Abstract
Objectives
To evaluate the periprocedural characteristics and outcomes of patients supported with Impella 2.5 prior to percutaneous coronary intervention (pre-PCI) versus those who received it after PCI (post-PCI) in the setting of cardiogenic shock (CS) complicating an acute myocardial infarction (AMI).
Background
Early mechanical circulatory support may improve outcome in the setting of CS complicating an AMI. However, the optimal timing to initiate hemodynamic support has not been well characterized.
Methods
Data from 154 consecutive patients who underwent PCI and Impella 2.5 support from 38 US hospitals participating in the USpella Registry were included in our study. The primary end-point was survival to discharge. Secondary end-points included assessment of patients’ hemodynamics and in-hospital complications. A multivariate regression model was used to identify independent predictors for mortality.
Results
Both groups were comparable except for diabetes (P = 0.02), peripheral vascular disease (P = 0.008), chronic obstructive pulmonary disease (P = 0.05), and prior stroke (P = 0.04), all of which were more prevalent in the pre-PCI group. Patients in the pre-PCI group had more lesions (P = 0.006) and vessels (P = 0.01) treated. These patients had also significantly better survival to discharge compared to patients in the post-PCI group (65.1% vs.40.7%, P = 0.003). Survival remained favorable for the pre-PCI group after adjusting for potential confounding variables. Initiation of support prior to PCI with Impella 2.5 was an independent predictor of in-hospital survival (Odds ratio 0.37, 95% confidence interval: 0.17–0.79, P = 0.01) in multivariate analysis. The incidence of in-hospital complications included in the secondary end-point was similar between the 2 groups.
Conclusions
The results of our study suggest that early initiation of hemodynamic support prior to PCI with Impella 2.5 is associated with more complete revascularization and improved survival in the setting of refractory CS complicating an AMI.
Introduction
Prompt revascularization with percutaneous coronary intervention (PCI) has significantly reduced the incidence of cardiogenic shock (CS) and improved survival in the setting of acute myocardial infarction (AMI).1,2 However, when it occurs, CS remains a highly fatal complication following an AMI,3 despite aggressive revascularization and other adjunctive therapies.3,4 Percutaneous ventricular assist devices (pVADs) have been shown to provide superior hemodynamic support compared to intraaortic balloon pump (IABP) counterpulsation.4,5 In light of the recently reported studies that have failed to show a hemodynamic or survival benefit of IABP in the setting of post-AMI CS,6–10 physicians might adopt a reflexive strategy to use pVADs in this setting more often. While prospective randomized and adequately powered clinical trials remain warranted to evaluate the potential benefits of these new devices, real-world observational data can be useful to provide clinical insights on their use in daily routine practice. In the present study, we sought to evaluate the current use of Impella 2.5 in patients with confirmed CS complicating an AMI undergoing PCI. In particular, we were interested in evaluating the outcomes of patients who received hemodynamic support with Impella 2.5 (Abiomed, Inc., Danvers, MA, USA), prior to PCI versus those who received Impella 2.5 after PCI.
Methods
Study Population
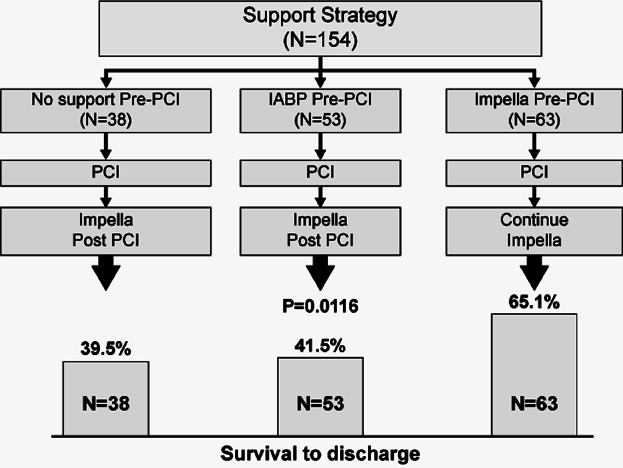
A total of 154 consecutive unselected patients who were reported in the USpella Registry to have undergone a PCI and Impella 2.5 hemodynamic support for a confirmed AMI with a CS indication were included in our study. The USpella Registry is an on-going multicenter voluntary registry open to all sites in the United States that have used the Impella 2.5 for all indications in more than 10 patients. Sites were invited to report all their consecutive Impella 2.5 cases without pre-selection of indication or patients. Between June 2009 and March 2012, 47 US sites had joined the USpella Registry. Of these, 38 sites had contributed 1 or more cases of patients who met the study inclusion criteria of CS complicating an AMI who underwent PCI. The remaining 9 sites reported no patients who met the study AMI with CS definition. The flow of patients is depicted in Figure 1.
Figure 1.
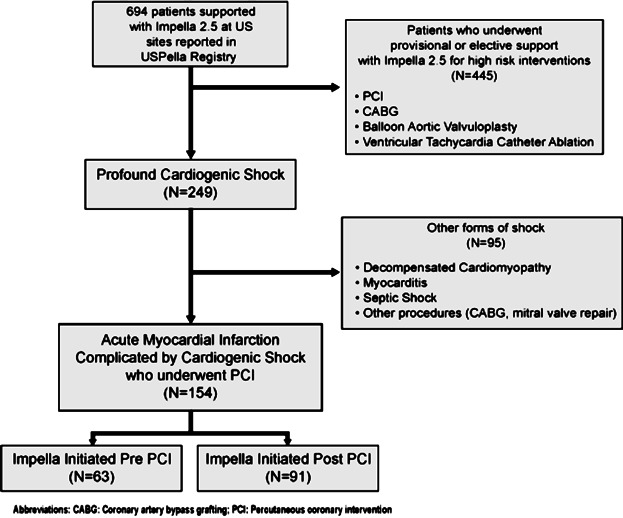
Patient flow chart.
The diagnosis of AMI was confirmed by electrocardiographic changes indicative of new or presumed new ischemia (significant new ST-T changes or new left bundle branch block), the presence of elevated cardiac biomarker values or based on coronary angiography. CS was defined by the presence of the following criteria: (1) systolic blood pressure <90 mmHg for >30 min or the need for vasopressor and/or inotropic therapy and/or IABP to maintain a systolic blood pressure greater than 90 mmHg; (2) signs of organ hypoperfusion such as oliguria/anuria, altered mental status, or cold extremities. All study patients underwent a PCI. Patients who underwent other means of revascularization than PCI or etiologies of shock other than AMI were not included in this analysis.
Timing of Impella insertion was left at the operating physician’s discretion. Coronary angiography and PCI were performed in a conventional manner. Patients were treated with drug-eluting stents and/or bare metal stents and/or percutaneous transluminal coronary angioplasty according to individual experience and local institutional guidelines. The number of vessels and lesions treated and the use of adjunctive therapies were left at the physician’s discretion.
Study End-points
The primary end-point aimed to assess the potential difference in survival rate to discharge between patients who received Impella 2.5 pre-PCI and those who received it post-PCI. The secondary end-points included the assessment of patients’ hemodynamics with Impella 2.5 support and in-hospital incidence of myocardial reinfarction, stroke, repeat revascularization, renal insufficiency, bleeding, hemolysis, limb ischemia, vascular complications requiring surgical repair, and infection. Myocardial reinfarction was defined as the recurrence of an MI distinct from the index event. Stroke was defined as an ischemic or hemorrhagic cerebrovascular accident that persisted beyond 24 hours or less than 24 hours associated with infarction on an imaging study. Renal insufficiency was defined as abnormal kidney function requiring dialysis (including hemofiltration) in patients who did not require dialysis prior to implant, or a rise in serum creatinine of greater than 2.5 mg/dL or greater than 2 times baseline. Bleeding was defined as blood loss requiring blood transfusion or surgical exploration for resolution. Hemolysis was defined by abnormal plasma free hemoglobin values greater than 40 mg/dL or presence of hematuria. Limb ischemia was reported whenever noted in the patient’s chart as new incidences of hypoperfusion of the leg requiring treatment and marked by such symptoms as decreased skin temperature of the limb or decreased peripheral pulses. Vascular complication requiring surgical repair was defined as a surgical intervention on a pseudoaneurysm, an arteriovenous fistula, a vessel dissection/perforation, or an access site thrombosis. Infection was defined as a clinical infection accompanied by pain, fever, drainage, and/or leukocytosis treated with nonprophylactic antimicrobial agents. Survival rate was reported at 30-day post-Impella implant when available at the time of data collection.
Device
The Impella 2.5 device (Abiomed, Inc.) has been described in detail elsewhere.11 Briefly, the 12 Fr microaxial pump is mounted on a 9 Fr catheter. It is inserted through the femoral artery using a modified Seldinger technique. The pump is advanced retrogradely across the aortic valve into the left ventricle under fluoroscopy guidance. It generates up to 2.5 L/min of forward flow directly in the ascending aorta. Heparinized dextrose fluid is purged through the pump and released in the general circulation at the usual rate of 4–12 mL/hr to prevent clot formation in the motor and early pump wear. The manufacturer recommends an activated thrombin time (ACT) of 160–180 seconds during pump support.
Data Collection
The USpella Registry was designed by an Executive Steering Committee and supervised by a Scientific Advisory Board that oversaw its ongoing conduct. The investigators had full access to the data. The registry protocol was reviewed and approved by the Institutional Review Board at each site. Investigators were asked to report in the registry all patients who had been supported with Impella 2.5 at their institution regardless of indication. Data were abstracted retrospectively from the medical record onto a standard case report form by the sites’ study coordinators who were centrally trained. Information was collected retrospectively on patient’s demographic characteristics, medical history, clinical presentation, hemodynamic, echocardiographic, and angiographic characteristics, treatment during hospitalization, hospital discharge status, and 30-day follow-up when available at the time of data collection. Data were monitored against source documentation to maximize accuracy. All patients reported in the registry that met the above definition of AMI CS and underwent PCI were included in the current analysis without pre-selection of patients or sites. An independent clinical event committee, consisting of 1 cardiovascular surgeon and 2 interventional cardiologists, adjudicated the in-hospital study end-points (all-cause of death, reinfarction, stroke, repeat revascularization, renal insufficiency, and vascular complications requiring surgical repair).
Statistical Analysis
Data are expressed as mean ± standard deviation (SD), median with quartiles or frequencies and percentages, as appropriate. Univariate analysis comparing Impella pre-PCI versus Impella post-PCI was performed with a chi-square test on discrete variables and with Student’s t-test or Wilcoxon rank sum test for independent samples for continuous variables, as appropriate. Survival to discharge is reported as the proportion of patients who were discharged alive from the hospital. As a supporting analysis, a Kaplan-Meier estimate with a log-rank test was used to compare survival rates up to 30 days between patients who received Impella prior to PCI (pre-PCI) versus those who received Impella after PCI (post-PCI). Surviving patients were censored at 30 days or last known follow-up, whichever is earlier in this analysis. pre-PCI time interval was defined as time preceding the first PCI balloon inflation. post-PCI interval was defined as the time interval after first balloon inflation. A multivariate forward stepwise logistic regression analysis was performed to identify the strongest independent predictors for in-hospital mortality while adjusting for potential confounding variables; the model included baseline, procedural, and hemodynamic characteristics known to impact patient’s outcome (see Results section for list of variables included in the model). A P-value of 0.05 was set as a forward stepping criterion to add sequentially variables from the model. All P values were 2-tailed and considered significant when P < 0.05. Data analysis was performed using JMP version 10.0 and SAS version 9.2 statistical software packages (SAS Institute, Inc., Cary, NC, USA).
Results
Between July 2008 and May 2012, a total of 154 consecutive unselected patients from 38 US hospitals received mechanical hemodynamic support with Impella 2.5 for CS complicating an AMI. Demographics and baseline characteristics are presented in Table 1 and Figure 2. Of the 154 patients, 63.4% were in CS at admission, 48.9% were transferred from an outlying facility to the institution where they received Impella 2.5, and 22.5% had sustained 1 or multiple witnessed out-of-hospital cardiac arrests (OHCA). At the time of Impella insertion, 88.3% continued to be in sustained CS despite high-dose inotropes and/or IABP support. In the remaining patients (11.7%), the use of inotropes or IABP could not be documented in the case report forms. [Correction added on 2 Jan 2014, after first online publication: In the first paragraph of the Results section, Impella 2.5% has been changed to Impella 2.5.]
Table 1.
Baseline Characteristics
| All | Impella Pre-PCI | Impella Post-PCI | ||
|---|---|---|---|---|
| N = 154 (mean ± SD, median [IQR], or %) | N = 63 (mean ± SD, median [IQR], or %) | N = 91 (mean ± SD, median [IQR], or %) | P-Value | |
| Age, years | 64.0 ± 12.7 | 66 ± 12 | 63 ± 13 | 0.12 |
| Gender, male | 71.4% | 73.0% | 70.3% | 0.73 |
| Hypertension | 77.3% | 82.3% | 73.9% | 0.23 |
| Diabetes | 44.6% | 56.7% | 36.4% | 0.02 |
| PVD | 21.4% | 32.2% | 13.6% | 0.008 |
| COPD | 15.9% | 22.9% | 10.7% | 0.05 |
| Stroke | 9.4% | 15.3% | 5.1% | 0.04 |
| Renal Insufficiency | 23.9% | 27.9% | 21.2% | 0.35 |
| Myocardial infarction | 38.6% | 43.3% | 35.3% | 0.33 |
| STEMI | 74.7% | 55.6% | 87.9% | <0.0001 |
| Prior CABG | 14.2% | 19.4% | 10.5% | 0.13 |
| Prior PCI | 38.5% | 37.1% | 39.5% | 0.77 |
| Preadmission cardiogenic shock | 63.4% | 63.5% | 63.3% | 0.98 |
| Preadmission cardiac arrest | 22.5% | 15.5% | 27.5% | 0.1 |
| Transfer admission | 48.9% | 57.1% | 42.9% | 0.09 |
| Duration of shock (hours) | 0.2 | |||
| <6 | 47.2% | 40.4% | 51.7% | |
| 6–12 | 14.6% | 19.3% | 11.5% | |
| 12–24 | 12.5% | 8.8% | 14.9% | |
| >24 | 25.7% | 31.6% | 21.8% | |
| Anoxic brain damage | 21.0% | 19.6% | 21.9% | 0.74 |
| Number of inotropes | 1.6 ± 1.1 | 1.4 ± 1.1 | 1.7 ± 1.2 | 0.17 |
| IABP prior to Impella | 48.7% | 34.9% | 58.2% | 0.004 |
| Mechanical ventilation | 65.5% | 54.8% | 73.3% | 0.02 |
| LVEF (%) | 26.4 ± 13.4 | 25.6 ± 12.9 | 27.0 ± 13.8 | 0.56 |
| Troponin, ng/mL | 5.7 [0.6 23.1] | 5.7 [0.9 24.7] | 5.7 [0.4 23.3] | 0.82 |
| Serum creatinine, mg/dL | 1.4 [1.1 2.0] | 1.3 [1.1 2.0] | 1.5 [1.1 1.9] | 0.41 |
| eGFR, mL/min/m2 | 49 [27.5 60] | 49 [25 60] | 48 [29.5 60] | 0.91 |
| Serum lactate, mmol/L | 4.1 [2.4 7.2] | 4.3 [1.6 10.2] | 3.8 [2.5 5.9] | 0.65 |
| STS mortality score | 21.7 ± 15.2 | 22.6 ± 14.4 | 21.0 ± 15.8 | 0.55 |
| STS morbidity score | 64.2 ± 18.2 | 67.1 ± 16.9 | 62.1 ± 18.9 | 0.11 |
CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; IABP, intra-aortic balloon pump; IQR, inter-quartile range; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; SD, standard deviation; STEMI, ST elevation myocardial infarction; STS, Society of Thoracic Surgeons.
Figure 2.
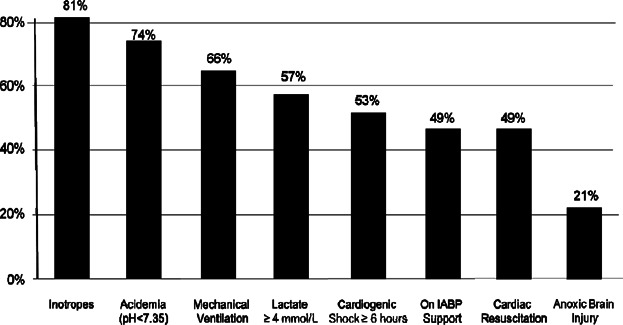
Baseline characteristics.
A total of 63 patients received Impella 2.5 support prior to PCI (pre-PCI group, 40.9%) and 91 patients received Impella post-PCI (post-PCI group, 59.1%). Patients presenting with an ST-segment elevation myocardial infarction (STEMI) were more likely to receive Impella after PCI (87.9% vs. 55.6%, P < 0.0001). The pre-PCI and post-PCI groups were similar in terms of baseline characteristics, duration of shock, extent of the infarct size (as determined by baseline plasma troponin level), and PCI success rate as measured by post-PCI TIMI grade flow 0–1, except for higher rate of diabetes (56.7% vs. 36.4%, P = 0.02), peripheral vascular disease (PVD) (32.2% vs. 13.6%, P = 0.0008), chronic obstructive pulmonary disease (COPD) (22.9% vs. 10.7%, P = 0.05), and prior stroke (15.3% vs. 5.1%, P = 0.04) in the pre-PCI group (Table 1). The number of inotropes prior to Impella use was similar between the 2 groups. Both groups had poor hemodynamics prior to Impella support but with a greater magnitude when the Impella insertion was delayed to after PCI despite the more frequent use of IABP in this group (Table 2). Mechanical ventilation was required more often when Impella insertion was delayed after PCI (54.8% vs. 73.3%, P = 0.02). More extensive revascularization was performed in patients who received Impella pre-PCI with more lesions (2.33 ± 1.40 vs. 1.77 ± 1.02, P = 0.006), more vessels treated (1.57 ± 0.67 vs. 1.30 ± 0.57, P = 0.01), and more stents placed (1.94 ± 1.15 vs. 1.47 ± 0.85, P = 0.007) compared with patients who received Impella post-PCI. Trends for more complete revascularization in pre-PCI group were similar in both STEMI and NSTEMI groups.
Procedural Characteristics
| All | Impella Pre-PCI | Impella Post-PCI | ||
|---|---|---|---|---|
| N = 154 (mean ± SD, median [IQR], or %) | N = 63 (mean ± SD, median [IQR], or %) | N = 91 (mean ± SD, median [IQR], or %) | P-Value | |
| Duration of Impella support, hours | 23.7 [3.5 62.7] | 22.8 [1.6 52.8] | 24.2 [4.2 69.2] | 0.39 |
| Median door-to-balloon time,* min | 63.5 [40.3 113.5] | 112 [79 112] | 52 [34 81] | <0.0001 |
| Suspected infarct related artery territory | ||||
| Left main | 16.1% | 23.8% | 9.5% | 0.02 |
| Left anterior descending | 52.6% | 53.9% | 51.4% | 0.76 |
| Left circumflex | 10.9% | 4.8% | 16.2% | 0.03 |
| Right coronary | 16.8% | 12.7% | 20.3% | 0.24 |
| Graft | 3.7% | 4.8% | 2.7% | 0.52 |
| Number of diseased vessels | 1.8 ± 0.76 | 1.94 ± 0.72 | 1.70 ± 0.79 | 0.07 |
| Number of significant lesions (≥70%) | 2.57 ± 1.39 | 2.74 ± 1.49 | 2.42 ± 1.28 | 0.19 |
| Number of vessel treated | 1.42 ± 0.63 | 1.57 ± 0.67 | 1.30 ± 0.57 | 0.01 |
| Number of lesions treated | 2.02 ± 1.24 | 2.33 ± 1.40 | 1.77 ± 1.02 | 0.006 |
| Number of stents | 1.68 ± 1.02 | 1.94 ± 1.15 | 1.47 ± 0.85 | 0.007 |
| TIMI flow [0–1] prior to PCI | 80.2% | 71.9% | 84.8% | 0.14 |
| TIMI flow [0–1] post-PCI | 8.7% | 4.6% | 11.9% | 0.19 |
PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction; SD, standard deviation.
Door-to-balloon time data are presented when available for patients admitted with STEMI or diagnosed with STEMI on admission.
Patients’ hemodynamics improved significantly after initiation of hemodynamic support with Impella 2.5 to the same magnitude in both groups (Table 3). The median duration of support was similar between the 2 groups (Table 2).
Table 3.
Hemodynamics
| All Patients | Impella Pre-PCI | Impella Post-PCI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N = 154 (mean ± SD or %) | N = 63 (mean ± SD or %) | N = 91 (mean ± SD or %) | |||||||
| Pre-support | On Support | P-Value | Pre-support | On Support | P-Value | Pre-support | On Support | P-Value | |
| SBP, mmHg | 85.4 ± 25.6 (143) | 126.7 ± 31.4 (144) | <0.0001 | 92.9 ± 27.7 (59) | 127.5 ± 30.6 (59) | <0.0001 | 80.2 ± 22.9 (84) | 126.8 ± 32.2 (84) | <0.0001 |
| DBP, mmHg | 50.8 ± 18.6 (143) | 78.7 ± 21.1 (143) | <0.0001 | 55.2 ± 18.6 (59) | 79.7 ± 18.5 (59) | <0.0001 | 47.8 ± 18.0 (84) | 78.0 ± 22.8 (84) | <0.0001 |
| MAP, mmHg | 62.7 ± 19.2 (143) | 94.4 ± 23.1 (143) | <0.0001 | 67.9 ± 20.7 (59) | 94.5 ± 21.3 (59) | <0.0001 | 59.1 ± 17.3 (84) | 94.4 ± 24.4 (84) | <0.0001 |
| PCWP, mmHg | 31.9 ± 11.1 (25) | 19.2 ± 9.7 (25) | <0.0001 | 30.8 ± 7.8 (11) | 19.7 ± 7.9 (11) | 0.004 | 32.7 ± 13.4 (14) | 18.9 ± 11.1 (14) | 0.004 |
| Cardiac output, L/min | 3.4 ± 1.3 (23) | 5.3 ± 1.7 (23) | <0.0001 | 3.6 ± 1.9 (7) | 4.4 ± 2.2 (7) | 0.022 | 3.4 ± 0.9 (16) | 5.8 ± 1.3 (16) | <0.0001 |
| Cardiac index, L/min/m2 | 1.9 ± 0.7 (23) | 2.7 ± 0.7 (23) | <0.0001 | 1.9 ± 0.9 (7) | 2.3 ± 0.8 (7) | 0.055 | 1.9 ± 0.6 (16) | 2.9 ± 0.6 (16) | <0.0001 |
| Cardiac power output, W | 0.48 ± 0.17 (23) | 1.06 ± 0.48 (23) | <0.0001 | 0.54 ± 0.2 (7) | 0.83 ± 0.4 (7) | 0.035 | 0.46 ± 0.1 (16) | 1.2 ± 0.5 (16) | <0.0001 |
SBP, Systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PCWP, pulmonary capillary wedge pressure.
The overall survival rate to discharge was 50.7%. A higher survival rate was observed in the pre-PCI group as compared with post-PCI group (65.1% vs. 40.7%, P = 0.003). Thirty-day follow-up data was reported for 145 (94.2%) patients (nine patients were lost to follow-up at 30-day visit). The Kaplan-Meier analysis confirmed the difference in mortality trends in favor of the pre-PCI group up to 30 days (57.4% vs. 38.2%, log-rank test P = 0.004, Fig. 3). The odds ratio of discharge survival for Impella post-PCI versus pre-PCI was 0.37 (95% CI: 0.19–0.72), again indicating lower survival for Impella post-PCI vs. pre-PCI. Odds ratios remained consistently favorable to the Impella pre-PCI group, either significantly or with strong favorable trends, within subgroups based on baseline, procedural, and hemodynamic variables that could be perceived as confounding variables (Fig. 4).
Figure 3.
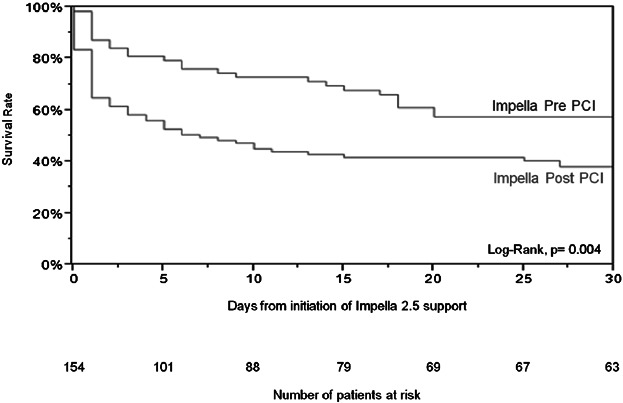
Kaplan-Meier curve survival to 30 days. CS, cardiogenic shock; DBP, diastolic blood pressure; MAP, mean arterial pressure; MV, mechanical ventilation; NSTEMI, non-ST elevation myocardial infarction; PVD, peripheral vascular disease; SBP, systolic blood pressure.
Figure 4.
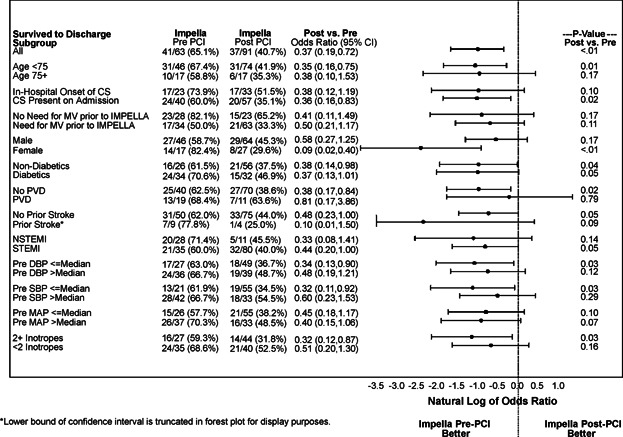
Sub-group outcome to discharge. CS, cardiogenic shock; DBP, diastolic blood pressure; MAP, mean arterial pressure; MV, mechanical ventilation; NSTEMI, non-ST elevation myocardial infarction; PVD, peripheral vascular disease; SBP, systolic blood pressure.
A multivariate forward stepwise logistic regression analysis was conducted to identify independent predictors of mortality in our study. Adjustments to correct for potential confounding variables in the model were made. The multivariate analysis model included the following as candidates for entry: age, gender, history of chronic obstructive pulmonary disease, diabetes, PVD or prior stroke, STEMI versus NSTEMI presentations, cardiac arrest prior to admission, onset and duration of CS, patient transfer from outlying facility, evidence of anoxic brain injury pre-Impella support, need for mechanical ventilation, systolic and diastolic blood pressure levels pre-Impella support, level of inotropic support pre-Impella support and potential use of IABP prior to Impella support, and baseline serum creatinine levels. Timing of initiation of Impella support (pre-PCI vs. post-PCI) was also included as a candidate for entry in the model. The timing of initiation of Impella 2.5 support relative to PCI was identified as an independent predictor of improved survival to hospital discharge (P = 0.01) while older age (OR 1.05, 95% CI: 1.02–1.08, P = 0.003), number of inotropes (P = 0.01), CS onset prior to admission (P = 0.03), and need for mechanical ventilation (P = 0.0003) were found to be independent predictors for increased in-hospital mortality (Table 4). There was no difference between the pre-PCI group and the post-PCI group in the occurrence of in-hospital complications included in the secondary end-point (Table 5).
Table 4.
Multivariate Analysis for Predictors of In-Hospital Mortality
| Odds Ratio | 95% Confidence Interval | P-Value | |
|---|---|---|---|
| Initiation of Impella support prior to PCI | 0.37 | 0.17–0.79 | 0.01 |
| Age | 1.05 | 1.02–1.08 | 0.003 |
| Number of inotropes | 1.56 | 11–2.18 | 0.01 |
| Cardiogenic shock onset prior to admission | 2.42 | 1.12–5.24 | 0.03 |
| Mechanical ventilation | 4.59 | 2.02–10.42 | 0.0003 |
Table 5.
In-Hospital Outcomes
| All (N = 154), % | Impella Pre-PCI (N = 63), % | Impella Post-PCI (N = 91), % | P-Value | |
|---|---|---|---|---|
| Survival to discharge | 50.7 | 65.1 | 40.7 | 0.003 |
| In-hospital adverse events | ||||
| Stroke | 1.9 | 1.6 | 2.2 | 0.79 |
| Reinfarction | 0.7 | 0.0 | 1.1 | 0.4 |
| Acute renal dysfunction/failure | 18.1 | 12.7 | 22.0 | 0.14 |
| Infection | 12.9 | 17.5 | 9.9 | 0.17 |
| Limb ischemia | 3.9 | 3.2 | 4.4 | 0.7 |
| Repeat revascularization | 2.6 | 3.2 | 2.2 | 0.71 |
| Vascular complication with surgical repair | 9.7 | 9.5 | 9.9 | 0.94 |
| Hemolysis | 10.3 | 11.1 | 9.9 | 0.81 |
| Bleeding requiring transfusion | 17.5 | 12.7 | 20.8 | 0.14 |
| Bleeding requiring surgery | 2.6 | 1.6 | 3.3 | 0.51 |
Discussion
This is the largest cohort to date documenting the use of Impella 2.5 in the setting of AMI complicated by CS. The analysis provides insight into the presentation, management, and outcomes of patients supported with Impella 2.5 at a large number of high- and low-volume PCI centers over multiple years, thus providing a fair representation of the current use of the device in routine practice in AMI CS across the United States. The results of our study showed that: (1) the use of Impella 2.5 was primarily restricted to a rescue population that failed to respond to conventional measures including inotropic and IABP support; and (2) although the risk for imminent death remained particularly high in this selected population, our findings suggest that the prompt use of Impella prior to PCI may significantly improve survival.
Our study showed that patients who received Impella 2.5 in daily routine practice presented with greater risk features than those who were reported in recent AMI CS randomized trials, reflecting a higher mortality observed in routine practice.12 Indeed, 21% of our study patients presented with anoxic brain damage and 38.2% had a shock duration >12 hours prior to Impella support. Thus, at least 38.2% of the patients included in our study would have been considered too sick and not eligible for enrollment in the IABP-SHOCK II trial.6 Moreover, at presentation, the incidences of diabetes (44.6% vs. 35.4%, P = 0.05), prior MI (38.6% vs. 23.7%, P < 0.0001), and prior coronary artery bypass graft (14.2% vs. 6.7%, P = 0.01), prior PCI (38.5% vs. 21.1%, P < 0.0001) were significantly higher in the patients included in our study compared to the patients randomized in the IABP-SHOCK II Trial.6 More importantly, 88.4% of our patients received Impella after the first-line therapy with inotropes and/or IABP failed to stabilize their hemodynamics. Despite these high-risk features, the overall survival rate was favorable in our study when compared to other AMI CS series.12–14
In our study, survival was significantly higher in the pre-PCI group compared with the post-PCI group. We hypothesized that active unloading of the left ventricle, hemodynamic stabilization of the patients with the Impella prior to undergoing PCI, and more complete revascularization may have led to improved outcomes in our study of AMI CS. Indeed, nonculprit intervention in the setting of CS is a reasonable approach after careful consideration, but may be limited in the clinical setting by worsening hemodynamics. Considering the limitations inherent to the retrospective nature of our investigation, one may argue that patients in the post-PCI group were simply sicker accounting for the higher in-hospital mortality observed in this group. Therefore, the validity of our hypothesis rests largely on the determination of whether: (1) the pre-PCI and post-PCI groups were comparable with respect to baseline characteristics, and (2) whether the prompt initiation of hemodynamic support with Impella may have resulted in treatment differences that may have influenced outcomes.
In our series, patients who received Impella prior to PCI appeared to be as sick as those who received Impella after PCI while accounting for baseline characteristics, although they presented more often with higher-risk features such as diabetes, COPD, PVD, or prior stroke compared to the latter group. Patients in the pre-PCI group had also more extensive coronary artery disease burden, a parameter that has been shown to be associated with increased mortality in CS.15,16 Our results suggest that early hemodynamic support prior to PCI has the potential to improve outcomes by enabling stable hemodynamics during the intervention and therefore probably allowing for more complete revascularization. This notion is supported by recent studies in the setting of AMI CS that showed improved outcomes associated with a more complete revascularization strategy.17 The current societal guidelines support nonculprit PCI at the time of primary PCI in the setting of CS complicating an AMI2 in the case of severe stenosis in arteries supplying large territory of myocardium. Additionally, active left ventricular unloading and increased forward flow to the systemic circulation may have prevented further hemodynamics deterioration and progression of the downward spiral of shock and increased risk of peri procedural death. This might explain also the large difference in death events between the 2 groups observed within the first 48 hours of PCI (Fig. 3).
The survival to discharge rate in the post-PCI group was comparable to the results reported in the SHOCK Registry (40.7% vs. 39.8%, P = 0.87)12 the EUROSHOCK Impella Registry reported by Lauten et al.13 or the single-site experience reported by Engström et al.14 in the SHOCK Registry,12 IABP was used as the predominant means of hemodynamic support. Unfortunately, IABP use has not been shown to significantly improve hemodynamics7 or clinical outcomes in the setting of AMI CS.6,8,9 In our study, patients in the post-PCI group had poor outcomes whether medical therapy alone or combined with IABP was used to support the patient hemodynamics during PCI (Fig. 5). In the EUROSHOCK Impella Registry, an overall survival rate of 35.8% to 30 days was reported by Lauten et al. In a series of 144 patients with refractory CS who had failed first-line therapy with inotropes and IABP support.13 The authors of this series concluded that Impella 2.5 was implanted in patients with a particularly poor hemodynamic profile and as a last-resort option. Moreover, the number of lesions treated in this study was also comparable to the one observed in the post-PCI group in our study (1.7 ± 1.2 vs. 1.8 ± 1.0); however, the timing of Impella insertion relative to the revascularization procedure was not specified. In the Engström et al.14 series, the authors reported that the decision to implant Impella was delayed to after PCI and dependent upon the diagnosis of profound CS and confirmed to be refractory to inotropic and IABP support.
Figure 5.
Treatment strategies.
In the current era where time to reperfusion has become a quality metric, physicians may adopt a reflexive approach to minimize door-to-balloon time and therefore delay Impella insertion, despite the fact that in general this quality metric does not apply to patients in CS. The results of our study showed that STEMI patients who received Impella pre-PCI had a door-to-balloon time about an hour longer compared with those who received Impella post-PCI, yet had better outcomes. This may further reinforce the assumption that the pre-PCI patients were perhaps more hemodynamically embarrassed and required more time to be stabilized before a PCI could be undertaken compared to the post-PCI group. Indeed, it would not be reasonable for a physician to explant an IABP to insert an Impella and delay the reperfusion if the patient had been stable enough with IABP therapy and conventional measures prior to PCI.
In our study, a majority of patients were admitted in CS and were in shock for more than 6 hours before revascularization with a large number of them being transferred from an outlying facility. These patients do not represent the typical STEMI patients in whom the door-to-balloon time metric has been shown to correlate with outcomes. Indeed, the extended door-to-balloon time did not seem to be a determinant factor in outcomes in this rescue population, suggesting that early stabilization of patients with effective hemodynamic support prior to PCI would be a more appropriate goal rather than the current door-to-balloon time performance metric to affect the outcomes of these sick patients.
Study Limitations
Our study includes several limitations to be considered: (1) the observational approach of our investigation cannot infer a definitive causal relationship between the use of Impella pre-PCI and subsequent reduced mortality; thus, our results will need to be confirmed by a prospective investigation; (2) we cannot exclude the presence of potential patient or site selection biases; and (3) the timing of Impella 2.5 insertion, the completeness of revascularization, and all adjunctive therapies were left to the discretion of the physician. Thus, we cannot totally eliminate the risk of potential treatment biases, and finally, (4) the duration of the follow-up was limited to the in-hospital phase and long-term follow-up could not be obtained.
Conclusion
The results of our study suggest that early initiation of hemodynamic support prior to PCI with Impella 2.5 is associated with more complete revascularization and improved survival in the setting of refractory CS complicating an AMI.
Acknowledgments
The authors would like to thank Drs. William Bachinsky, Randolph C. Hubbard, and Mubashir Mumtaz, members of the Independent CEC, for their time in the audit and adjudication of all adverse events. We gratefully acknowledge the patients, physician investigators, and research coordinators who participated in the USpella Registry.
References
- Jeger RV, Radovanovic D, Hunziker PR, et al. Ten-year trends in the incidence and treatment of cardiogenic shock. Ann Intern Med. 2008;149:618–626. doi: 10.7326/0003-4819-149-9-200811040-00005. [DOI] [PubMed] [Google Scholar]
- Levine GN, Bates ER, Blankenship JC, et al. ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44–e122. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Goldberg R, Spencer FA, Gore JM, et al. Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction. A population-based perspective. Circulation. 2009;119:1211–1219. doi: 10.1161/CIRCULATIONAHA.108.814947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfarth M, Sibbing D, Bauer I, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52:1584–1588. doi: 10.1016/j.jacc.2008.05.065. [DOI] [PubMed] [Google Scholar]
- O’Neill WW, Kleiman NS, Moses J, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: The PROTECT II study. Circulation. 2012;126:1717–1727. doi: 10.1161/CIRCULATIONAHA.112.098194. [DOI] [PubMed] [Google Scholar]
- Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- Prondzinsky R, Unverzagt S, Russ M, et al. Hemodynamic effects of intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: The prospective, randomized IABP shock trial. Shock. 2012;37:378–384. doi: 10.1097/SHK.0b013e31824a67af. [DOI] [PubMed] [Google Scholar]
- Sjauw KD, Engström AE, Vis MM, et al. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: Should we change the guidelines. Eur Heart J. 2009;30:459–468. doi: 10.1093/eurheartj/ehn602. [DOI] [PubMed] [Google Scholar]
- Romeo F, Acconcia MC, Sergi D, et al. The outcome of intra-aortic balloon pump support in acute myocardial infarction complicated by cardiogenic shock according to the type of revascularization: A comprehensive meta-analysis. Am Heart J. 2013;165:679–682. doi: 10.1016/j.ahj.2013.02.020. [DOI] [PubMed] [Google Scholar]
- Zeymer U, Hochadel M, Hauptmann KE, et al. Intra-aortic balloon pump in patients with acute myocardial infarction complicated by cardiogenic shock: Results of the ALKK-PCI registry. Clin Res Cardiol. 2013;102:223–227. doi: 10.1007/s00392-012-0523-4. [DOI] [PubMed] [Google Scholar]
- Henriques JP, Remmelink M, Baan J, Jr, et al. Safety and feasibility of elective high-risk percutaneous coronary intervention procedures with left ventricular support of the Impella Recover LP 2.5. Am J Cardiol. 2006;97:990–992. doi: 10.1016/j.amjcard.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Webb JG, Sleeper LA, Buller CE, et al. Implications of the timing of onset of cardiogenic shock after acute myocardial infarction: A report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK. J Am Coll Cardiol. 2000;36:1084–1090. doi: 10.1016/s0735-1097(00)00876-7. [DOI] [PubMed] [Google Scholar]
- Lauten A, Engström AE, Jung C, et al. Percutaneous left-ventricular support with the Impella-2.5-assist device in acute cardiogenic shock: Results of the Impella-EUROSHOCK-registry. Circ Heart Fail. 2013;6:23–30. doi: 10.1161/CIRCHEARTFAILURE.112.967224. [DOI] [PubMed] [Google Scholar]
- Engström AE, Cocchieri R, Driessen AH, et al. The Impella 2.5 and 5.0 devices for ST-elevation myocardial infarction patients presenting with severe and profound cardiogenic shock: The Academic Medical Center intensive care unit experience. Crit Care Med. 2011;39:2072–2079. doi: 10.1097/CCM.0b013e31821e89b5. [DOI] [PubMed] [Google Scholar]
- Sanborn TA, Sleeper LA, Webb JG, et al. Correlates of one-year survival in patients with cardiogenic shock complicating acute myocardial infarction: Angiographic findings from the SHOCK trial. J Am Coll Cardiol. 2003;42:1373–1379. doi: 10.1016/s0735-1097(03)01051-9. [DOI] [PubMed] [Google Scholar]
- van der Schaaf RJ, Claessen BE, Vis MM, et al. Effect of multivessel coronary disease with or without concurrent chronic total occlusion on one-year mortality in patients treated with primary percutaneous coronary intervention for cardiogenic shock. Am J Cardiol. 2010;105:955–959. doi: 10.1016/j.amjcard.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Hussain F, Philipp RK, Ducas RA, et al. The ability to achieve complete revascularization is associated with improved in-hospital survival in cardiogenic shock due to myocardial infarction: Manitoba cardiogenic SHOCK Registry investigators. Catheter Cardiovasc Interv. 2011;78:540–548. doi: 10.1002/ccd.23006. [DOI] [PubMed] [Google Scholar]


