Abstract
Background
Cluster immunotherapy represents an interesting alternative to conventional up-dosing schedules because it allows achieving the maintenance dose within a shorter time interval. In this study, the efficacy and safety of cluster immunotherapy with a high polymerized allergen extract of a grass/rye pollen mixture have been evaluated in a randomized, double-blind, placebo-controlled, multicenter study.
Methods
In total, 121 patients with allergic rhinoconjunctivitis due to grass pollen were randomized 1 : 1 to verum or placebo group. A short cluster up-dosing schedule of only 1 week was applied to achieve the maintenance dose which was administered monthly during the study period of 1 year. Total combined symptom and medication score (TCS) was defined as primary outcome parameter. Secondary outcome parameters were individual symptom and medication scores, ‘well days,’ global improvement as well as immunological effects and nasal allergen challenge. The safety profile was evaluated based on the European academy of allergy and clinical immunology grading system.
Results
Significant reduction in the verum compared to the placebo group (intention-to-treat, population, verum: n = 55; placebo: n = 47) was found regarding TCS (P = 0.005), rhinoconjunctivitis total symptom score (RTSS, P = 0.006), and total rescue medication score (TRMS, P = 0.002). Additionally, secondary outcomes such as ‘well days,’ nasal challenge results, and increase of specific IgG4 were in favor of the active treatment. All systemic adverse reactions (0.8% of all injections in the verum group) were of mild intensity. No severe reactions related to the study medication were observed.
Conclusion
Cluster immunotherapy with high polymerized grass pollen extracts resulted in significant clinical efficacy and has been shown to be a safe treatment for grass pollen-allergic patients.
Keywords: allergic rhinoconjunctivitis, cluster immunotherapy, glutardialdehyde-polymerized allergen extract, grass pollen, specific subcutaneous immunotherapy
Grass pollen is the most important aeroallergen in many European countries 1, and sufficient allergen avoidance of pollen is hardly possible 2,3. Allergen-specific immunotherapy (SIT) as described for the first time more than a century ago by Noon 4 is currently the only causal treatment for patients suffering from allergic rhinoconjunctivitis aimed to ameliorate their allergic symptoms and to reduce the need of symptomatic, anti-allergic medication 3,5,6.
However, the subcutaneous form of this therapy (SCIT) has the drawback of rare but potentially life-threatening risks of systemic reactions 7,8. Therefore, chemically modified allergen extracts (allergoids) have been developed to reduce the IgE reactivity of these extracts contributing to a stepwise shortening of the initiation phase of SIT 9. Moreover, innovative modifications of the (initial) up-dosing schedules in SCIT have been explored, such as cluster schedules with multiple injections per treatment day to reach the maintenance dose within a short period of time 10,11.
Cluster-protocols have been investigated in several clinical trials using native or chemically modified allergen extracts 12–21. However, up to now, only few data have been published related to the clinical efficacy and tolerability of accelerated schedules in SCIT with chemically modified grass pollen allergen extracts 16,21.
Therefore, the aim of this randomized, double-blind, placebo-controlled, multicenter study was to assess clinical efficacy, immunological effects as well as tolerability and safety of SCIT with a high polymerized allergen extract initiated with an accelerated cluster schedule within 1 week.
Materials and methods
Study design
The study was designed as a 1-year (October 2008 to October 2009) multicenter, prospective, 1 : 1 randomized, double-blind, placebo-controlled (DBPC) trial, performed according to Good Clinical Practice 22 and the Declaration of Helsinki 23 in three study centers in Germany, approved by the responsible ethical committees.
Inclusion criteria were as follows: allergic rhinitis and/or allergic rhinoconjunctivitis, 18–75 years of age, clinically relevant sensitization to grass pollen possibly with additional controlled seasonal asthma as defined by the GINA guideline 2007 24, a positive skin prick test for grass pollen (wheal diameter >3 mm), specific IgE to grass pollen ≥CAP class 2, and written informed consent signed prior to inclusion into the study.
The most important exclusion criteria were as follows: predominant perennial allergic rhinitis, commonly accepted contraindications for specific immunotherapy, previous immunotherapy with grass or rye pollen extracts within the last 3 years, partly controlled or uncontrolled asthma as defined by the GINA guideline 2007 24, pregnancy or lack of adequate contraceptive protection.
Immunotherapy and treatment schedule
Subcutaneous immunotherapy was performed with a glutardialdehyde-modified high polymerized allergen extract especially developed for cluster immunotherapy using a special manufacturing approach to polymerize the allergens in order to obtain the highest polymerization resulting in less binding sites available for specific IgE. The study medication (verum) contained a standardized mixture of six grasses and rye pollen (60% grasses in equal parts: Holcus lanatus, Dactylis glomerata, Lolium perenne, Phleum pratense, Poa pratensis, Festuca elatior; 40% Secale cereale), adsorbed onto aluminum hydroxide (CLUSTOID®, ROXALL Medizin, Germany), and provided in one strength (10 000 TU/ml; 24 μg of Group 1 plus 5 allergens/ml). Placebo was identical to verum in appearance and content, but without containing allergens, and the investigational medicinal products were labeled with a randomization number.
The study started preseasonally in October 2008 following the cluster schedule during the initiation phase with two increasing doses injected on the first treatment day: 0.1 ml + 0.2 ml with an interval of 30 min in one and the other arm 16, 1 week later on the second treatment day 0.3 ml + 0.5 ml, also with an interval of 30 min. Thereafter, patients received the maintenance dose of 0.5 ml (= 5000 TU) every 4 weeks (±2 weeks) until October 2009. If considered necessary, any dose adjustment could be applied in the responsibility of the investigators. Altogether, 14 visits were scheduled during the 1-year study period. Few patients with longer intervals between injections received the last injection in November 2009, one patient in December 2009. After each injection, patients were under medical observation for at least 30 min 25.
Efficacy assessments
Symptom and medication score
The primary outcome parameter applied was the total combined score (TCS) taken into account the rhinoconjunctivitis total symptom score (RTSS) and the total rescue medication score (TRMS), which were evaluated separately.
The RTSS covers the six rhinoconjunctivitis symptoms (sneezing, rhinorrhea, nasal itching, nasal congestion, ocular tearing, and ocular itching) which were graded daily by the patients during the grass pollen season in a diary using the 4-point rating scale: 0 = no symptoms; 1 = mild symptoms; 2 = moderate symptoms; 3 = severe symptoms 26,27. The daily rhinoconjunctivitis symptom score was calculated as the mean of patients' valid symptom scores as proposed 28: Dividing the results by the number of symptoms (six symptoms, mentioned above) has the advantage that the daily RTSS always takes values between 0 and 3. The RTSS was then determined as the mean of the valid daily symptom scores during focusing on the peak pollen period (PPP) as well as on the pollen season (PS). Single rhinoconjunctivitis symptoms were analyzed separately as secondary outcome parameters.
During the grass pollen season, rescue medication for the management of allergic rhinoconjunctivitis was provided to the patients. The use of rescue and other concomitant medication was documented daily along with allergic symptoms in the same diary.
The daily rescue medication score was calculated as the weighted sum of the daily documented rescue medication (4 drops/eye of cromoglycate eye drops: 1 point; 1 tablet of oral antihistamines: 2 points; application of 4 puffs/nostril of nasal corticosteroids: 3 points; 1 tablet of leukotriene antagonists only on demand for asthmatic patients: 5 points).
The TRMS was determined as the mean of the valid daily rescue medication scores during PPP and PS, respectively.
Well days
A well day was defined to be a day without use of rescue medication and with ‘0 = no symptoms’ recorded for each of the six individual rhinoconjunctivitis symptoms according to our previous reporting and European medicines agency (EMA) guidelines 27,29; a similar definition was called ‘symptom-free day’ 30. The proportion of well days (%) was assessed for PPP and PS based on days with valid RTSS and TRMS scores.
Patients' contentment
At the end of the study, the patients rated their overall contentment with the treatment (not satisfied, less satisfied, satisfied, very satisfied).
Nasal challenge test
The nasal challenge test (NCT) was performed in a titrated way before and at the end of the study using two serial increasing concentrations (500 and 5000 BU/ml) of a six grass pollen mixture (ROXALL Medizin, Germany). Reactions were measured by rhinomanometry and by symptoms according to the German NCT guideline 31.
The allergen concentration provoking at first a positive reaction was defined to be the threshold of the NCT (0 = positive reaction after NaCl; 1 = positive reaction after 500 BU/ml; 2 = positive reaction after 5000 BU/ml; 3 = negative reaction after 5000 BU/ml). As NCT was performed only twice, missing values were not imputed; thus, main results are based on patients with values at both measurements only.
Immunological parameters
Before start and at the end of the study, specific IgE and IgG4 levels for Phleum pratense as well as for rye (Secale cereale) were determined. As immunological parameters were determined only twice, only patients with valid values at both measurements were included into the corresponding analyses.
Safety assessment
Grading of local and systemic adverse events was classified according to the European academy of allergy and clinical immunology (EAACI) Position Paper 32 and additionally related to the time of onset (immediate reactions = within 30 min after injection; late-type reactions = later than 30 min after injection). Furthermore, the global tolerability of the treatment (bad, satisfactory, good, very good) was assessed by the investigators at the last study visit.
Pollen counts
The relevant grass pollen counts were obtained from the ‘Stiftung Deutscher Polleninformationsdienst’ (http://www.pollenstiftung.de/) collected at Burkard pollen traps (Burkard Scientific Ltd., Uxbridge, UK) located close to each study center. According to the German Weather Forecast Institution ‘Deutscher Wetterdienst’ (DWD, http://www.dwd.de), the risk of grass pollen stress can be categorized as follows: ‘0 = no’ (= 0 pollen/m3 per 24 h), ‘1 = low’ (= 1–5 pollen/m3 per 24 h), ‘2 = moderate’ (= 6–30 pollen/m3 per 24 h), and ‘3 = high’ (>30 pollen/m3 per 24 h).
In the trial protocol, the PPP was defined as the month with at least 10 consecutive days with a risk of grass pollen stress of at least moderate (i.e., ≥6 pollen/m3 per 24 h). Based on this definition and on the actually observed grass pollen data, June 2009 (June 01, 2009 to June 30, 2009) was identified as PPP, comprising 30 days. A high pollen load was also observed during the period from May 20, 2009 to May 31, 2009 (cf. Fig.2), but to adhere strictly to the trial protocol according to current recommendations June 2009 was used as PPP.
Figure 2.
Mean daily rhinoconjunctivitis total symptom score (RTSS, left Y-axis, upper part) and mean daily total rescue medication score (TRMS, left Y-axis, middle part) in line with the mean daily pollen counts (right Y-axis at the bottom) during the peak pollen period (PPP) and during the pollen season (PS) for the intention-to-treat (ITT) population.
In addition, the whole grass PS was identified as the period from May 20, 2009 till July 05, 2009 comprising 47 days; on almost all of these 47 days, the pollen load was ≥6 pollen/m3 per 24 h in all three centers. Before and after PS, the pollen load for grass pollen was mainly ‘low’.
Sample size estimation
In a meta-analysis of several randomized placebo-controlled clinical SCIT application, studies 33 found mean effect sizes of 0.73 for RTSS and 0.57 for TRMS. Assuming the application of the two-sided Wilcoxon–Mann–Whitney U-test with α = 0.05 and power 1-ß = 0.8, sample sizes of 2 × 31 and 2 × 51, respectively, were determined for these two parameters, assuming a dropout rate of about 15% for this study.
Statistical analyses
All results are described by the usual descriptive statistics depending on the scale level of the corresponding parameter (counts, percentages, or mean ± standard deviation).
The primary endpoint was the TCS for the intention-to-treat (ITT) population taking into account the RTSS and TRMS during PPP. The following closed two-step multiple testing procedure controlling the overall α-level in multiple testing 34,35 was applied: In the first step, the combined hypotheses are tested by comparing the total combined score (TCS = sum of RTSS and TRMS after z-transformation) between study groups by means of a two-sided Wilcoxon–Mann–Whitney U-test with α = 0.05; if this overall test is significant, both the RTSS and TRMS scores are then compared between study groups by means of a two-sided Wilcoxon–Mann–Whitney U-test with α = 0.05.
Secondary endpoints were TCS, RTSS, and TRMS during PS, the single rhinoconjunctivitis symptom scores during PPP and PS, and all other parameters described above. For these, study groups were compared using the Mann–Whitney U-test in case of ordinal or continuous and the chi-square test in case of categorical variables (α = 0.05 for each test carried out without α-adjustment for multiple testing). For continuous outcomes, the estimated effect size (ES, Hedges's g) and its 95% confidence interval (95% CI) were determined as described in 33. Analyses of safety parameters were of descriptive nature.
Randomization and blinding
For allocation of the participants to the trial groups, a computer-generated list of random numbers was used prepared by an independent biometrical institute; randomization sequence was stratified by center with a 1 : 1 allocation using random block sizes of 4. Placebo and verum were in vials, packed in two boxes per patient. Vials and boxes were labeled according to national regulations and consecutively numbered according to the randomization schedule.
Clinical supplies were sent to the investigators along with the appropriate documents and sealed emergency envelopes and had to be handled in accordance with national regulations. Eligible patients who fulfilled all of the inclusion criteria and none of the exclusion criteria were randomized. The patients were assigned the next random number available at the corresponding study center in a consecutive and ascending way.
Results
Patients
Altogether, 121 patients (18–69 years) screened and randomized 1 : 1 either to the verum or to the placebo group. Due to a screening failure, one randomized patient was excluded before any further treatment. Thus, 120 patients (verum: n = 61; placebo: n = 59) received the study medication as randomized and at least once and were evaluated as safety population. One hundred and two patients were analyzed in the ITT population (Fig.1), comprising all patients of the safety population with a diary compliance ≥50% during the peak pollen period (verum: n = 55; placebo: n = 47). All patients achieved and received later injections of the maintenance dose of 0.5 ml. Both treatment groups were balanced with respect to demographic, physical, and anamnestic characteristics (Table1).
Figure 1.

Overall participant flow during study.
Table 1.
Patients characteristics (ITT population; n = 102)
| Intention-to-treat (ITT) group | Verum | Placebo |
|---|---|---|
| Number of patients | 55 | 47 |
| Gender, n (%) | ||
| Female | 31 (56.4) | 21 (44.7) |
| Male | 24 (43.6) | 26 (55.3) |
| Age (mean ± SD) [years] | 37.1 ± 10.4 | 36.2 ± 10.7 |
| Range | 18.1–65.4 | 19.5–69.5 |
| Height [cm] | 175.0 ± 9.6 | 174.6 ± 10.0 |
| Weight [kg] | 74.6 ± 15.7 | 75.6 ± 14.0 |
| BMI | 24.2 ± 3.8 | 24.7 ± 3.7 |
| Clinical symptoms, n (%)* | ||
| Rhinitis | 55 (100.0) | 47 (100.0) |
| Rhinoconjunctivitis | 54 (98.2) | 44 (93.6) |
| Allergic asthma | 8 (14.6) | 3 (6.4) |
| Skin prick test results to grass pollen extract [mean wheal diameter in mm] | 7.3 ± 2.8 | 7.2 ± 3.4 |
Multiple symptoms possible.
In both ITT study groups, the mean number of injections (about 16), the mean diary compliance (PPP: about 97%, PS: about 96%), the mean number of days with valid diary data (PPP: about 29.5 days, PS: 46), and the mean pollen counts (PPP: 25.5/m3, PS: 29/m3) were comparable (U-tests: p-values always >α = 0.10).
Clinical efficacy: symptom and medication score
Evaluation of the primary objective in the ITT population revealed a significant difference for the TCS between verum and placebo group during PPP (verum: −0.44 ± 1.69, placebo: 0.54 ± 1.85; P = 0.006; ES = −0.5509, 95% CI = [−0.9475, −0.1543]) as well as during the whole pollen season (PS; verum: −0.45 ± 1.66, placebo: 0.51 ± 1.87; P = 0.004; ES = −0.5414, 95% CI = [−0.9378, −0.1451]). In addition, RTSS and TRMS were evaluated and described separately.
A significant mean reduction for the RTSS of 36% in the verum group compared to placebo was obtained during PPP (verum: 0.55 ± 0.43, placebo: 0.86 ± 0.58; P = 0.006; ES = −0.6098, 95% CI = [−1.0080, −0.2115]) as well as of 34% during PS (verum: 0.57 ± 0.41, placebo: 0.86 ± 0.55; P = 0.004; ES = −0.6002, 95% CI = [−0.9982, −0.2023]).
The use of rescue medication in both treatment groups was significantly lower in the verum group than in the placebo group during PPP (verum: 0.64 ± 1.36, placebo: 1.13 ± 1.16, 43% reduction, P = 0.002; ES = −0.3824, 95% CI = [−0.7752, +0.0105]) as well as during PS (verum: 0.70 ± 1.28, placebo: 1.17 ± 1.16, 40% reduction, P = 0.004; ES = −0.3804, 95% CI = [−0.7732, +0.0124]).
In Fig.2, symptom scores and medication scores are presented separately: the originally obtained (not divided) scores of the mean daily RTSS for both treatment groups are shown in the upper part, the mean daily TRMS for both treatment groups in the middle part and the daily pollen counts in the bottom line, showing the close relation of allergic symptoms and use of rescue medication to the pollen load; on 27 of 30 days within PPP, significant differences between both treatment groups were observed (PS: 35 of 47 days). Similar results were obtained for the per-protocol (PP) population (data not shown).
The means of all six single-symptom scores during PPP were significantly lower in the verum group as compared to placebo (P ≤ 0.028 for each score) as shown in Fig.3.
Figure 3.
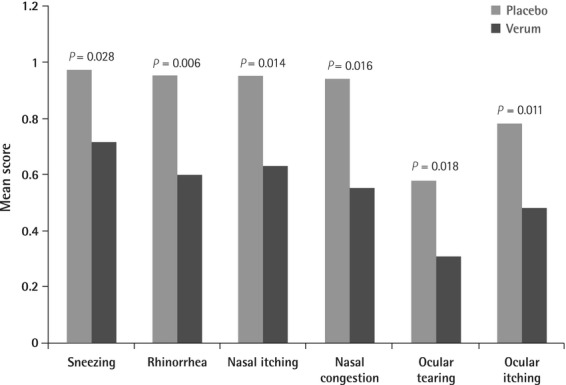
Mean single-symptom scores during peak pollen period (intention-to-treat population), P-values from U-tests between study groups.
Well days
On average, patients in the verum group had significantly more well days in relation to all days under consideration than patients in the placebo group during PPP (verum: 25.59 ± 29.40%, placebo: 18.47 ± 29.42%; P = 0.033) as well as during PS (verum: 24.08 ± 27.44%, placebo: 18.33 ± 28.70%; P = 0.025, respectively).
Patients' contentment
At the end of the study, significantly more patients from the verum group (90.9%) were satisfied or very satisfied with the treatment compared to the patients of the placebo group (59.6%; P < 0.001).
Nasal Challenge Test
Sixty two patients of the ITT population (verum: n = 34; placebo: n = 28) had evaluable data before and after treatment. At the initial visit, the mean threshold was 1.38 ± 1.13 in the verum and 1.36 ± 1.16 in the placebo group with no significant difference between study groups. At the end of the study, the threshold increased by 0.56 ± 1.31 in the verum and by 0.11 ± 1.31 in the placebo group. The difference between groups was, however, not significant (P = 0.122).
Immunological parameters
Seventy four patients of the ITT population (verum: n = 39; placebo: n = 35) had evaluable pre- and posttreatment IgG4 (μg/L). Both groups were comparable before treatment for specific IgG4 to Phleum pratense (verum: 463.31 ± 628.69, placebo: 515.83 ± 666.69) as well as to rye (verum: 251.23 ± 230.54, placebo: 326.17 ± 374.96). Pre- and postchanges were significantly higher in the verum compared to the placebo group with regard to Phleum pratense-specific IgG4 (verum: 3061.36 ± 5005.24, placebo: 35.49 ± 316.92; P < 0.001) and rye-specific IgG4 (verum: 3019.44 ± 4941.66, placebo: 37.17 ± 207.77; P < 0.001).
Eighty eight patients of the ITT population (verum: n = 49; placebo: n = 39) had evaluable data before and after treatment regarding specific IgE to Phleum pratense and specific IgE to rye. Specific IgE (kU/L) to Phleum pratense (verum: 21.76 ± 27.63, placebo: 19.00 ± 28.09) and to rye (verum: 17.13 ± 25.40, placebo: 12.86 ± 18.85) was comparable between the study groups at the beginning of the study.
There were no significantly different pre- and postchanges when comparing verum and placebo group for specific IgE to Phleum pratense (verum: 4.30 ± 15.58; placebo: 1.18 ± 5.35) and to rye (verum: 7.42 ± 13.49, placebo: 3.79 ± 8.13).
Safety and tolerability
Safety of treatment was evaluated for 120 patients (verum: n = 61; placebo: n = 59) receiving a total of 1778 injections (verum: n = 928, placebo: n = 850). The majority of the patients received 16 injections throughout the study period: 84% in the verum group and 75% in the placebo group. The mean dose for these patients in the verum group was 6.69 ± 1.39 ml and 6.30 ± 1.66 ml in the placebo group, respectively, corresponding to a mean total dose of 66 900 TU per patient in the verum group.
Immediate local reactions ≥ grade 1 occurred after 0.7% of verum injections during the up-dosing phase. Two immediate systemic reactions of mild intensity (rhinitis, nasal obstruction) were reported during the up-dosing phase (0.2% of verum injections), whereas 0.6% late-phase systemic reactions grade 1 were described (fatigue, nasal obstruction, skin reaction), one patient reported about circulatory dysregulation after having performed sports. Systemic reactions > grade 2 as classified in the EAACI Position Paper 32 have not been observed.
Additionally, the global evaluation of the tolerability of the treatment was assessed as good/very good by the investigators for 95% of verum and 100% of placebo patients.
No severe adverse events related to the study medication occurred at all.
Discussion
This is the first randomized, double-blind, placebo-controlled study with a high polymerized grass/rye pollen mixture developed for cluster immunotherapy (‘clustoid’).
Previously, the clinical effect of cluster immunotherapy with grass pollen clustoids has been shown using the nasal challenge test as an objective outcome parameter 16. However, this was an open controlled trial without a placebo group.
Thus, in the present study, efficacy of the investigational product was shown according to the actual ‘gold standard’ using symptom and medication scores as primary endpoints as recently defined 26,27,36,37.
Few double-blind, placebo-controlled studies with modified grass pollen extracts (‘allergoids’) have been published; moreover, in these studies, different allergoid preparations with different characteristics, varying study designs, and treatment schedules 6,21,38–40 were applied.
In our study, we could show a significant reduction of total symptom and medication scores verum vs placebo during PPP in the ITT population by 36% and 43%, respectively, even very early during the first pollen season following the initiation of treatment (Fig.2).
Moreover, we found significant improvements for ‘well days’ and ‘patient's contentment’ evaluated as secondary endpoints according to the EMA guideline 27. These data confirm the results of symptom and medication scores from the patients' diaries. In this study, ‘well days’ were defined to be symptom- and medication-free days, but are defined in other studies as days with no intake of medication and with a symptom score ≤2 41–43.
In addition, we found a trend toward an improved tolerance in nasal challenge with grass pollen in verum patients, although significance was not achieved probably due to the small number of valid pre- and postdata. Due to technical and practical faults (e.g., malfunction of the rhinomanometer, errors in performing the NCT or in the documentation of results), evaluable data were obtained only from 61% of the ITT study population. Furthermore, for future studies, we recommend to include more than two different dilution steps for the titrated nasal challenge test to obtain more differentiated effects. Nevertheless, these findings support the results from symptom and medication scores and comply with the formerly described data 16.
Increase of Phleum pratense-specific IgG4 as well as rye-specific IgG4 was significantly higher in the verum compared to the placebo group; this result might be viewed as a marker of introduced allergen dose 44–46 reflecting an immunological effect of the treatment. These findings are in concordance with previous studies showing an increase of specific IgG4 after SCIT with modified pollen extracts 21,40,47. However, a direct correlation between clinical improvement and increase of specific IgG4 could not be determined as no patient-related baseline data regarding the severity of symptoms were included in the study design.
Safety aspects of cluster immunotherapy have been investigated mostly in studies using unmodified, but also using modified allergen extracts with a wide variety of cluster schedules up to 5 weeks with up to three injections per treatment day 12–14,18,19. In our study, we applied a rapid up-dosing schedule for cluster immunotherapy to reach the maintenance dose within 1 week, which was achieved in all patients. Immediate and late local reactions ≥grade 1 were observed in 1.2% of verum injections. Five verum patients developed mild systemic reactions of grade 1 such as nasal irritation or nasal obstruction. Additionally, one of these patients reported also about dyspnoea and circulatory dysfunction after having sports; however, this was a protocol violence by the patient as it was strictly not advised at the day of the SIT injections during this study and the relationship with the injection was questionable for the investigator. The majority of reactions related to the study medication (94%) were immediate-type reactions occurring within 30 min after injection, when the patients were under medical observation. No severe adverse events related to the study medication occurred at all.
In conclusion, in our double-blind, placebo-controlled phase III clinical trial, cluster immunotherapy with a modified, high polymerized grass/rye pollen mixture has been shown to be clinically and immunologically effective in the first grass pollen season after initiation of SCIT and, moreover, revealed a good safety profile although a rapid up-dosing schedule achieving the maintenance dose within one week was performed.
Acknowledgments
The authors wish to thank all participants for collaboration in the study, the investigators Dr. Daniela Kasche and Dr. Norbert Pasch as well as the study nurses.
Glossary
- EAACI
European academy of allergy and clinical immunology
- EMA
European medicines agency
- Ig
immunoglobulin
- ITT
intention-to-treat
- NCT
nasal challenge test
- PP
per protocol
- PPP
peak pollen period
- PS
pollen season
- RTSS
rhinoconjunctivitis total symptom score
- SCIT
subcutaneous immunotherapy
- SIT
specific immunotherapy
- TCS
total combined score
- TRMS
total rescue medication score
Authors contributions
LK was coordinating investigator of the trial. OP participated in the trial as recruiting investigator. JU was the sponsor's project manager for the trial, involved in trial design and conduct. RM was responsible for biometrical planning and involved in trial design and conduct. KR was responsible for data management and final statistical analyses. JU wrote the first draft of the manuscript. All authors contributed to the manuscript preparation from the draft stage by reviewing, commenting, critically revising the text where applicable, and approving journal submission. LK took overall responsibility for manuscript finalization and submission.
Funding
This trial was funded by ROXALL Medizin, Hamburg, Germany. The full trial protocol can be accessed at ROXALL Medizin, Hamburg, Germany.
Conflicts of interest
L Klimek (LK) and O Pfaar (OP) have received research grants from ALK-Abelló, Denmark; Allergopharma, Germany; Bionorica, Germany; Dr. Pfleger, Germany; Stallergenes, France; HAL, the Netherlands; Artu Biologicals, the Netherlands; Allergy Therapeutics/Bencard, Great Britain/Germany; Hartington, Spain; Lofarma, Italy; MEDA, Sweden; MSD, USA; Novartis/Leti, Germany/Spain; ROXALL, Germany; GlaxoSmithKline (GSK), Great Britain; Essex-Pharma, Germany; Cytos, Switzerland; Curalogic, Denmark, and have served as advisors and on the speaker's bureau for some of the above-mentioned pharmaceutical companies.
OP has received travel grants from HAL-Allergy (the Netherlands/Germany) and Allergopharma (Germany) and is a consultant for Bencard (Germany), HAL-Allergy (the Netherlands), Novartis (Germany), MEDA (Germany), and Stallergenes (France). OP is the current chairman of the EAACI Immunotherapy Interest Group and secretary of section ENT of DGAKI.
Ralph Mösges (RM) reports personal fees and nonfinancial support from ALK-Abello, personal fees and nonfinancial support from Allergy Therapeutics/Bencard, personal fees from Allergopharma, grants and personal fees from Biotech Tools, nonfinancial support from ROXALL/Dr. Beckmann, grants, personal fees, and nonfinancial support from Lofarma, personal fees from MSD, personal fees from Leti, personal fees from Novartis, personal fees from Paladin, grants and personal fees from Stallergenes, outside the submitted work.
J Uhlig (JU) is employee of ROXALL Medizin, Hamburg, Germany.
K Rettig (KR) is a consultant working for GEM, Meerbusch, Germany. He was engaged by ROXALL Medizin to carry out the data management and the statistical analyses.
References
- 1.D'Amato G, Cecchi L, Bonini S, Nunes C, Annesi-Maesano I, Behrendt H, et al. Allergenic pollen and pollen allergy in Europe. Allergy. 2007;62:976–990. doi: 10.1111/j.1398-9995.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 2.Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(Suppl. 5):S147–S334. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- 3.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl. 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 4.Noon L. Prophylactic inoculations against hay fever. Lancet. 1911;1:1572–1573. [Google Scholar]
- 5.Bousquet J, Lockey RF, Malling HJ. WHO position paper: allergen immunotherapy: therapeutic vaccines for allergic diseases. Allergy. 1998;53:1–42. [Google Scholar]
- 6.Cox L, Calderon M, Pfaar O. Subcutaneous allergen immunotherapy for allergic disease: examining efficacy, safety and cost-effectiveness of current and novel formulations. Immunotherapy. 2012;6:1–16. doi: 10.2217/imt.12.36. [DOI] [PubMed] [Google Scholar]
- 7.Bukantz SC, Bagg AS, Lockey RF. Adverse effects and fatalities associated with subcutaneous allergen immunotherapy. Clin Allergy Immunol. 2008;21:455–468. [PubMed] [Google Scholar]
- 8.Stewart GE, 2nd, Lockey RF. Systemic reactions from allergen immunotherapy. J Allergy Clin Immunol. 1992;90:567–578. doi: 10.1016/0091-6749(92)90129-p. [DOI] [PubMed] [Google Scholar]
- 9.Marsh DG. Preparation and properties of allergoids, derived from native pollen allergens by mild formalin treatment. Int Arch Allergy Appl Immunol. 1971;41:199–215. doi: 10.1159/000230518. [DOI] [PubMed] [Google Scholar]
- 10.Cox L. Accelerated immunotherapy schedules: review of efficacy and safety. Ann Allergy Asthma Immunol. 2006;97:126–137. doi: 10.1016/S1081-1206(10)60003-8. [DOI] [PubMed] [Google Scholar]
- 11.Pfaar O, Leitzbach S, Hormann K, Klimek L. Cluster protocols in SCIT: enough evidence for practical use? Curr Opin Allergy Clin Immunol. 2010;10:188–193. doi: 10.1097/ACI.0b013e328339505c. [DOI] [PubMed] [Google Scholar]
- 12.Hansen I, Hormann K, Stuck BA, Schneider-Gene S, Mösges R, Klimek L. Cluster-Immuntherapie bei allergischer Rhinokonjunktivitis. Allergologie. 2002;25:549–556. [Google Scholar]
- 13.Serrano P, Algorta J, Martinez A, Gonzalez-Quevedo T, Velazquez E, Diaz M. Prospective safety study of immunotherapy administered in a cluster schedule. J Investig Allergol Clin Immunol. 2004;14:312–319. [PubMed] [Google Scholar]
- 14.Tabar AI, Echechipia S, Garcia BE, Olaguibel JM, Lizaso MT, Gomez B, et al. Double-blind comparative study of cluster and conventional immunotherapy schedules with Dermatophagoides pteronyssinus. J Allergy Clin Immunol. 2005;116:109–118. doi: 10.1016/j.jaci.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Casanovas M, Martin R, Jimenez C, Caballero R, Fernández-Caldas E. Safety of an ultra-rush immunotherapy build-up schedule with therapeutic vaccines containing depigmented and polymerized allergen extracts. Int Arch Allergy Immunol. 2006;139:153–158. doi: 10.1159/000090392. [DOI] [PubMed] [Google Scholar]
- 16.Subiza J, Feliú A, Subiza JL, Uhlig J, Fernández-Caldas E. Cluster immunotherapy with a glutaraldehyde-modified mixture of grasses results in an improvement in specific nasal provocation tests in less than 2.5 months of treatment. Clin Exp Allergy. 2008;38:987–994. doi: 10.1111/j.1365-2222.2008.02995.x. [DOI] [PubMed] [Google Scholar]
- 17.Pfaar O, Klimek L, Fischer I, Sieber J, Amoroso S, Moreno AC, et al. Safety of two Cluster schedules for subcutaneous immunotherapy in allergic rhinitis or asthma patients sensitized to inhalant allergens. Int Arch Allergy Immunol. 2009;150:102–108. doi: 10.1159/000210436. [DOI] [PubMed] [Google Scholar]
- 18.Schubert R, Eickmeier O, Garn H, Baer PC, Mueller T, Schulze J, et al. Safety and immunogenicity of a cluster specific immunotherapy in children with bronchial asthma and mite allergy. Int Arch Allergy Immunol. 2009;148:251–260. doi: 10.1159/000161585. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Wang C, Han D, Wang X, Zhao Y, Liu J. Comparative study of cluster and conventional immunotherapy schedules with Dermatophagoides pteronyssinus in the treatment of persistent allergic rhinitis. Int Arch Allergy Immunol. 2009;148:161–169. doi: 10.1159/000155747. [DOI] [PubMed] [Google Scholar]
- 20.Pfaar O, Mösges R, Hörmann K, Klimek L. Safety aspects of Cluster immunotherapy with semi-depot allergen extracts in seasonal allergic rhinoconjunctivitis. Eur Arch Otorhinolaryngol. 2010;267:245–250. doi: 10.1007/s00405-009-1077-6. [DOI] [PubMed] [Google Scholar]
- 21.Pfaar O, Urry Z, Robinson DS, Sager A, Richards D, Hawrylowicz CM, et al. A randomized placebo-controlled trial of rush preseasonal depigmented polymerized grass pollen immunotherapy. Allergy. 2012;67:272–279. doi: 10.1111/j.1398-9995.2011.02736.x. [DOI] [PubMed] [Google Scholar]
- 22.European Medicines Agency. Committee for Proprietary Medicinal Products (CPMP) and International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH): ICH Topic E 6 (R1)/Note for Guidance on Good Clinical Practice. 2002. ; CPMP/ICH/135/95.
- 23.World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. Adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, and amended by the 59th WMA General Assembly, Edinburgh, Scotland, October 2000 and Washington 2002.
- 24.Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2007. . [Available from: http://www.ginasthma.org ]
- 25.Alvarez-Cuesta E, Bousquet J, Canonica GW, Durham SR, Malling HJ, Valovirta E. Standards for practical allergen-specific immunotherapy. Allergy. 2006;61(Suppl. 82):1–20. doi: 10.1111/j.1398-9995.2006.01219_1.x. [DOI] [PubMed] [Google Scholar]
- 26.Canonica GW, Baena-Cagnani CE, Bousquet J, Bousquet PJ, Lockey RF, Malling HJ, et al. Recommendations for standardization of clinical trials with Allergen Specific Immunotherapy for respiratory allergy. A statement of a World Allergy Organization (WAO) taskforce. Allergy. 2007;62:317–324. doi: 10.1111/j.1398-9995.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- 27.Committee for Medicinal Products for Human Use (CHMP) Guideline on the Clinical Development of Products for Specific Immunotherapy for the Treatment of Allergic Diseases. London: CHMP/EWP/18504/2006; 2008. [Google Scholar]
- 28.Clark J, Shall R. Assessment of combined symptom and medication scores for rhinoconjunctivitis immunotherapy clinical trials. Allergy. 2007;62:1023–1028. doi: 10.1111/j.1398-9995.2007.01469.x. [DOI] [PubMed] [Google Scholar]
- 29.Vorwalder S, Pfaar O, Klimek L. Klinische parameter zur Beurteilung der Wirksamkeit einer spezifischen Immuntherapie bei polleninduzierter Rhinitis allergica. Bestimmung von “well days” als ergänzendes Konzept. Allergologie. 2010;33:35–42. [Google Scholar]
- 30.Didier A, Malling HJ, Worm M, Horak F, Jäger S, Montagut A, et al. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5-grass pollen tablet for seasonal allergic rhinitis. J Allergy Clin Immunol. 2007;120:1338–1345. doi: 10.1016/j.jaci.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 31.Riechelmann H, Bachert C, Goldschmidt O, Hauswald B, Klimek L, Schlenter WW, et al. Application of the nasal provocation test on diseases of the upper airways. Position paper of the German Society for Allergology and Clinical Immunology (ENT Section) in cooperation with the Working Team for Clinical Immunology. Laryngorhinootologie. 2003;82:183–188. doi: 10.1055/s-2003-38411. [DOI] [PubMed] [Google Scholar]
- 32.Malling HJ, Weeke B. Immunotherapy. Position paper of the European Academy of Allergology and Clinical Immunotherapy. Allergy. 1993;48:7–35. [PubMed] [Google Scholar]
- 33.Turner HM, Bernard RM. Calculating and synthesizing effect sizes. Contemp Issues Commun Sci Disord. 2006;33:42–55. [Google Scholar]
- 34.Lehmacher W, Wassmer G, Reitmeir P. Procedures for two-sample comparisons with multiple endpoints controlling the experimentwise error rate. Biometrics. 1991;47:511–521. [PubMed] [Google Scholar]
- 35.Vent J, Lehmacher W. The problem of placebo-controlled trials allowing rescue medication: how to assess efficacy in rhinosinusitis and allergic rhinoconjunctivitis when both groups receive active rescue treatment. Curr Opin Allergy Clin Immunol. 2009;9:222–227. doi: 10.1097/ACI.0b013e32832aa5cf. [DOI] [PubMed] [Google Scholar]
- 36.Draft guidance; 2000. U.S. Department of Health and Human Serviced. FDA Food and Drug Administration. Center for Drug Evaluation and Research (CDER), Guidance for Industry. Allergic Rhinitis: Clinical development programs for drug products. [Google Scholar]
- 37.Pfaar O, Demoly P, Gerth van Wijk R, Bonini S, Bousquet J, Canonica GW, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy. 2014;69:854–867. doi: 10.1111/all.12383. [DOI] [PubMed] [Google Scholar]
- 38.Drachenberg KJ, Wheeler AW, Stuebner P, Horak F. A well-tolerated grass pollenspecific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy. 2001;56:498–505. doi: 10.1034/j.1398-9995.2001.056006498.x. [DOI] [PubMed] [Google Scholar]
- 39.Alvarez-Cuesta E, Aragoneses-Gilsanz E, Martín-Garcia C, Berges-Gimeno P, Gonzalez-Mancebo E, Cuesta-Herranz J. Immunotherapy with depigmented glutaraldehyde-polymerized extracts: changes in quality of life. Clin Exp Allergy. 2005;35:572–578. doi: 10.1111/j.1365-2222.2005.02245.x. [DOI] [PubMed] [Google Scholar]
- 40.Corrigan CJ, Kettner J, Doemer C, Cromwell O, Narkus A. Efficacy and safety of preseasonal-specific immunotherapy with an aluminium-adsorbed six-grass pollen allergoid. Allergy. 2005;60:801–807. doi: 10.1111/j.1398-9995.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 41.Bufe A, Eberle P, Franke-Beckmann E, Funck J, Kimmig M, Klimek L, et al. Safety and efficacy in children of an SQ-standardized grass allergen tablet for sublingual immunotherapy. J Allergy Clin Immunol. 2009;123:167–173. doi: 10.1016/j.jaci.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 42.Pfaar O, Kleine-Tebbe J, Hörmann K, Klimek L. Allergen-specific immunotherapy: which outcome measures are useful in monitoring clinical trials? Immunol Allergy Clin North Am. 2011;31:289–309. doi: 10.1016/j.iac.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Calderon MA, Eichel A, Makatsori M, Pfaar O. Comparability of subcutaneous and sublingual immunotherapy outcomes in allergic rhinitis clinical trials. Curr Opin Allergy Clin Immunol. 2012;12:249–256. doi: 10.1097/ACI.0b013e32835358b3. [DOI] [PubMed] [Google Scholar]
- 44.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2007;119:780–789. doi: 10.1016/j.jaci.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 45.Shamji MH, Durham SR. Mechanisms of immunotherapy to aeroallergens. Clin Exp Allergy. 2011;41:1235–1246. doi: 10.1111/j.1365-2222.2011.03804.x. [DOI] [PubMed] [Google Scholar]
- 46.Shamji MH, James LK, Durham SR. Serum immunologic markers for monitoring allergen-specific immunotherapy. Immunol Allergy Clin North Am. 2011;31:311–323. doi: 10.1016/j.iac.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Pfaar O, Robinson DS, Sager A, Emuzyte R. Immunotherapy with depigmented-polymerized mixed tree pollen extract: a clinical trial and responder analysis. Allergy. 2010;65:1614–1621. doi: 10.1111/j.1398-9995.2010.02413.x. [DOI] [PubMed] [Google Scholar]



