrome | 2 |
| X-linked ichthyosis | 1 |
| Non-ketotic hyperglycinemia | 1 |
| Alagille syndrome | 1 |
| Treacher-Collins syndrome | 1 |
| Akinesia syndrome | 1 |
| Noonan syndrome | 1 |
| Mowat–Wilson syndrome | 1 |
| Brachman-De Lange syndrome | 1 |
| Apert syndrome | 3 |
| Amniotic band syndrome | 2 |
| Leigh syndrome | 1 |
| Meckel–Gruber Syndrome | 1 |
| TAR syndrome | 1 |
| Duchenne muscular dystrophy | 1 |
| Von Willebrand disease | 1 |
| Fanconi anemia | 1 |
| Sickle-cell homozygous | 1 |
| Thalassemia major | 2 |
| Total | 30 |
Table 3.
Structural anomalies (N = 494)
| Neural tube defects and CNS anomalies | N = 252 |
| Neural tube defects (anencephaly, acrania, encephalocele, spina bifida) | 97 |
| Hydrocephaly and ventriculomegaly | 68 |
| Porencephaly and Hydranencephaly | 3 |
| Microcephaly | 10 |
| Holoprosencephaly (alobar or semilobar) | 15 |
| Agenesis of the corpus callosum | 13 |
| Arachnoid cysts | 6 |
| Dandy–Walker malformation | 27 |
| Arnold–Chiari I and II malformations | 4 |
| Cerebral hemorrhage (stroke) | 9 |
| Cardiac and lung anomalies | N = 49 |
| Hypoplastic left or right ventricle | 16 |
| Tetralogy of Fallot | 2 |
| Univentricular heart | 1 |
| Ebstein’s anomaly | 2 |
| Aortic stenosis | 3 |
| Cardiosplenic syndromes/heterotaxy | 4 |
| Complete heart block | 1 |
| Other cardiac anomalies | 18 |
| Lung hypoplasia | 2 |
| Abdominal Wall anomalies | N = 18 |
| Omphalocele | 7 |
| Gastroschisis | 5 |
| Extrophy | 1 |
| Diaphragmatic hernia | 5 |
| Gastrointestinal anomalies | N = 4 |
| Duodenal atresia | 2 |
| Abdominal cyst | 1 |
| Echogenic bowel | 1 |
| Urinary Tract and adrenal anomalies | N = 31 |
| Polycystic kidneys | 5 |
| Bilateral renal agenesis | 3 |
| Potter syndrome and caudal regression | 7 |
| Hydronephrosis and other obstructions | 10 |
| Prune belly syndrome | 2 |
| Congenital adrenal hyperplasia/ambiguous genitalia | 4 |
| Skeletal dysplasias | N = 91 |
| Achondroplasia; phocomelia, micromelia, hemimelia | 70 |
| Osteogenesis imperfecta | 6 |
| Polydactyly, syndactyly, ectrodactyly | 5 |
| Arthrogryposis | 5 |
| Rocker bottom feet | 1 |
| Scoliosis | 1 |
| Craniosynostosis | 3 |
| Facial anomalies | N = 10 |
| Bilateral cleft lip and palate | 2 |
| Cleft face | 4 |
| Other craniofacial defects | 4 |
| Other malformations | N = 3 |
| Multiple malformations | N = 36 |
There were 160 diagnostic categories of fetal abnormality, including spontaneous intrauterine fetal demise, among these 1005 patients. The categories utilized in Tables 4 were generated for this report to group patients with similar diagnoses. Where possible, the correct terminology was substituted for the referral diagnosis. Whereas some preoperative diagnoses provided by referral sources and patients were unequivocal and highly specific, other women were referred with less specific information (i.e. ‘cerebral abnormalities’ and ‘chromosome anomaly’). Diagnostic terms reported here were provided in all cases by the referring physician or genetic counselor although some patients, whose physicians did not support termination of the pregnancy and refused to provide a formal report, had only a limited or general idea of the fetal abnormality.
Patients were not refused assistance because of inadequate documentation of fetal abnormality or lack of cooperation by the patient’s physician. Preoperative requests for postoperative diagnostic laboratory studies were accommodated whenever possible, but obtaining a satisfactory specimen was secondary to the safest procedure for the patient. Fetal abnormalities discovered postoperatively are not included in this report.
The proportions of preoperative diagnosis of fetal disorder changed over time (Figure 2) and by week of gestation (Figure 3), with chromosomal disorders being discovered earlier in pregnancy and other problems such as CNS abnormalities or skeletal dysplasia being discovered later in pregnancy.
Figure 2.
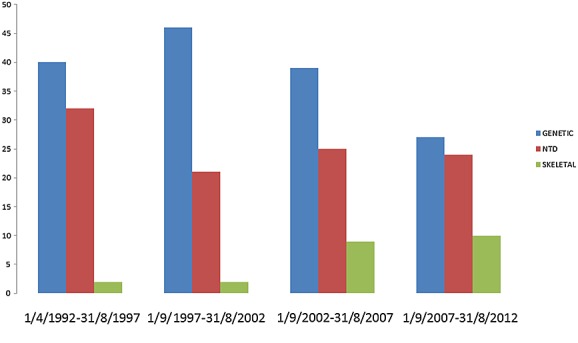
Proportion (%) of fetal disorder by 5-year time interval
Figure 3.
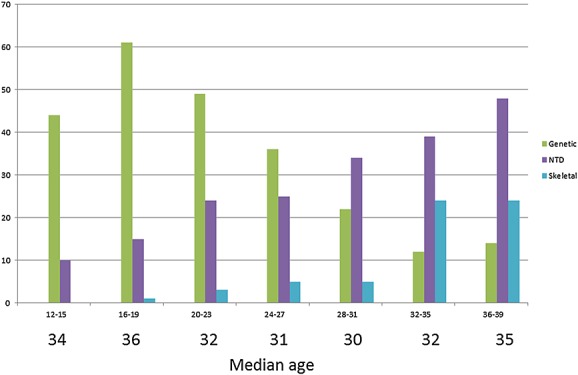
Proportion (%) of fetal disorder by menstrual weeks gestation and median age in years
Median operative measured blood loss for abortion patients was 150 mL (range: 10–2000 mL). Forty-nine patients (5%) experienced an operative blood loss of more than 500 mL. Median procedure duration for D & E was 9 min (range: 5 to 194 min).
Five of the abortion patients (0.5%) experienced a major complication (blood product transfusion) using the previously cited CDC definition. Thirteen other patients were hospitalized postoperatively for observation but did not experience a major complication. There were no uterine perforations. One patient with twins discordantly affected by abnormality and who experienced selective termination developed a postoperative amnionitis that required a cesarean delivery of the healthy fetus several days following the procedure.
In numerous cases, the patient requesting termination of the pregnancy for reasons of fetal abnormality concomitantly had a severe medical condition such as pre-eclampsia, multiple sclerosis, lupus erythematosis, severe hyperemesis gravidarum, massive uterine fibroids, morbid obesity, coagulation disorder, placenta previa, or diabetes exacerbated by pregnancy. Many of these conditions precluded termination of the pregnancy by labor induction alone.
DISCUSSION
For the women and their families who seek late abortion, the experience represents pain, loss, grief, and suffering, as these were all desired pregnancies. For women in their late 30s or early 40s, the pregnancy to be terminated represented perhaps their last opportunity to have a child. At the other extreme, the youngest patient with a fetal diagnosis of trisomy 21 was 16 years old. Two 17-year-old patients had a fetal diagnosis of trisomy 18, and one 19 year-old patient had a fetal diagnosis of trisomy 13.
As for gestational age, many of the patients whose diagnoses of fetal disorder were not made until after the 30th week of gestation reported that ultrasound examination had been evaluated as ‘normal’ at 18 or 20 weeks, and the diagnosis of fetal anomaly was made in late pregnancy when a repeat ultrasound scan was carried out in connection with evaluation or treatment of some other unrelated problem. Some patients reported that a diagnosis of fetal disorder had been made early in the pregnancy, at the time of the 20th-week fetal anatomy survey, but they were not informed of the diagnosis until it was too late to terminate the pregnancy according to local law or hospital regulations because the patient’s physician was opposed to abortion. Such patients reported that this delay and deliberate withholding of information added to the stress of terminating the pregnancy. Other patients had a timely diagnosis with full information and support from family members, physicians, and genetic counselors but could not decide whether to seek termination of the pregnancy. Optimization of the timing of the initial diagnostic process is thus unlikely to reduce significantly the number of cases seen at our clinic. Pregnancies affected by fetal genetic disorders were diagnosed earlier in gestation than those affected by structural anomalies such as neural tube defects or skeletal dysplasia.
A possible limitation of this study is the lack of specific diagnoses in a minority of cases. Our clinic however operates downstream from the centers, located throughout the entire United States, in which the original diagnostic and genetic information was generated. Our goal is not to compensate for any diagnostic or counseling deficiencies in such centers, but rather to satisfy the need of women seeking late termination of pregnancy for fetal anomalies.
There were many factors affecting the numbers of patients, average length of gestation, and kinds of fetal diagnoses seen in this series over the observation period. These include changes in policies and personnel within this private practice, changes in the legal availability of late abortion services in other states and regions, and events such as the assassination of Dr. George Tiller on 31 May 2009. At the time of his assassination, Dr. Tiller was the only other physician in the United States offering pregnancy termination services for fetal anomaly in very advanced gestations. Some of the patients in this series had been on Dr. Tiller’s operative schedule for the week following the day of his assassination and were referred by his office for care.
Outpatient termination for fetal disorder in a specialized private facility offers many advantages over hospital care. In a specialized clinic such as ours, medical care is completely oriented toward assisting and supporting women who have decided to terminate a pregnancy and to giving each woman and her family individual attention. All clinic personnel have a positive attitude toward the patients who have made this decision including those with desired but complicated pregnancies. Staff members are employed specifically because of supportive attitudes in addition to professional competence. Patients having questions, complaints, or complications who call after discharge speak with the same physician and staff members who took care of them at the clinic. Continuity of care is a basic principle. Disadvantages of outpatient care consist primarily of increasingly heightened security concerns, needs, and costs as a result of anti-abortion harassment and violence directed toward patients, staff, the physician, and support personnel. Response to these issues includes the provision of secure private transportation to and from the clinic for patients staying at local hotels, especially during infrequent anti-abortion demonstrations.
Except for some university hospitals with abortion training programs that care for patients up to 24 weeks gestation, choices of hospital access for abortion patients after 16 weeks’ gestation and for physicians are increasingly restricted by the acquisition of private hospitals by sectarian agencies that are officially opposed to abortion and by aggressive legislative restriction of access to abortion in various states. For women seeking termination of an advanced pregnancy for reasons of a fetal abnormality, this means that there are fewer choices for individual care among a diminishingly small number of experienced private physicians specializing in this service.
CONCLUSION
Women seeking pregnancy termination for fetal disorder do so for a broad variety of diagnostic reasons. Underlying the delay in seeking termination of pregnancy are different factors, which for the most part are not preventable.
WHAT’S ALREADY KNOWN ABOUT THIS TOPIC?
Very few reports have been published on fetal diagnosis in abortions throughout the duration of pregnancy.
The published series have small numbers.
WHAT DOES THIS STUDY ADD?
This study adds detailed information on the preoperative diagnosis for the indication for termination of more than 1000 pregnancies up to 39 weeks’ gestation at a single private medical facility.
Performance of these procedures in a unique setting with a consistent protocol resulted in a low major complication rate (0.5%).
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher’s web site.
REFERENCES
- 1.Shulman LP, Ling RW, Meyers CM, et al. Dilation and evacuation for second-trimester genetic pregnancy termination. Obstet Gynecol. 1990;75(6):1037–40. [PubMed] [Google Scholar]
- 2.Dickinson JE, Evans SF. A Comparison of oral misoprostol with vaginal misoprostol administration in second-trimester pregnancy termination for fetal abnormality. Obstet Gynecol. 2003;101(6):1294–9. doi: 10.1016/s0029-7844(03)00357-0. [DOI] [PubMed] [Google Scholar]
- 3.Bryant AG, Grimes DA, Garrett JM, Stuart GS. Second-trimester abortion for fetal anomalies or fetal death: labor induction compared with dilation and evacuation. Obstet Gynecol. 2011;117(4):788–92. doi: 10.1097/AOG.0b013e31820c3d26. [DOI] [PubMed] [Google Scholar]
- 4.Borgatta L. Labor induction termination of pregnancy. Glob. libr. women’s med. 2011 (ISSN 1756-2228) DOI: 10.3843/GLOWM.10444. [Google Scholar]
- 5.Barel O, Vaknin Z, Smorgick N, et al. Fetal abnormalities leading to third trimester abortion: nine-year experience from a single medical center. Prenat Diagn. 2009;29(3):223–8. doi: 10.1002/pd.2188. DOI: 10.1002/pd.2188. [DOI] [PubMed] [Google Scholar]
- 6.Vaknin Z, Lahat Y, Barel O, et al. Termination of pregnancy due to fetal abnormalities performed after 23 weeks’ gestation: analysis of indications in 144 cases from a single medical center. Fetal Diagn Ther. 2009;25(2):291–6. doi: 10.1159/000229501. DOI: 10.1159/000229501. [DOI] [PubMed] [Google Scholar]
- 7.Milunsky A, Milunsky JM, editors. Genetic Disorders and the Fetus: Diagnosis, Prevention and Treatment. 6th edn. Oxford: Wiley-Blackwell; 2010. [Google Scholar]
- 8.Tonks AM, Gornall AS, Larkins SA, Gardosi JO. Trisomies 18 and 13: trends in prevalence and prenatal diagnosis – population based study. Prenat Diagn. 2013;33(8):742–50. doi: 10.1002/pd.4117. [DOI] [PubMed] [Google Scholar]
- 9.Verweij EJ, Oepkes D, de Boer MA. Changing attitudes towards termination of pregnancy for trisomy 21 with non-invasive prenatal trisomy testing: a population-based study in Dutch pregnant women. Prenat Diagn. 2013;33(4):397–9. doi: 10.1002/pd.4063. DOI: 10.1002/pd.4063. [DOI] [PubMed] [Google Scholar]
- 10.Tararbi CK, Thao Bui TT, Lelong N, et al. Clinical and socioeconomic predictors of pregnancy termination for fetuses with congenital heart defects: a population-based evaluation. Prenat Diagn. 2013;33(2):179–86. doi: 10.1002/pd.4043. DOI: 10.1002/pd.4043. [DOI] [PubMed] [Google Scholar]
- 11.Natoli JL, Ackerman DL, McDermott S, Edwards JG. Prenatal diagnosis of Down syndrome: a systematic review of termination rates (1995–2011) Prenat Diagn. 2012;33(2):142–53. doi: 10.1002/pd.2910. DOI: 10.1002/pd.2910. [DOI] [PubMed] [Google Scholar]
- 12.Chenni N, Lacroze V, Pouet C, et al. Fetal heart disease and interruption of pregnancy: factors influencing the parental decision-making process. Prenat Diagn. 2012;33(2):168–72. doi: 10.1002/pd.2923. DOI: 10.1002/pd.2923. [DOI] [PubMed] [Google Scholar]
- 13.Hern WM, Zen C, Ferguson KA, et al. Outpatient abortion for fetal anomaly and fetal death from 15–34 menstrual weeks’ gestation: Techniques and clinical management. Obstet Gynecol. 1993;81:301–6. [PubMed] [Google Scholar]
- 14.Hern WM. Selective termination for fetal anomaly/genetic disorder in twin pregnancy at 32+ menstrual weeks. Fetal Diagn Ther. 2004;19:292–5. doi: 10.1159/000076714. [DOI] [PubMed] [Google Scholar]
- 15.Hadlock FP, Harrist RB, Deter RL, Park SK. Fetal femur length as a predictor of menstrual age: sonographically measured. Am J Roentgenol. 1982;138(5):875–8. doi: 10.2214/ajr.138.5.875. [DOI] [PubMed] [Google Scholar]
- 16.Altman DG, Chitty LS. New charts for ultrasound dating of pregnancy. Ultrasound Obstet Gynecol. 1997;10:174–91. doi: 10.1046/j.1469-0705.1997.10030174.x. [DOI] [PubMed] [Google Scholar]
- 17.Chitty LS, Altman DG. Charts of fetal size: limb bones. Br J Obstet Gynaecol. 2002;109:919–29. doi: 10.1111/j.1471-0528.2002.01022.x. [DOI] [PubMed] [Google Scholar]
- 18.Hern WM. Outpatient second-trimester D & E abortion through 24 menstrual weeks’ gestation. Adv Plan Parent. 1981;16:7–13. [Google Scholar]
- 19.Hern WM. Serial multiple laminaria and adjunctive urea in late outpatient dilatation and evacuation abortion. Obstet Gynecol. 1984;63:543–9. [PubMed] [Google Scholar]
- 20.Hern WM. Dilapan vs. laminaria in second trimester abortion: randomized controlled cohort of 1001 patients. Am J Obstet Gynecol. 1994;171(5):1324–8. doi: 10.1016/0002-9378(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 21.Hern WM. Laminaria, induced fetal demise and misoprostol in late abortion. Int Gynecol Obstet. 2001;75:279–86. doi: 10.1016/s0020-7292(01)00478-7. [DOI] [PubMed] [Google Scholar]
- 22.Hern WM. Second-Trimester Surgical Abortion. In: Sciarra JJ, editor. Gynecology and Obstetrics. Revised edn. Philadelphia: J.B. Lippincott Company; 2002. Glob. libr. women’s med., (ISSN 1756-2228) 2011, DOI: 10.3843/GLOWM.10442. [Google Scholar]
- 23.Hern WM. Misoprostol as an adjunctive medication in late surgical abortion. Int J Gynecol Obstet. 2005;88:327–8. doi: 10.1016/j.ijgo.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Hern WM. Abortion Practice. Philadelphia (PA): JB Lippincott Co; 1984. Boulder (CO) 1990; Alpenglo Graphics (2 printing). p 130–132. [Google Scholar]
- 25.Hern WM. Correlation of fetal age and measurements between 10 and 26 weeks of gestation. Obstet Gynecol. 1984;63:26–32. [PubMed] [Google Scholar]
- 26.Shepard MJ, Richards VA, Berkowitz RL, et al. An evaluation of two equations for predicting fetal weight by ultrasound. Am J Obstet Gynecol. 1982;142(1):47–52. doi: 10.1016/s0002-9378(16)32283-9. From Gordon JD, Rydfors JT, Druzin ML, Tadir Y (eds): Obstetrics Gynecology & Infertility (4 Edition). Menlo Park, CA: Scrub Hill Press, Inc, 1995. p 73. [DOI] [PubMed] [Google Scholar]
- 27.Grimes DA, Schulz KF, Cates W, Jr, Tyler CW. The Joint Program for the Study of Abortion/CDC: a preliminary report. In: Hern WM, Andrikopoulos B, editors. Abortion in the Seventies. New York (NY): National Abortion Federation; 1977. pp. 41–6. [Google Scholar]
- 28.Evans MI, Goldberg JD, Horenstein J, et al. Selective termination for structural, chromosomal, and mendelian anomalies: international experience. Am J Obstet Gynecol. 1999;181:893–7. doi: 10.1016/s0002-9378(99)70321-2. [DOI] [PubMed] [Google Scholar]
- 29.Evans MI, Rosner M, Andriole S, et al. Evolution of gender options in multiple pregnancy management. Prenat Diagn. 2013;33(10):935–9. doi: 10.1002/pd.4167. [DOI] [PubMed] [Google Scholar]
- 30.Rosner M, Pergament E, Andriole S, et al. Detection of genetic abnormalities by using CVS and FISH prior to fetal reduction in sonographically normal appearing fetuses. Prenat Diagn. 2013;33(10):940–4. doi: 10.1002/pd.4213. [DOI] [PubMed] [Google Scholar]
- 31.Yinon Y, Katorza E, Nassie DI, et al. Late diagnosis of fetal central nervous system anomalies following a normal second trimester anatomy scan. Prenat Diagn. 2013;33(10):929–34. doi: 10.1002/pd.4163. [DOI] [PubMed] [Google Scholar]
- 32.Luchi C, Monacci F, Schifano M, Gadducci A. ‘Bat-like’ choroid plexus and other sonographic features in trisomy 22 at the first trimester of pregnancy. Prenat Diagn. 2013;33(10):1013–4. doi: 10.1002/pd.4180. DOI: 10.1002/pd.4180. [DOI] [PubMed] [Google Scholar]
- 33.Alamillo CM, Krantz D, Evans M, et al. Nearly a third of abnormalities found after first-trimester screening are different than expected: 10-year experience from a single center. Prenat Diagn. 2013;33(3):251–6. doi: 10.1002/pd.4054. [DOI] [PubMed] [Google Scholar]
- 34.Kontopoulos E, Odibo A, Wilson RD. Current controversies in prenatal diagnosis 2: are we ready to screen for fetal anomalies with first trimester ultrasound? Prenat Diagn. 2013;33(1):9–12. doi: 10.1002/pd.4030. DOI: 10.1002/pd.4030. [DOI] [PubMed] [Google Scholar]
- 35.Ferreira AFA, Syngelaki A, Smolin A, et al. Posterior brain in fetuses with trisomy 18, trisomy 13 and triploidy at 11 to 13 weeks’ gestation. Prenat Diagn. 2012;32(9):854–8. doi: 10.1002/pd.3920. DOI: 10.1002/pd.3920. [DOI] [PubMed] [Google Scholar]
- 36.Lachmann R, Sinkovskaya E, Abuhamad A. Posterior brain in fetuses with Dandy–Walker malformation with complete agenesis of the cerebellar vermis at 11–13 weeks: a pilot study. Prenat Diagn. 2012;32(8):765–9. doi: 10.1002/pd.3899. DOI: 10.1002/pd.3899. [DOI] [PubMed] [Google Scholar]
- 37.Karadzov-Orlic N, Egic A, Milovanovic Z, et al. Improved diagnostic accuracy by using secondary ultrasound markers in the first-trimester screening for trisomies 21, 18 and 13 and Turner syndrome. Prenat Diagn. 2012;32(7):638–43. doi: 10.1002/pd.3873. DOI: 10.1002/pd.3873. [DOI] [PubMed] [Google Scholar]
- 38.Miron JP, Cuckle H, Miron P. Prenasal thickness in first-trimester screening for Down syndrome. Prenat Diagn. 2012;32(7):695–7. doi: 10.1002/pd.3879. DOI: 10.1002/pd.3879. [DOI] [PubMed] [Google Scholar]
- 39.Persico N, Molina F, Azumendi G, et al. Nasal bone assessment in fetuses with trisomy 21 at 16–24 weeks of gestation by three-dimensional ultrasound. Prenat Diagn. 2012;32(3):240–4. doi: 10.1002/pd.2938. DOI: 10.1002/pd.2938. [DOI] [PubMed] [Google Scholar]
- 40.Shaffer LG, Dabell MP, Fisher AJ, et al. Experience with microarray-based comparative genomic hybridization for prenatal diagnosis in over 5000 pregnancies. Prenat Diagn. 2012;32(10):976–85. doi: 10.1002/pd.3945. DOI: 10.1002/pd.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wapner RJ, Driscoll DA, Simpson JL. Integration of microarray technology into prenatal diagnosis: counseling issues generated during the NICHD clinical trial. Prenat Diagn. 2012;32(4):396–400. doi: 10.1002/pd.3863. DOI: 10.1002/pd.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pergament E, Alamilo C, Sak K, Fiddler M. Genetic assessment following increased nuchal translucency and normal karyotype. Prenat Diagn. 2011;31:307–10. doi: 10.1002/pd.2718. [DOI] [PubMed] [Google Scholar]
- 43.Yinon Y, Katorza E, Nassie DI, et al. Late diagnosis of fetal central nervous system anomalies following a normal second trimester anatomy scan. Prenat Diagn. 2013;33(10):929–34. doi: 10.1002/pd.4163. [DOI] [PubMed] [Google Scholar]
- 44.Lim J, Whittle WL, Lee YM, et al. Early anatomy ultrasound in women at increased risk of fetal anomalies. Prenat Diagn. 2013;33(9):863–8. doi: 10.1002/pd.4145. DOI: 10.1002/pd.4145. [DOI] [PubMed] [Google Scholar]
- 45.Engels MAJ, Twisk JWR, Blankenstein MA, van Vugt JMG. Age independent first trimester screening for Down syndrome: improvement in test performance. Prenat Diagn. 2013;33(9):884–8. doi: 10.1002/pd.4153. DOI: 10.1002/pd.4153. [DOI] [PubMed] [Google Scholar]
- 46.Picone O, Vauloup-Fellous C, Cordier AG, et al. A series of 238 cytomegalovirus primary infections during pregnancy: description and outcome. Prenat Diagn. 2013;33(8):751–8. doi: 10.1002/pd.4118. DOI: 10.1002/pd.4118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


