In a recent paper in Nature, Venkatesh et al. (2014) cast valuable new light on the molecular underpinnings of vertebrate evolution with their publication of the genome of the elephant shark Callorhinchus. Noting that among the gene families involved in bone formation, the SCPP family is the only one absent in Callorhinchus (and probably other chondrichthyans), they argue that this absence is primitive for gnathostomes, and that the origin of the family in osteichthyans by tandem duplication of Sparcl1 provided a basis for the evolution of endochondral ossification in this group. They provide experimental evidence in support of their argument by disrupting the function of the bone-specific SCPP gene spp1 in zebrafish by targeted mutagenesis, resulting in reduced bone formation. However, a careful examination of their experimental data, and of the phylogenetic framework of known hard-tissue phenotypes, suggests a different scenario: SCPP genes originated in the gnathostome stem group and were secondarily lost in chondrichthyans.
The evolutionary history of bone is well known. Cellular dermal and perichondral bone is present and well developed in placoderms, which are derived members of the gnathostome stem group (Zhu et al. 2013; Venkatesh et al. 2014). Dermal bone identities and histological architectures appear to be substantially conserved between placoderms and osteichthyans (Fig. 1), arguing for conserved molecular patterning (Sanchez et al. 2012, 2013; Zhu et al. 2013). Within the gnathostome crown group, the Osteichthyes have retained dermal and perichondral bone, and added endochondral bone—a novel tissue, wholly distinct from perichondral bone although spatially associated with it—to this osteogenetic repertoire. Chondrichthyes (including Callorhinchus), on the other hand have lost perichondral bone and all large dermal bones with distinct morphological identities. They exhibit an acellular bone-like tissue in the base of their dentine scales and a cellular mineralized perichondral tissue in their neural arches, but neither can be identified convincingly as bone: the scale tissue appears to be a modified dentine whereas the perichondral tissue has characteristics of mineralized fibrocartilage (Eames et al. 2007). This pattern of character distribution suggests that an osteogenic molecular regulatory network was present in placoderms, retained and further elaborated in osteichthyans, but lost or substantially deconstructed in chondrichthyans.
Figure 1.
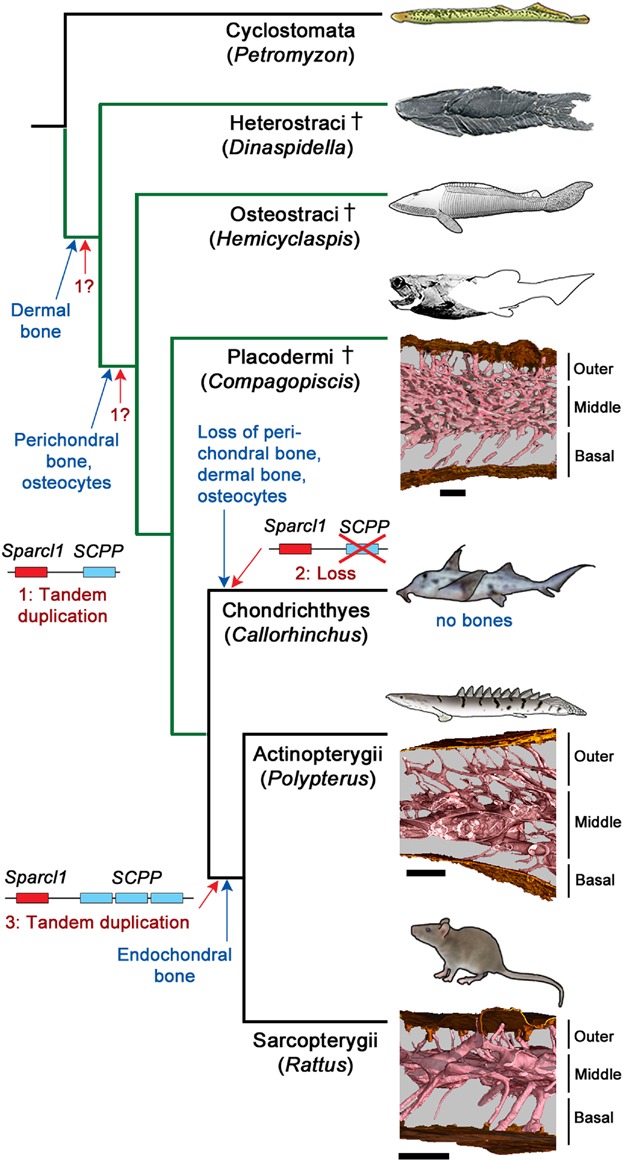
Simplified vertebrate phylogeny showing evolution of bone and inferred evolution of SCPP genes. Compagopiscis, Polypterus, and Rattus accompanied by block models of dermal bone microarchitecture, derived from synchrotron microtomography scans, showing conserved organization into basal, middle (or cancellous), and outer layers. Gnathostome stem group indicated in green. Blue legends and arrows, evolutionary changes in bone phenotype; red legends and arrows, inferred evolutionary changes of SCPP gene family. Scale bars, 250 µm.
Venkatesh et al. (2014) present 5-dpf and (in Supplementary Information) 15-dpf zebrafish larvae, wild-type, and spp1-deficient, as evidence for the supposed role of spp1 in endochondral bone formation. However, the spp1 phenotypes show no specific endochondral effects; bone formation in general is strongly suppressed, which affects the limited endochondral ossifications as well as the much larger dermal and perichondral elements, but there is no preferential loss of endochondral bone. Spp1 thus seems to have a general role in bone development rather than a specifically endochondral role.
Taken together, these data suggest (1) that the absence of spp1 in Callorhinchus and other chondrichthyans is functionally related to the lack of perichondral and dermal bones in these fishes, and (2) that this represents a loss rather than a primitive absence (Fig. 1). The alternative hypothesis proposed by Venkatesh et al. (2014), that spp1 is primitively absent in chondrichthyans and that bone formation in stem gnathostomes may have been regulated by sparc or sparcl1, provides no mechanism to explain the known evolutionary bone loss in chondrichthyans, where these genes are still present.
We suggest that the tandem duplication of Sparcl1 producing the ancestral SCPP gene occurred in the gnathostome stem group, which is compatible with the lamprey data (Fig. 1), and that this gene was lost in the Chondrichthyes (leading to the loss of dermal and perichondral bone) but retained in the Osteichthyes and further duplicated to produce the SCPP family. The origin of endochondral bone may be associated with this osteichthyan-specific elaboration of the SCPP family, although this remains to be demonstrated. The zebrafish experiment by Venkatesh et al. effectively replicates the evolutionary loss of spp1 and ossification in chondrichthyans; it provides an elegant demonstration of the explanatory power of developmental, paleontological, and genomic data brought together in the analytical framework of phylogeny.
MATERIALS AND METHODS
Dermal bone samples of Compagopiscis (anterior ventrolateral plate), Polypterus (Cleithrum) and Rattus (Frontal) were imaged at beamline ID19, European Synchrotron Radiation Facility (ESRF), by propagation phase contrast synchrotron microtomography (PPC-SRµCT) (Tafforeau et al. 2006). Samples were scanned with different set-ups according to size and density, with voxel sizes of 0.678 µm (Rattus), 0.744 µm (Polypterus), and 5.05 µm (Compagopiscis). After ring-artefact correction, all data were converted from 32 to 8/16 bits for 3D processing. The scans were processed and rendered into three-dimensional virtual models using VGStudioMax 2.2 (Volume Graphics, Inc., Heidelberg, Germany). The data will be made available through the ESRF palaeontology database (http://paleo.esrf.eu).
Acknowledgments
We gratefully acknowledge the support of ERC Advanced Investigator Grant 233111 (P.E.A., S.S.), Swedish Research Council Grant 2012-4673 (T.H.) and ESRF proposals EC203 and EC519 (S.S., P.E.A.), as well as institutional support from Uppsala University and ESRF. Zerina Johanson (Natural History Museum, London) and Jan Ove Ebbestad (Museum of Evolution, Uppsala University) kindly lent us specimens in their care.
Author contribution
B.R. and P.E.A. wrote the text, with contributions from T.H., S.S., and P.T.; S.S., & P.T. conceived, designed, and performed the synchrotron experiments and reconstructed the raw scan data; S.S. & P.E.A. segmented, analyzed and interpreted the scan data; P.E.A. & S.S. composed the figure; all the authors provided a critical review of the draft and approved the final version.
REFERENCES
- Eames BF, Allen N, Young J, Kaplan A, Helms JA. Schneider RA. Skeletogenesis in the swell shark Cephaloscyllium ventriosum. J. Anat. 2007;210:542–554. doi: 10.1111/j.1469-7580.2007.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez S, Ahlberg PE, Trinajstic KM, Mirone A. Tafforeau P. Three dimensional synchrotron virtual paleohistology: a new insight into the world of fossil bone microstructures. Microsc. Microanal. 2012;18:1095–1105. doi: 10.1017/S1431927612001079. [DOI] [PubMed] [Google Scholar]
- Sanchez S, et al. 3D microstructural architecture of muscle attachments in extant and fossil vertebrates revealed by synchrotron microtomography. PLoS ONE. 2013;8(2):e56992. doi: 10.1371/journal.pone.0056992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafforeau P, et al. Applications of X-ray synchrotron microtomography for nondestructive 3D studies of paleontological specimens. Appl. Phys. A Mater. 2006;83:195–202. [Google Scholar]
- Venkatesh B, et al. Elephant shark genome provides unique insights into gnathostome evolution. Nature. 2014;505:174–179. doi: 10.1038/nature12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, et al. A Silurian placoderm with osteichthyan-like marginal jaw bones. Nature. 2013;502:188–193. doi: 10.1038/nature12617. [DOI] [PubMed] [Google Scholar]


