Abstract
Parvovirus B19 has been linked with various clinical syndromes including neurological manifestations. However, its role in the latter remains not completely understood. Although the last 10 years witnessed a surge of case reports on B19-associated neurological aspects, the literature data remains scattered and heterogeneous, and epidemiological information on the incidence of B19-associated neurological aspects cannot be accurately extrapolated. The aim of this review is to identify the characteristics of cases of B19-associated neurological manifestations. A computerized systematic review of existing literature concerning cases of B19-related neurological aspects revealed 89 articles describing 129 patients; 79 (61.2%) were associated with CNS manifestations, 41 (31.8%) were associated with peripheral nervous system manifestations, and 9 (7.0%) were linked with myalgic encephalomyelitis. The majority of the cases (50/129) had encephalitis. Clinical characteristic features of these cases were analyzed, and possible pathological mechanisms were also described. In conclusion, B19 should be included in differential diagnosis of encephalitic syndromes of unknown etiology in all age groups. Diagnosis should rely on investigation of anti-B19 IgM antibodies and detection of B19 DNA in serum or CSF. Treatment of severe cases might benefit from a combined regime of intravenous immunoglobulins and steroids. To confirm these outcomes, goal-targeted studies are recommended to exactly identify epidemiological scenarios and explore potential pathogenic mechanisms of these complications. Performing retrospective and prospective and multicenter studies concerning B19 and neurological aspects in general, and B19 and encephalitic syndromes in particular, are required. © 2014 The Authors. Reviews in Medical Virology published by John Wiley & Sons, Ltd.
INTRODUCTION
Since its discovery in the 1970s of last century 1, human parvovirus B19 (B19) has been linked with a broad spectrum of clinical syndromes, including erythema infectiosum (EI), transient aplastic crisis, persistent infection manifesting as pure red cell aplasia in immunocompromised individuals, nonimmune hydrops fetalis, and arthritis.
Less commonly recognized, but receiving increasing attention recently, are the neurological manifestations, a variety of which have been described in patients with either clinically diagnosed or laboratory-confirmed B19 infection. The last 10 years witnessed a surge of case reports on the association of B19 with neurological aspects. However, the literature on B19 infection and its association with neurological aspects continue to be heterogeneous, and epidemiological data on the incidence of B19-associated neurological aspects cannot be accurately extrapolated. Therefore, the role of B19 in neurological diseases remains incompletely described and understood.
The pathogenesis of B19 infection is complex and variable, so it is likely that a combination of mechanisms contribute to the development of neurological manifestations 2, although there is a lack of detailed descriptions of autopsy reports.
The objectives of this systematic review are to search for cases of B19-related neurological aspects and identify the clinical characteristics of those patients that could be associated with B19 infection.
METHODS
A computerized search was conducted using all databases included in Web of Knowledge in addition to PubMed database. The search was performed combining the terms (‘human parvovirus’ or ‘parvovirus B19’ or ‘B19’ or ‘erythema infectiosum’) and (‘neurologic complication’ or ‘neurological disorder’ or ‘neurological manifestation’ or ‘central nervous system’ or ‘peripheral nervous system’ or ‘a specific term for a specific neurological disorder’) without language and time restrictions. The specific terms for neurological disorders used in the search were obtained from the website of National Institute of Neurological Disorders and Stroke 3, with a total of 442 disorders and manifestations. In addition, all cited references listed in the identified papers were hand-searched for other relevant articles. An article was considered for inclusion in the systematic review if it reported cases with B19 infection that presented with neurological manifestations. A case was considered eligible for the following reasons: (i) if data of age, sex, immune status, description of manifestations and investigation, treatment, and outcomes were presented and (ii) if B19 infection was diagnosed in the presence of B19 DNA or anti-B19 IgM specific antibodies in the serum or the CSF. Exceptions included cases with neurological manifestations associated with the presence of clinical presentation of EI while laboratory tests were not performed or available. The legitimacy behind that relies on the fact that B19 is the sole agent for EI. In the absence of B19 specific markers, other common B19-related clinical manifestations, such as transient aplastic crisis, persistent infection manifesting as pure red cell aplasia, nonimmune hydrops fetalis, and arthritis, were not considered as indicators of B19 infection because the latter is not their sole etiological agent. Cases of B19-associated neurological manifestations that result from intrauterine infection were also excluded. B19-associated myalgic encephalomyelitis (ME) cases were included because of the neurological classification of ME in the World Health Organization's International Classification of Diseases (ICD G93.3) but classified and labeled separately. Cases that did not fulfill the International Consensus Criteria of ME 4 were excluded. The computerized search was conducted for the last time on 30 June 2013. The preferred reporting items for systematic review and meta-analysis guidelines were followed 5.
Data were summarized using percentages and cross tabulations. Comparisons between subgroups were made using Fisher's exact tests. The 95% confidence intervals (CIs) for percentages were calculated using the Wilson method. All statistical analyses used the conventional two-sided 5% significance level and were carried out using spss version 20 and cia version 2.0.
RESULTS
As shown in Figure 1, the search using Web of Knowledge databases identified 998 publications, whereas PubMed database search identified 903 publications, with a combined search result of 1065 publications. A scrupulous analysis resulted in 89 eligible articles 6–94 describing the history of 129 patients, published between the years 1970 and 2012, which were further evaluated.
Figure 1.
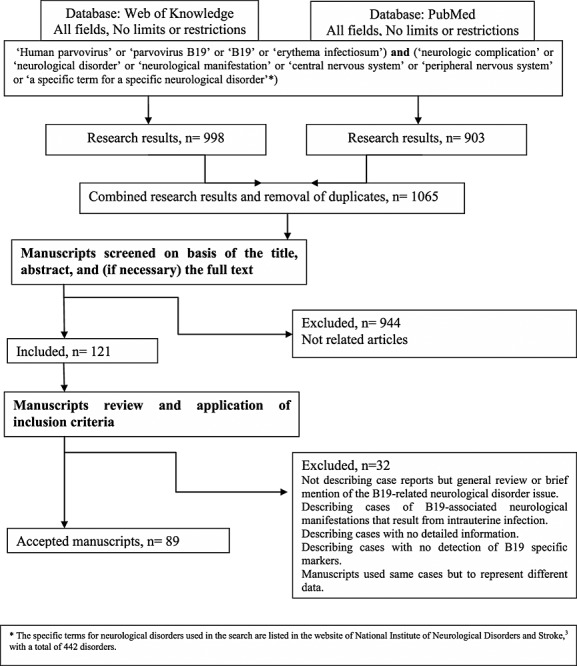
Flow diagram of information through the different phases of the review
Seventy nine of the eligible cases (61.2%) were associated with CNS manifestations (Table 1), whereas 41 (31.8%) were associated with peripheral nervous system (PNS) manifestations (Table 2), and nine cases (7.0%) were linked with ME (Table 3). Many of the cases (50/129) had encephalitis, encephalopathy, or meningoencephalitis. The patients age ranged from 1 day to 75 years; median age of 12.5 years, with 70 (54.3%) children (<18 years) and 59 (45.7%) adults (≥18 years). The male-to-female ratio was 4 : 5 (55.8% female). One hundred cases (77 · 5%) were immunocompetent, whereas 29 cases (22.5%) were patients with suppressed immune status.
Table 1.
Cases of B19 infection and manifestations related to the central nervous system
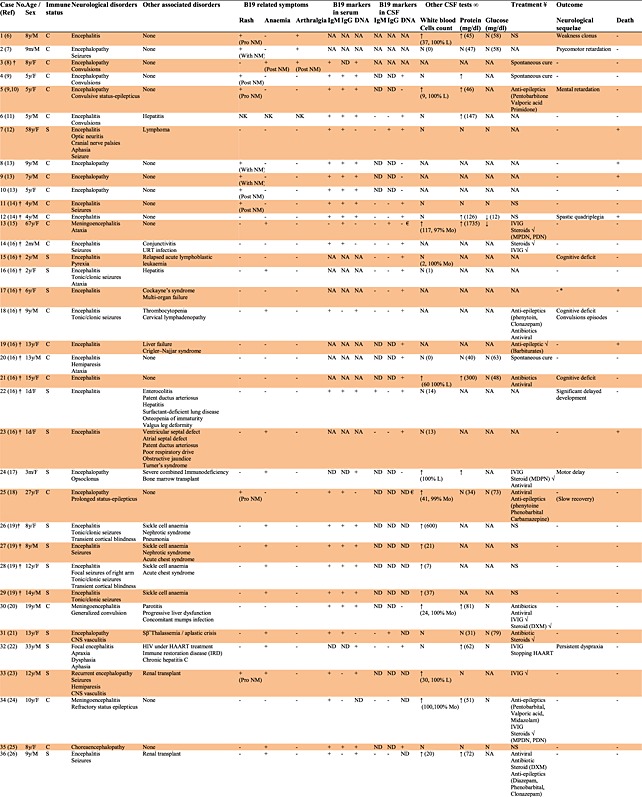 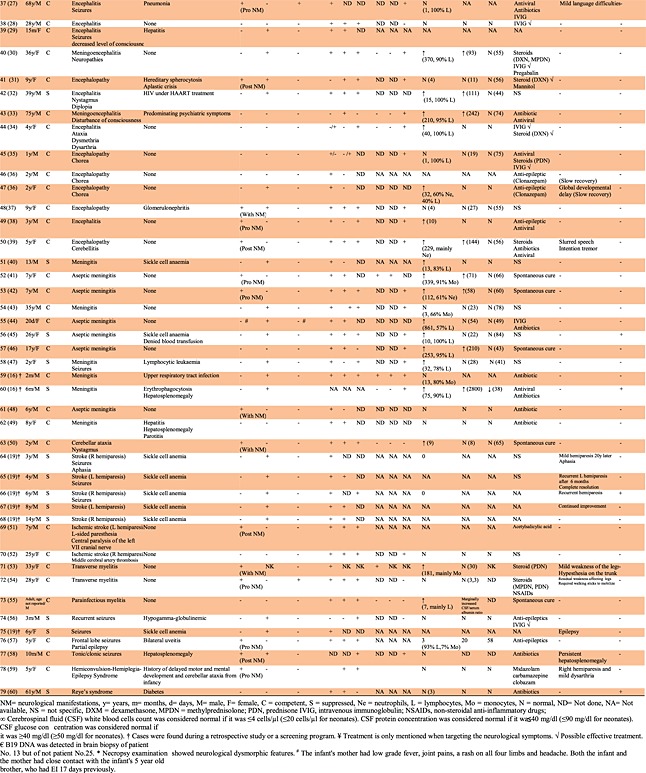
|
Table 2.
Cases of B19 infection and manifestations related to the peripheral nervous system
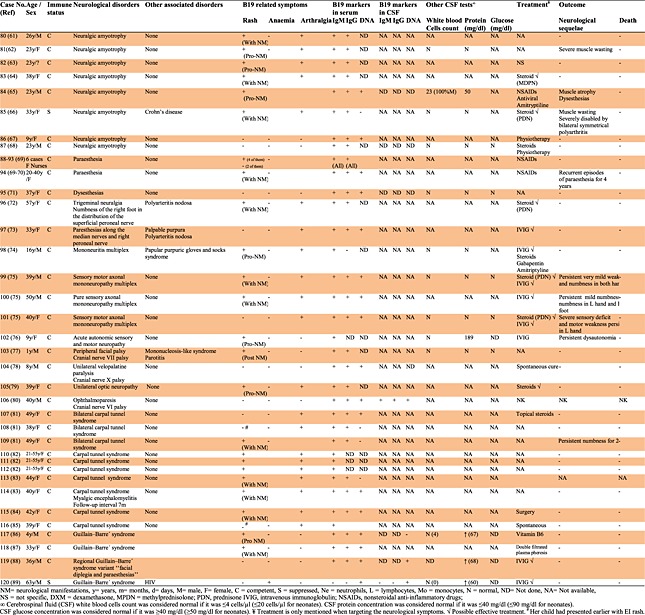
|
Table 3.
Cases of B19 infection and myalgic encephalomyelitis

|
Clinical features of B19-associated encephalitis, encephalopathy, and meningoencephalitis cases were subject to comprehensive analysis in this review because many of the B19-associated neurological aspects were cases belonging to this category. For readers who are interested in other B19-related neurological aspects, they should refer to Tables 3 and to the Discussion Section of this review.
B19 and encephalitis, encephalopathy, and meningoencephalitis
Fifty cases of encephalitic syndromes were found and reviewed 6–39, representing 63.3% of B19-related CNS cases and 38.8% of total B19-related neurological cases currently found in the literature (Table 1). In most of these cases (33/50), B19 was sought after other possible pathogens were proved to be negative, and the etiological cause was not determined. In addition, B19 was investigated in these cases because of the appearance of B19-related symptoms or merely because of a suspicion of B19 infection. This association was confirmed by the detection of at least one specific marker for B19 infection, with the exception of two cases of encephalitis associated with EI prior to the recognition of the etiological role of B19 in this disease 6,7. However, 12 cases were identified during two retrospective studies of 43 and 282 patients, respectively, with etiologically undiagnosed neurological symptoms and with no sufficient clinical information to support the detection of a recent B19 infection [14,16]. Four more cases were detected in another retrospective study that targeted 346 patients with aplastic crisis 19. An additional case was detected during a screening program of 1572 sera from hospitalized pediatric patients with various presentations submitted for viral investigation 8.
Analysis of CSF for cell count and protein and glucose concentrations varied according to the cases. From those who were subject to CSF analysis, 19 cases had normal white cells count, whereas 21 cases had raised count, 15 had normal protein concentration whereas 16 had higher concentration, and 23 had normal glucose concentration whereas only two had lower concentrations.
The majority of the cases (34, 68.0%) were immunocompetent and 16 (32.0%) were immunocompromised. Typical EI rash was observed in 15 cases (30.0%), 13 cases among children (33.0%, 95% CI 20.6–49.0%) and two cases among adults (20%, 95% CI 5.7–51.0%). Only one of these (no. 33) had suppressed immunity, as might be expected from the immunopathological nature of the rash. All 17 cases detected during screening programs or retrospective studies (except one) were free from the rash 8,14,16,19. The timing of neurological symptoms in relation to the rash varied considerably. B19-associated encephalitis presented prior to the appearance of the rash in five cases, contemporaneously with the appearance of the rash in four cases, or following the appearance of the rash in six cases. There were statistically significant differences between patients with competent and suppressed immune status and symptoms of rash (p = 0.018); rash was observed by 42.4% of patients with competent immune status, compared with only 6.2% with suppressed immune status.
Anemia was detected in 21 cases (42.0%), 17 cases among children (43.6%, 95% CI 29.3–59.0%) and four cases among adults (40.0%, 95% CI 16.8–68.7). There were statistically significant differences between patients with competent and suppressed immune status and symptoms of anemia (p = 0.002). The majority of the cases with anemia were observed at reduced immune status (12/16, 75%) comparing with the immunocompetent group (9/34, 26.5%).
Arthralgia was present only in three cases among children (7.7%, 95% CI 2.7–20.3%) and one case among adults (10.0%, 95% CI 1.8–40.4). There were no statistically significant differences between patients with competent and suppressed immune status and symptoms of arthralgia (p = 0.289).
Neuroimaging studies (Table 4) were performed on 34 cases using computed axial tomography (20 cases), electroencephalogram (20 cases), and/or magnetic resonance imaging (27 cases). Among the computed axial tomography cases, 12 were normal whereas eight showed a range of abnormalities including enlarged ventricles, lesions, frontal, and occipital vasogenic swelling. By electroencephalogram, only three cases were normal whereas 17 showed abnormal activities. Using magnetic resonance imaging, 11 cases were normal whereas 16 showed various abnormalities including enlarged ventricles and increased signal from the white matter. Two cases were normal with all three types of neuroimaging studies.
Table 4.
Neuroimaging studies for B19-associated encephalitis, encephalopathy and meningoencephalitis cases
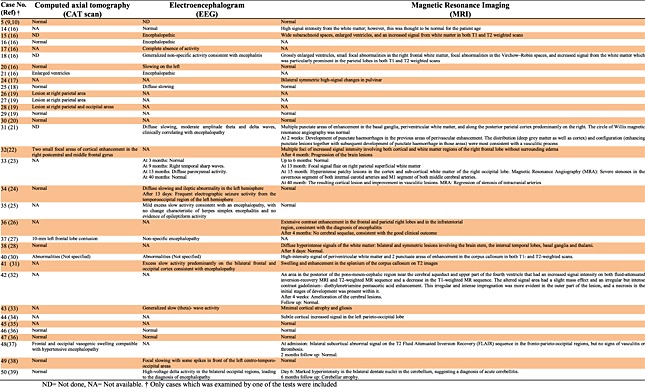
|
Excluding 25 cases in which treatment regimen was not known (12 cases), not specific (10 cases), or the encephalitis presentation resolved spontaneously without medical intervention (three cases), most cases were initially treated with antivirals (11 cases) and/or antibiotics (eight cases) to cover a possibility of unidentified viral and/or bacterial encephalitis, respectively, accompanied sometimes with an addition of antiepileptics (10 cases) to relieve the symptoms. However, when B19 involvement was either suspected or confirmed, 16 cases were treated with intravenous immunoglobulins (IVIGs) and/or steroids. Four cases were treated only with IVIG, two of them showing concomitant clinical improvement. Four other cases were treated only with steroids, two of them showing associated clinical improvement. Eight cases were treated with IVIG and steroids (according to the physicians reports about these cases, two showed improvement most probably due to IVIG, two showed improvement most probably due to steroid treatment, while three showed improvement due to combined IVIG and steroids treatment). None of the cases treated with IVIG and/or steroids died but four of them (no. 24, 32, 37, and 50) treated in later stage with either IVIG or steroids (but not both) had mild neurological sequelae. It should be noted that two of these cases had underlying immunodeficiency (no. 24 and 32) while the other two were treated with IVIG alone (no. 50) or steroids alone (no. 37). In contrast, seven out of nine patients who did not receive IVIG and/or steroids were either slow in their recovery (three cases), had a form of neurological sequelae (three cases), or even died (one case). There were no statistically significant differences between patients with competent and suppressed immune status and the success of the treatment (IVIG and/or steroids) (p = 0.188).
The prognosis of the encephalitis associated with B19 appears to vary. Although in some cases, complete recovery without neurological sequelae was achieved, there were seven deaths (14.0%) following the illness, and in 13 cases (26.0%), long-term neurological sequelae were observed, ranging from mild language learning difficulties and slurred speech to mental and motor retardation.
DISCUSSION
This is the first systematic review that targets the association between B19 infection and neurological aspects. We identified in this review 129 cases, reported in 89 publications including case reports, brief communications, comments or letters to the editors, retrospective studies or screening programs, and follow-up studies. These publications linked the virus with various neurological aspects either confined to CNS (including encephalitis; 50 cases, meningitis; 12 cases (no. 51–62), cerebellar ataxia as isolated neurological event; one case (no. 63), stroke; seven cases (no. 64–70), transverse myelitis; three cases (no. 71–73), seizures; five cases (no. 74–78), Reye's syndrome; one case (no. 79)) or related to PNS (including neuralgic amyotrophy; eight cases (no. 80–87), peripheral neuropathy; 19 cases (no. 88–106), carpal tunnel syndrome (CTS); 10 cases (no. 107–116), Guillain–Barré syndrome (GBS); four cases (no. 117–120)), in addition to nine cases that reported an association between B19 infection and ME (no. 121–129).
The most common B19-associated neurological manifestation was encephalitic syndromes, representing 38.8% of the total B19-related neurological cases that are currently in the literature. B19 is not usually investigated during encephalitis episodes, and in most of the cases reported, it was only sought after other pathogens that are commonly involved in encephalitis were proved to be negative, and the etiological cause remained undetermined. In addition, in other cases, B19 was suspected because of the appearance of one of its related symptoms or merely because of the physician anticipation of or interest in B19 infection. This could explain to some extent the rarity of B19 involvement in such a widespread neurological disorder, which occurs at a rate of six to seven cases among 100 000 individuals worldwide 95. That means if B19 is investigated at the same rate as other pathogens that commonly cause encephalitis, more cases could be detected. This is supported by the fact that when B19 was sought retrospectively in encephalitis cases, a 4% detection rate was found in the two retrospective studies performed so far, detecting 12 cases with etiologically undiagnosed encephalitis and with no sufficient information to support the detection of a recent B19 infection 14,16. Therefore, we suggest performing larger multicenter retrospective studies and further prospective studies to support these findings. Because the cost of detecting anti-B19 IgM antibodies and B19 DNA in serum or CSF is relatively low, we recommend at this stage that detection of B19 should be incorporated in the differential diagnosis of encephalitis cases.
The represented cases of B19-associated encephalopathy clearly indicate that there are no distinguishing features of B19-associated encephalitis compared with encephalitis caused by many other viral pathogens, except for the presence of rash, anemia, and arthritis in some patients. It is clear from the evidence of B19 infection upon retrospective analyses that there are no clinical clues regarding the diagnosis 14,16. For example, physicians cannot depend only on typical EI rash to confirm the concurrent B19 infection with encephalitis because many case reports, especially those identified retrospectively, failed to identify the coexistence of the rash with the encephalitic episode. In addition, the timing of neurological symptoms in relation to the rash varied considerably without a clear pattern. Furthermore, in immunocompromised individuals, B19-specific rash is usually absent around the time of neurological illness because of the immunopathological nature of the rash. Also, a physician cannot depend on the appearance of other B19-related symptoms, such as arthralgia, which is present in few cases, and anemia, which is detected mostly in cases with reduced immune status because of various conditions other than neurological symptoms. On the other hand, analysis of CSF for cell count and protein and glucose concentrations vary among cases, and significant indications cannot be obtained from these data and thus cannot be used in supporting the association of B19 infection in such cases. In addition, physicians cannot rely on neuroimaging studies in detecting specific abnormalities related to B19-associated encephalitis. Therefore, we conclude that the detection or confirmation of associated B19 infection with encephalopathy should always depend on the presence of B19 specific markers, namely, the anti-B19 IgM antibodies and B19 DNA in serum or CSF. However, B19 DNA may be detectable for extended periods, even in healthy individuals 96. Therefore, the presence of low levels of B19 DNA alone cannot be used to diagnose B19 infection with encephalopathy.
Intravenous immunoglobulin are considered the only treatment option for many clinical syndromes associated with B19 infection because it is believed that they include a good source of antibodies to neutralize the virus, although the mechanism of IVIG action is not precisely known. On the other hand, steroid therapy was also suggested for treatment of neurological disorders in general and encephalopathy in particular. When B19 involvement was either suspected or confirmed, 16 cases of encephalopathy were treated with IVIG and/or steroids. Given the clinical cases of encephalopathy presented, we are clearly not in a position to fully support the use of IVIG, steroids, or their combination in encephalitis associated with B19 infection, although 12 cases showed clinical improvement, because favorable outcomes with full recovery seem to be another distinctive feature for many encephalopathy reported cases without IVIG and/or steroids treatment, which may suggest a casual association with IVIG and/or steroids when they are used. In addition, IVIG and steroids were given together in eight cases showing clinical improvement, and therefore, an objective assessment of efficacy of either treatment cannot be obtained. However, with the absence of other effective treatment regimens, the use of combined treatment of IVIG and steroids in B19-associated encephalopathy could be considered. We, therefore, recommend that treatment of severe cases might benefit from a combined regime of IVIG and steroids, until a randomized prospective clinical trial of this regimen can be conducted.
There were seven deaths following encephalitis associated with B19, and in 12 cases, long-term neurological sequelae were observed, urging the necessity of rapid diagnosis of B19 infection and swift clinical intervention with combined IVIG and steroids regimen. In contrast to encephalitis cases, prognosis appears to be good in cases of B19-associated meningitis with a high rate of spontaneous cure and no sequelae reported.
In addition to one case of ataxia as an individual isolated event in association with B19 50, there are also six cases where cerebellar involvement was additionally suggested either clinically (no. 16, 20, 44, and 50) or pathologically (no. 17 and 60). Although aplastic crisis can be, by itself, a risk factor for stroke, B19 could participate in the latter in patients without aplastic crisis. Isolated events of seizures were also reported, although episodes of seizures seemed more part of a wider neurological picture. Scattered case reports have linked B19 infection with transverse myelitis and Reye's syndrome. We are not in a position to confirm these associations because of the low number of cases reported in the literature. Large-scale retrospective studies are required to confirm the association of B19 infection with these CNS presentations.
B19 is generally not regarded to be neurotropic, but direct infection and local replication of the virus could not be ruled out as possible pathogenesis mechanism for B19-associated CNS aspects. There have been controversial reports regarding the detection of B19 DNA in brain tissues. In one study, no evidence of B19 invasion of the brain was observed using relatively insensitive techniques 97. However, in other reports, B19 DNA was detected in a brain biopsy specimen from a 67-year-old woman with severe meningoencephalitis by PCR 15 and in the nucleus of the multinucleated giant cells and solitary endothelial cells for a hydropic fetus by in situ PCR 98. These data were supported by recent large-scale studies that used a highly specific nested PCR and nucleotide sequencing to detect B19 sequences in the dorsolateral prefrontal cortex 99 and cerebellum 100 of postmortem adult human brains. However, concerns should be cast over the fact that B19 DNA could persist in many tissues in detectable levels for years. In these cases, B19 DNA is likely to be existing in the circulation at high levels after infections and which becomes sequested and persists in the tissues as a result. Distinguishing that remnant DNA from the products of an active infection in a tissue has plagued many studies of B19 pathogenesis. B19 DNA persistence in brain tissues, however, could by itself provoke the pathogenic action of the virus through inducing chromosomal defect or damage 2. On the other hand, immune-related pathogenic mechanisms cannot be ruled out, supported by complete resolution of symptoms in cases treated with steroids. In addition, recent reports suggest that some cases of anti-N-methyl-d-aspartate receptor encephalitis could be triggered by B19 35,36,101. Vascular injury, particularly in the cerebellum, could also be involved in the pathogenesis. Therefore, we conclude that complex and variable pathogenesis is likely to contribute to the CNS manifestations. Exact mechanisms of actions through thorough prospective and retrospective pathological studies on sera, CSF, and postmortem tissues are required.
The number of B19-associated neuralgic amyotrophy cases does not necessarily reflect the extent of B19 involvement in this neurological disorder of unknown etiology because all were case reports. Therefore, retrospective and prospective studies are required to give a more comprehensive picture of the involvement of B19 in this disorder. The pathological mechanism of brachial plexus neuritis after B19 infection is also not known. However, clinical characteristics of reported cases show some striking similarities: All cases but one concerned adults, whereas the majority of parvovirus infections usually occur in children. All patients but one presented symptoms of brachial plexus neuritis coinciding with or immediately after the appearance of EI rash, which is interesting because the typical rash is believed to be immune mediated and generally coincides with the appearance of viral antibodies. It is likely that either autoantibodies or immune complex deposition are involved in these cases while direct infection and local replication of the virus could be ruled out.
Reported B19-associated peripheral neuropathy cases occurred as paraesthesia, dysesthesia, cranial nerve palsy, optic neuropathy, mononeuritis multiplex (MM), or acute autonomic sensory and motor neuropathy (AASM). In general, these cases followed a subacute but progressive course with unpredictable extension and severity ranging from complete recovery with no further neurological symptoms to pure limited sensory disorders and could end with severe multifocal sensory motor axonal loss with marked functional disability. The way in which B19 is able to trigger neuropathy is not fully elucidated. Possible mechanisms may involve necrotizing vasculitis through immune complex deposition or hypersensitivity vasculitis secondary to B19 infection. Persistence of B19 infection may play a role in prolongation of the disorder. When followed up, B19 DNA was present in serum beyond 6 months after onset of neuropathy in at least five cases (no. 94–95 and 99–101). This could provide a rationale for the treatment of such patients with IVIG. However, and as discussed in B19-associated encephalitis cases, its combination with steroids in three patients and the possibility of a spontaneous progressive recovery after acute nerve injury does not allow definite conclusions to be made about the efficacy of IVIG in B19-associated neuropathy. Complete neurological, neurophysiological, and nerve histological examination of B19-associated neuropathy should be performed. Further epidemiological studies are required to confirm the link between B19 and the neuropathy and to assess the frequency of B19-related neuropathy in comparison with other causes of B19-free neuropathy. However, B19 infection should be routinely considered in the etiological assessment, especially in the event of initial sensory symptoms with concurrent rash, as this could lead to an early appropriate treatment with IVIG.
There are several causes of CTS, but in many cases, the etiology remains unknown. The assumptions that B19 can be the infectious agent that triggers CTS, and that the coexistence of B19 infection and CTS was not causal in the reported cases, require prospective and follow-up studies that compare B19 markers (detection and quantification) between CTS patients and a CTS-free control group, preferably during an epidemic peak of B19 infection. This is because most CTS cases are usually confirmed long time after laboratory detection of B19 acute infection.
B19 is not usually cited as a cause of GBS compared with Campylobacter jejuni and cytomegalovirus that constitute the most frequent bacterial and viral triggers 102. Only four cases linked to B19 infection are reported in the literature, and retrospective studies have yet to prove any association. However, it is worth mentioning that, in a relatively old prospective study, serum samples taken from a group of GBS patients were examined for the presence of a variety of pathogens 103. Anti-B19 IgM antibodies were detected in four patients (4%) but not in controls. Although this was statistically insignificant, this finding suggests that parvovirus B19 may be an important cause of some cases of GBS. These four cases were not included in this study because of insufficient data concerning them.
Few case reports and follow-up studies have documented an association between acute B19 infection and ME (no. 121–129), whereas others found no association 104,105. Reasons behind this disparity could be due to various groups promoting different nomenclature, diagnostic criteria, etiologic hypotheses, and treatments for ME, resulting in controversy about many aspects of this disorder. For example, ME is not always classified as a neurological disorder. In addition, although ME may follow acute B19 infection, attribution of a case of ME to B19 infection may be extremely difficult in the absence of serological confirmation of acute infection at fatigue onset. Although the gold standard method of diagnosis is to confirm acute B19 infection through positive anti-B19 IgM antibodies or B19 DNA at the time of onset of fatigue, this would be a rare occurrence, and a more practical method would is needed to detect other B19 markers during the illness. Kerr et al. 106 found out that antibodies against B19 nonstructural protein are associated with chronic and not acute B19 infection and therefore could be used as a marker for B19-assciated ME cases. Because several different infections may be involved in ME, the proportion resulting from any one agent, such as B19, is likely to vary, as evident in various follow-up studies, with regard to sampling strategy, time and place, and its relation to the prevalence of each infection. Two particularly important factors are whether there is an outbreak in progress at a particular location and the selection strategy for the control/comparison groups. These factors should be taken into consideration when attempting to confirm the possible link between B19 and ME. Interestingly, five out of nine patients were improved after IVIG 92,94,107, a matter that should be considered when B19 is suspected or confirmed to be associating with ME cases.
This study has various limitations: Firstly, the literature data on this topic are few and heterogeneous in terms of criteria used for characterization of B19-associated neurological symptoms, in addition to the differences in diagnosis, complementary investigation, treatment, and follow-up of these cases. Therefore, epidemiological data on the incidence of B19-associated neurological aspects cannot be accurately extrapolated. Prospective, structured, multicenter studies would be necessary to determine the real epidemiological scenario of these complications that are currently receiving increasing attention. Furthermore, although there are some hypotheses on the pathogenesis of B19-associated neurological aspects, lack of detailed descriptions of autopsy reports render the pathogenesis not completely understood, and therefore, thorough prospective and retrospective pathological studies on postmortem tissues using sensitive immunocytochemistry and in situ hybridization techniques are a priority.
In conclusion, pending answers to the questions raised, we recommend that B19 infection should be included in the differential diagnosis of encephalitic syndrome and some PNS manifestations regardless of the age. We recommend that diagnosis of B19-associated neurological aspect should solely depend on the investigation of anti-B19 IgM antibodies and B19 DNA in serum or CSF. We also suggest that severe cases of B19-associated neurological aspects might benefit from a combined regime of IVIGs, and steroids and a randomized controlled trial should be considered.
CONFLICT OF INTEREST
The authors have no competing interest.
Acknowledgments
The support by the projects WELCOMEII/6/CNC/1055/2011 and PEst-C/SAU/LA0001/2013-2014 “financiado por Fundos FEDER através do Programa Operacional Factores de Competitividade – COMPETE e por Fundos Nacionais através da FCT – Fundação para a Ciência e a Tecnologia” are gratefully acknowledged.
Glossary
- AASM
acute autonomic sensory and motor neuropathy
- B19
human parvovirus B19
- CAT
computed axial tomography
- CIs
confidence intervals
- CTS
carpal tunnel syndrome
- EEG
electroencephalogram
- EI
erythema infectiosum
- GBS
Guillain–Barré syndrome
- IVIGs
intravenous immunoglobulins
- ME
myalgic encephalomyelitis
- MM
mononeuritis multiplex
- MRI
magnetic resonance imaging
- NIHF
nonimmune hydrops fetalis
- PNS
peripheral nervous system
- PRCA
persistent infection manifesting as pure red cell aplasia
- PRISMA
preferred reporting items for systematic review and meta-analysis
- TAC
transient aplastic crisis
REFERENCES
- Cossart YE, Field AM, Cant B, Widdows D. Parvovirus-like particles in human sera. Lancet. 1975;1:72–73. doi: 10.1016/s0140-6736(75)91074-0. [DOI] [PubMed] [Google Scholar]
- Barah F, Vallely PJ, Cleator GM, Kerr JR. Neurological manifestations of human parvovirus B19 infection. Reviews in Medical Virology. 2003;13:185–199. doi: 10.1002/rmv.388. [DOI] [PubMed] [Google Scholar]
- National institute of neurological disorders and stroke http://www.ninds.nih.gov/disorders/disorder_index.htm (Accessed March 31st, 2013)
- Carruthers BM, van de Sande MI, De Meirleir KL, et al. Myalgic encephalomyelitis: international consensus criteria. Journal of Internal Medicine. 2011;270:327–338. doi: 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Balfour HH, Schiff GM, Bloom JE. Encephalitis associated with erythema infectiosum. Journal of Pediatrics. 1970;77:133–136. doi: 10.1016/s0022-3476(70)80059-2. [DOI] [PubMed] [Google Scholar]
- Breese C, Homer FA. Encephalopathy with erythema infectiosum. American Journal of Diseases of Children. 1977;131:65–67. [PubMed] [Google Scholar]
- Zerbini M, Musiani M, Venturoli S, et al. Different syndromes associated with parvovirus viraemia in paediatric patients: report of four cases. European Journal of Pediatrics. 1992;151:815–817. doi: 10.1007/BF01957931. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Satoh M, Oda Y. Human parvovirus B19 encephalopathy. Archives of Disease in Childhood. 1994;70:71. doi: 10.1136/adc.70.1.71-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T. Ulcerative colitis that developed 8 years after human parvovirus B19 encephalopathy. European Journal of Pediatrics. 2004;163:341–342. doi: 10.1007/s00431-004-1433-x. [DOI] [PubMed] [Google Scholar]
- Yoto Y, Kudoh T, Asanuma H, et al. Transient disturbance of consciousness and hepatic dysfunction associated with human parvovirus B19 infection. Lancet. 1994;344:624–625. [PubMed] [Google Scholar]
- Heegaard ED, Peterslund NA, Homsleth A. Parvovirus B19 infection associated with encephalitis in a patient suffering from malignant lymphoma. Scandinavian Journal of Infectious Diseases. 1995;27:631–633. doi: 10.3109/00365549509047080. [DOI] [PubMed] [Google Scholar]
- Umene K, Nunoue T. A new genome type of human parvovirus B19 present in sera of patients with encephalopathy. Journal of General Virology. 1995;76:2645–2651. doi: 10.1099/0022-1317-76-11-2645. [DOI] [PubMed] [Google Scholar]
- Haseyama K, Kudoh T, Yoto Y, Suzuki N, Chiba S. Detection of human parvovirus B19 DNA in cerebrospinal fluid. Pediatric Infectious Disease Journal. 1997;16:324–326. doi: 10.1097/00006454-199703000-00013. [DOI] [PubMed] [Google Scholar]
- Druschky K, Walloch J, Heckmann J, et al. Chronic parvovirus B19 meningoencephalitis with additional detection of Epstein–Bar virus DNA in the cerebrospinal fluid of an immunocompetent patient. Journal of NeuroVirology. 2000;6:418–422. doi: 10.3109/13550280009018306. [DOI] [PubMed] [Google Scholar]
- Barah F, Vallely PJ, Chiswick ML, Cleator GM, Kerr JR. Human parvovirus B19 infection associated with acute meningoencephalitis. Lancet. 2001;358:729–730. doi: 10.1016/S0140-6736(01)05905-0. [DOI] [PubMed] [Google Scholar]
- Gennery AJ, Cant A, Forsyth RJ. Development of parainfectious opsoclonus in an infant by a non-humoral immune mechanism. Developmental Medicine and Child Neurology. 2001;43:213–214. [PubMed] [Google Scholar]
- Skaff PT, Labiner DM. Status epilepticus due to human parvovirus B19 encephalitis in an immunocompetent adult. Neurology. 2001;57:1336–1337. doi: 10.1212/wnl.57.7.1336. [DOI] [PubMed] [Google Scholar]
- Wierenga KJ, Serjeant BE, Serjeant GR. Cerebrovascular complications and parvovirus infection in homozygous sickle cell disease. Journal of Pediatrics. 2001;139:438–442. doi: 10.1067/mpd.2001.117070. [DOI] [PubMed] [Google Scholar]
- Yazawa S, Kawasaki S, Fujimoto C, Ohi T. Case report of meningoencephalitis during a concomitant mumps and parvovirus B19 infection. Clinical Neurology and Neurosurgery. 2002;104:380–382. doi: 10.1016/s0303-8467(02)00030-6. [DOI] [PubMed] [Google Scholar]
- Bakhshi S, Sarnaik SA, Becker C, Shurney WW, Nigro M, et al. Acute encephalopathy with parvovirus B19 infection in sickle cell disease. Archives of Disease in Childhood. 2002;87:541–542. doi: 10.1136/adc.87.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan RC, Chidlow G, French MA. Parvovirus B19 encephalitis presenting as immune restoration disease after highly active antiretroviral therapy for human immunodeficiency virus infection. Clinical Infectious Diseases. 2003;36:1191–1194. doi: 10.1086/374603. [DOI] [PubMed] [Google Scholar]
- Bilge I, Sadikoglu B, Emre S, et al. Central nervous system vasculitis secondary to parvovirus B19 infection in a pediatric renal transplant patient. Pediatric Nephrology. 2005;20:529–533. doi: 10.1007/s00467-004-1736-1. [DOI] [PubMed] [Google Scholar]
- Erol I, Alehan F, Yalcin K. Refractory status epilepticus owing to human parvovirus B19 encephalitis in a child. Journal of Child Neurology. 2006;21:820–822. doi: 10.1177/08830738060210092301. [DOI] [PubMed] [Google Scholar]
- Fong CY, de Sousa C. Childhood chorea-encephalopathy associated with human parvovirus B19 infection. Developmental Medicine and Child Neurology. 2006;48:526–528. doi: 10.1017/S0012162206001101. [DOI] [PubMed] [Google Scholar]
- Laurenz M, Winkelmann B, Roigas J, et al. Severe parvovirus B19 encephalitis after renal transplantation. Pediatric Transplantation. 2006;10:978–981. doi: 10.1111/j.1399-3046.2006.00599.x. [DOI] [PubMed] [Google Scholar]
- Steinfort DP, Dixon B. Parvovirus encephalitis and pneumonia in an immunocompetent adult. Internal Medicine Journal. 2006;36:209–210. doi: 10.1111/j.1445-5994.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- Tonnellier M, Bessereau J, Carbonnell N, et al. A possible parvovirus B19 encephalitis in an immunocompetent adult patient. Journal of Clinical Virology. 2007;38:186–187. doi: 10.1016/j.jcv.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Giørtz-Carlsen B, Rittig S, Thelle T. Neurological symptoms and acute hepatitis associated with parvovirus B19. Ugeskrift for Laeger. 2007;169:4075–4077. [PubMed] [Google Scholar]
- Bonvicini F, Marinacci G, Pajno MC, et al. Meningoencephalitis with persistent parvovirus B19 infection in an apparently healthy woman. Clinical Infectious Diseases. 2008;47:385–387. doi: 10.1086/589863. [DOI] [PubMed] [Google Scholar]
- Oshima K, Kikuchi A, Mochizuki S, et al. Acute encephalopathy with human parvovirus B19 infection in hereditary spherocytosis. Pediatric Infectious Disease Journal. 2008;27:651–652. doi: 10.1097/INF.0b013e3181694fcf. [DOI] [PubMed] [Google Scholar]
- Moretti MV, Abbate I, Sciarrone MR, et al. Acute infection with parvovirus B19 manifesting as brain stem encephalitis. Infections in Medicine. 2008;25:173–179. [Google Scholar]
- Coskun O, Erdem H, Gul HC, et al. Meningoencephalitis associated with human parvovirus B19. Clinical Microbiology and Infection. 2008;14:1188–1190. doi: 10.1111/j.1469-0691.2008.02110.x. [DOI] [PubMed] [Google Scholar]
- Greco F, Barbagallo ML, Chiodo DC, Guglielmino R, Sorge G. Severe ataxia as a complication of human parvovirus B19 acute encephalitis in a child. Journal of Child Neurology. 2008;23:1078–1080. doi: 10.1177/0883073808315420. [DOI] [PubMed] [Google Scholar]
- Grillo E, da Silva RJ. Childhood chorea-encephalopathy and unremarkable MRI: an association suggesting parvovirus B19 infection. Developmental Medicine and Child Neurology. 2009;51:759–761. doi: 10.1111/j.1469-8749.2009.03388.x. [DOI] [PubMed] [Google Scholar]
- Melbourne Chambers RH, Gooden MA, Gilbert TD, Jackson ST. Childhood chorea-encephalopathy associated with recent parvovirus B19 infection in two Jamaican children. Annals of Tropical Paediatrics. 2010;30:339–344. doi: 10.1179/146532810X12858955921438. [DOI] [PubMed] [Google Scholar]
- Cugler T, Carvalho LM, Facincani I, et al. Severe glomerulonephritis and encephalopathy associated with parvovirus B19 infection mimicking systemic lupus erythematosus. Scandinavian Journal of Rheumatology. 2012;41:79–81. doi: 10.3109/03009742.2011.617315. [DOI] [PubMed] [Google Scholar]
- Meyer P, Jeziorski E, Bott-Gilton L, et al. Childhood parvovirus B19 encephalitis. Archives of Pediatrics. 2011;18:1315–1319. doi: 10.1016/j.arcped.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Matsubara K, Morio T, et al. Acute cerebellitis and concurrent encephalitis associated with parvovirus B19 infection. Pediatric Infectious Disease Journal. 2012;31:427. doi: 10.1097/INF.0b013e3182481e24. [DOI] [PubMed] [Google Scholar]
- Lefrere J, Oliver C, Courouce AM, Soulier JP. Erythroblastopenia and febrile meningeal syndrome. Presse Médicale. 1985;14:228–229. [PubMed] [Google Scholar]
- Tsuji A, Uchida N, Asamura S, Matsunaga Y, Yamazaki S. Aseptic meningitis with erythema infectiosum. European Journal of Pediatrics. 1990;149:449–450. doi: 10.1007/BF02009674. [DOI] [PubMed] [Google Scholar]
- Okumura A, Ichikawa T. Aseptic meningitis caused by human parvovirus B19. Archives of Disease in Childhood. 1993;68:784–785. doi: 10.1136/adc.68.6.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassinotti P, Schultze D, Schlageter P, Chevili S, Siegl G. Persistent human parvovirus B19 infection following an acute infection with meningitis in an immunocompetent patient. European Journal of Clinical Microbiology and Infectious Diseases. 1993;12:701–704. doi: 10.1007/BF02009384. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Terada S, Inoue M. Neonatal meningitis with human parvovirus B19 infection. Archives of Disease in Childhood - Fetal and Neonatal Edition. 1995;73:F196–F197. doi: 10.1136/fn.73.3.f196-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koduri PR, Naides SJ. Aseptic meningitis caused by parvovirus B19. Clinical Infectious Diseases. 1995;21:1053. doi: 10.1093/clinids/21.4.1053. [DOI] [PubMed] [Google Scholar]
- Tabak F, Mert A, Ozturk R, et al. Prolonged fever caused by parvovirus B19-induced meningitis: case report and review. Clinical Infectious Diseases. 1999;29:446–447. doi: 10.1086/520234. [DOI] [PubMed] [Google Scholar]
- Sinclair JP, Croxson MC, Thomas SM, Teague LR, Mauger DC. Chronic parvovirus B19 meningitis in a child with acute lymphocytic leukemia. Pediatric Infectious Disease Journal. 1999;18:395–396. doi: 10.1097/00006454-199904000-00024. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Barros RA, do Nascimento JP, de Oliveira SA. Two family members with a syndrome of headache and rash caused by human parvovirus B19. Brazilian Journal of Infectious Diseases. 2001;5:37–39. doi: 10.1590/s1413-86702001000100006. [DOI] [PubMed] [Google Scholar]
- Akin M, Carman KB, Karaturk AH, Ceran O. Mumps-like syndrome owing to parvovirus B19: a brief report. Annals of Tropical Paediatrics. 2002;22:57–58. doi: 10.1179/027249302125000166. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Ueno T, Komatsu H, Takada H, Nunoue T. Acute cerebelar ataxia with human parvovirus B19 infection. Archives of Disease in Childhood. 1999;80:72–73. doi: 10.1136/adc.80.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi B, Bergonzini P, Crisi G, Frigieri G, Portolani M. Case of stroke in a 7-year-old male after parvovirus B19 infection. Pediatric Neurology. 2003;28:69–71. doi: 10.1016/s0887-8994(02)00504-0. [DOI] [PubMed] [Google Scholar]
- Mandrioli J, Portolani M, Cortelli P, Sola P. Middle cerebral artery thrombosis in course of parvovirus B19 infection in a young adult: a new risk factor for stroke? Journal of Neurovirology. 2004;10:71–74. doi: 10.1080/13550280490261752. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Shimohama S, Kaji R, Akiguchi I, Kimura J. An adult case of transverse myelitis with erythema infectiosum. Rinshō Shinkeigaku. 1992;32:1035–1037. [PubMed] [Google Scholar]
- Earl SC, Zhang B, Thomas LM, Ledingham JM. A case of reactive arthritis secondary to parvovirus B19 infection complicated by an acute transverse myelitis. Rheumatology. 2007;46:i139. [Google Scholar]
- Scheibe F, Hofmann J, Ruprecht K. Parainfectious myelitis associated with parvovirus B19 infection. Journal of Neurology. 2010;257:1557–1558. doi: 10.1007/s00415-010-5536-1. [DOI] [PubMed] [Google Scholar]
- Nigro G, D'Eufemia P, Zerbini M, et al. Parvovirus B19 infection in a hypogammaglobulinemic infant with neurologic disorders and anemia: successful immunoglobulin therapy. Pediatric Infectious Disease Journal. 1994;13:1019–1021. [PubMed] [Google Scholar]
- Hsu D, Sandborg C, Hahn JS. Frontal lobe seizures and uveitis associated with acute human parvovirus B19 infection. Journal of Child Neurology. 2004;19:304–306. doi: 10.1177/088307380401900413. [DOI] [PubMed] [Google Scholar]
- Kamlesh Y, Pallav G, Manjula M, Rohan M. Seizure and hepatosplenomegaly-rare manifestation of parvovirus B-19: a case report and review of the literature. Journal of Tropical Medicine. 2011;2011:287914. doi: 10.1155/2011/287914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Ikeno K, Abe T, Tohyama J, Adachi Y. Hemiconvulsion-hemiplegia-epilepsy syndrome associated with CACNA1A S218L mutation. Pediatric Neurology. 2011;45:193–196. doi: 10.1016/j.pediatrneurol.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Costa PS, Ribeiro GM, Vale TC, Casali TG, Leite FJ. Adult Reye-like syndrome associated with serologic evidence of acute parvovirus B19 infection. Brazilian Journal of Infectious Diseases. 2011;15:482–483. doi: 10.1016/s1413-8670(11)70232-x. [DOI] [PubMed] [Google Scholar]
- Denning D, Amos A, Rudge P, Cohen B. Neuralgic amytrophy due to parvovirus infection. Journal of Neurology, Neurosurgery, and Psychiatry. 1986;50:641–642. doi: 10.1136/jnnp.50.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh KJ, Armstrong RD, Turner AM. Brachial plexus neuropathy associated with human parvovirus infection. British Medical Journal. 1988;296:896. doi: 10.1136/bmj.296.6626.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellas F, Olivares JP, Zandotti C, Delarque A. Neuralgic amytrophy after parvovirus B19 infection. Lancet. 1993;342:503–504. doi: 10.1016/0140-6736(93)91635-y. [DOI] [PubMed] [Google Scholar]
- Staud R, Davidson RA, Corman LC. Brachial plexitis in a patient with acute parvovirus B19 infection. British Journal of Rheumatology. 1995;34:480–481. doi: 10.1093/rheumatology/34.5.480. [DOI] [PubMed] [Google Scholar]
- Maas JJ, Beersma MF, Haan J, Jonkers GJ, Kroes AC. Bilateral brachial plexus neuritis following parvovirus B19 and cytomegalovirus infection. Annals of Neurology. 1996;40:928–932. doi: 10.1002/ana.410400618. [DOI] [PubMed] [Google Scholar]
- Puechal X, Hilliquin P, Kahan A, Menkes CJ. Neuralgic amyotrophy and polyarthritis caused by parvovirus B 19 infection. Annals of the Rheumatic Diseases. 1998;57:262. doi: 10.1136/ard.57.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff-Moradpour A, Huzly D, Korinthenberg R, Bener R. Neuralgic amytrophy associated with parvovirus B19 infection in a child. European Journal of Pediatrics. 2001;160:200–201. doi: 10.1007/pl00008427. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A, Carrillo-García F, Montes-Latorre E, Gómez-Aranda F. Bilateral neuralgic amyotrophy secondary to parvovirus B19 infection. Medicina Clínica (Barcelona) 2006;127:398. doi: 10.1157/13092441. [DOI] [PubMed] [Google Scholar]
- Faden H, Gary GW, Korman M. Numbness and tingling of fingers associated with parvovirus B19 infection. Journal of Infectious Diseases. 1990;161:354–355. doi: 10.1093/infdis/161.2.354. [DOI] [PubMed] [Google Scholar]
- Faden H, Gary GW, Jr, Anderson LJ. Chronic parvovirus infection in a presumably immunologically healthy woman. Clinical Infectious Diseases. 1992;15:595–597. doi: 10.1093/clind/15.4.595. [DOI] [PubMed] [Google Scholar]
- Rabar D, Peyramond D. Parvovirus B19 infection revealed by prolonged dysesthesia. Médecine et Maladies Infectieuses. 2005;35:91–94. doi: 10.1016/j.medmal.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Corman LC, Dolson DJ. Polyarteritis nodosa and parvovirus B19 infection. Lancet. 1992;339:491. doi: 10.1016/0140-6736(92)91096-q. [DOI] [PubMed] [Google Scholar]
- Viguier M, Guillevin L, Laroche L. Treatment of parvovirus B19-associated polyarteritis nodosa with intravenous immune globulin. New England Journal of Medicine. 2001;344:1481–1482. doi: 10.1056/NEJM200105103441919. [DOI] [PubMed] [Google Scholar]
- Aguilar-Bernier M, Bassas-Vila J, Torné-Gutiérrez JI. Presence of perineuritis in a case of papular purpuric gloves and socks syndrome associated with mononeuritis multiplex attributable to B19 parvovirus. Journal of the American Academy of Dermatology. 2006;54:896–899. doi: 10.1016/j.jaad.2005.10.012. , et al. ( [DOI] [PubMed] [Google Scholar]
- Lenglet T, Haroche J, Schnuriger A. Mononeuropathy multiplex associated with acute parvovirus B19 infection: characteristics, treatment and outcome. Journal of Neurology. 2011;258:1321–1326. doi: 10.1007/s00415-011-5931-2. , et al. ( [DOI] [PubMed] [Google Scholar]
- Hanai S, Komaki H, Sakuma H, et al. Acute autonomic sensory and motor neuropathy associated with parvovirus B19 infection. Brain Dev. 2011;33:161–165. doi: 10.1016/j.braindev.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Martinón-Torres F, Seara MJ, Del Río Pastoriza I, Mata MB, Castro-Gago M. Parvovirus B19 infection complicated by peripheral facial palsy and parotitis with intraparotid lymphadenitis. Pediatric Infectious Disease Journal. 1999;18:307–308. doi: 10.1097/00006454-199903000-00025. [DOI] [PubMed] [Google Scholar]
- Soares-Fernandes JP, Maré R. Isolated velopalatine paralysis associated with parvovirus B19 infection. Arquivos de Neuro-Psiquiatria. 2006;64:603–605. doi: 10.1590/s0004-282x2006000400015. [DOI] [PubMed] [Google Scholar]
- Wilhelm H, Hartmann C, Boesche-Abele V. Optic neuropathy after erythema infectiosum. Klinische Monatsblätter für Augenheilkunde. 1998;213:355–357. doi: 10.1055/s-2008-1035002. [DOI] [PubMed] [Google Scholar]
- Le Scanff J, Vighetto A, Mekki Y, et al. Acute ophthalmoparesis associated with human parvovirus B19 infection. European Journal of Ophthalmology. 2010;20:802–804. doi: 10.1177/112067211002000428. [DOI] [PubMed] [Google Scholar]
- Samii K, Cassinotti P, de Freudenreich J, et al. Acute bilateral carpal tunnel syndrome associated with human parvovirus B19 infection. Clinical Infectious Diseases. 1996;22:162–164. doi: 10.1093/clinids/22.1.162. [DOI] [PubMed] [Google Scholar]
- Gendi NS, Gibson K, Wordsworth BP. Effect of HLA type and hypocomplementaemia on the expression of parvovirus arthritis: one year follow up of an outbreak. Annals of the Rheumatic Diseases. 1996;55:63–65. doi: 10.1136/ard.55.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JR, Bracewell J, Laing I, et al. Chronic fatigue syndrome and arthralgia following parvovirus B19 infection. Journal of Rheumatology. 2002;29:595–602. [PubMed] [Google Scholar]
- Musiani M, Manaresi E, Gallinella G, Zerbini M. Persistent parvovirus b19 infection resulting in carpal tunnel syndrome. Journal of Clinical Pathology. 2007;60:1177–1178. doi: 10.1136/jcp.2007.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula JMP, Mayor-Toranzo E, Franco-Hidalgo S. Bilateral carpal tunnel syndrome and parvovirus B19 infection. Revista Clínica Española. 2012;212:221–222. doi: 10.1016/j.rce.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Minohara Y, Koitabashi Y, Kato T, et al. A case of Guillain-Barré syndrome associated with human parvovirus B19 infection. Journal of Infection. 1998;36:327–328. doi: 10.1016/s0163-4453(98)94531-5. [DOI] [PubMed] [Google Scholar]
- Yamaoka Y, Isozaki E, Kagamihara Y, et al. A case of Guillain–Barré syndrome (GBS) following human parvovirus B19 infection. Rinshō Shinkeigaku. 2000;40:471–475. [PubMed] [Google Scholar]
- Barbi F, Ariatti A, Funakoshi K, et al. Parvovirus B19 infection antedating Guillain–Barré syndrome variant with prominent facial diplegia. Journal of Neurology. 2011;258:1551–1552. doi: 10.1007/s00415-011-5949-5. [DOI] [PubMed] [Google Scholar]
- Bucher Praz C, Dessimoz C, Bally F, Reymond S, Troillet N. Guillain–Barré syndrome associated with primary parvovirus B19 infection in an HIV-1-infected patient. Medical Case Reports. 2012;2012:140780. doi: 10.1155/2012/140780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JR, Coyle PV, DeLeys RJ, Patterson CC. Follow-up study of clinical and immunological findings in patients presenting with acute parvovirus B19 infection. Journal of Medical Virology. 1996;48:68–75. doi: 10.1002/(SICI)1096-9071(199601)48:1<68::AID-JMV11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Kerr JR, Cunniffe VS. Antibodies to parvovirus B19 non-structural protein are associated with chronic but not acute arthritis following B19 infection. Rheumatology. 2000;39:903–908. doi: 10.1093/rheumatology/39.8.903. [DOI] [PubMed] [Google Scholar]
- Jacobson SK, Daly JS, Thorne GM, McIntosh K. Chronic parvovirus B19 infection resulting in chronic fatigue syndrome: case history and review. Clinical Infectious Diseases. 1997;24:1048–1051. doi: 10.1086/513627. [DOI] [PubMed] [Google Scholar]
- Matano S, Kinoshita H, Tanigawa K, Terahata S, Sugimoto T. Acute parvovirus B19 infection mimicking chronic fatigue syndrome. Internal Medicine. 2003;42:903–905. doi: 10.2169/internalmedicine.42.903. [DOI] [PubMed] [Google Scholar]
- McGhee SA, Kaska B, Liebhaber M, Stiehm ER. Persistent parvovirus-associated chronic fatigue treated with high dose intravenous immunoglobulin. Pediatric Infectious Disease Journal. 2005;24:272–274. doi: 10.1097/01.inf.0000155194.66797.20. [DOI] [PubMed] [Google Scholar]
- Jmor F, Emsley HC, Fischer M, Solomon T, Lewthwaite P. The incidence of acute encephalitis syndrome in Western industrialised and tropical countries. Virology Journal. 2008;5:134. doi: 10.1186/1743-422X-5-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heegaard ED, Brown KE. Human parvovirus B19. Clinical Microbiology Reviews. 2002;15:485–505. doi: 10.1128/CMR.15.3.485-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JR, Barah F, Chiswick ML, et al. Evidence for the role of demyelination, HLA-DR alleles, and cytokines in the pathogenesis of parvovirus B19 meningoencephalitis and its sequelae. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;73:739–746. doi: 10.1136/jnnp.73.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isumi H, Nunoue T, Nishida A, Takashima S. Fetal brain infection with human parvovirus B19. Pediatric Neurology. 1999;21:661–663. doi: 10.1016/s0887-8994(99)00055-7. [DOI] [PubMed] [Google Scholar]
- Hobbs JA. Detection of adeno-associated virus 2 and parvovirus B19 in the human dorsolateral prefrontal cortex. Journal of Neurovirology. 2006;12:190–199. doi: 10.1080/13550280600827351. [DOI] [PubMed] [Google Scholar]
- Grant JK, Yin NC, Zaytoun AM, Waseem H, Hobbs JA. Persistent adeno-associated virus 2 and parvovirus B19 sequences in post-mortem human cerebellum. Cerebellum. 2009;8:490–498. doi: 10.1007/s12311-009-0126-4. [DOI] [PubMed] [Google Scholar]
- Grillo E, da Silva RJ. Anti-N-methyl-d-aspartate receptor encephalitis and parvovirus B19: a possible link? Journal of Pediatrics. 2013;163:1233–1234. doi: 10.1016/j.jpeds.2013.06.079. [DOI] [PubMed] [Google Scholar]
- Dua K, Banerjee A. Guillain-Barré syndrome: a review. British Journal of Hospital Medicine. 2010;71:495–498. doi: 10.12968/hmed.2010.71.9.78159. [DOI] [PubMed] [Google Scholar]
- Winer JB, Hughes RA, Anderson MJ, et al. A prospective study of acute idiopathic neuropathy II. Antecedent events. Journal of Neurology, Neurosurgery, and Psychiatry. 1988;51:613–618. doi: 10.1136/jnnp.51.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilaria RL, Jr, Komaroff AL, Fagioli LR, et al. Absence of parvovirus B19 infection in chronic fatigue syndrome. Arthritis and Rheumatism. 1995;38:638–641. doi: 10.1002/art.1780380510. [DOI] [PubMed] [Google Scholar]
- Koelle DM, Barcy S, Huang ML, et al. Markers of viral infection in monozygotic twins discordant for chronic fatigue syndrome. Clinical Infectious Diseases. 2002;35:518–525. doi: 10.1086/341774. [DOI] [PubMed] [Google Scholar]
- Kerr JR, Gough J, Richards SC, et al. Antibody to parvovirus B19 nonstructural protein is associated with chronic arthralgia in patients with chronic fatigue syndrome/myalgic encephalomyelitis. Journal of General Virology. 2010;91:893–897. doi: 10.1099/vir.0.017590-0. [DOI] [PubMed] [Google Scholar]
- Kerr JR, Cunniffe VS, Kelleher P, Bernstein RM, Bruce IN. Successful intravenous immunoglobulin therapy in 3 cases of parvovirus B19-associated chronic fatigue syndrome. Clinical Infectious Diseases. 2003;36:e100–e106. doi: 10.1086/374666. [DOI] [PubMed] [Google Scholar]


