Abstract
Background
Hypophosphataemia after a hepatectomy suggests hepatic regeneration. It was hypothesized that the absence of hypophosphataemia is associated with post-operative hepatic insufficiency (PHI) and complications.
Methods
Patients who underwent a major hepatectomy from 2000–2012 at a single institution were identified. Post-operative serum phosphorus levels were assessed. Primary outcomes were PHI (peak bilirubin >7 mg/dl), major complications, and 30- and 90-day mortality.
Results
Seven hundred and nineteen out of 749 patients had post-operative phosphorus levels available. PHI and major complications occurred in 63 (8.8%) and 169 (23.5%) patients, respectively. Thirty- and 90-day mortality were 4.0% and 5.4%, respectively. The median phosphorus level on post-operative-day (POD) 2 was 2.2 mg/dl; 231 patients (32.1%) had phosphorus >2.4 on POD2.
Patients with POD2 phosphorus >2.4 had a significantly higher incidence of PHI, major complications and mortality. On multivariate analysis, POD2 phosphorus >2.4 remained a significant risk factor for PHI [(hazard ratio HR):1.78; 95% confidence interval (CI):1.02–3.17; P = 0.048], major complications (HR:1.57; 95%CI:1.02–2.47; P = 0.049), 30-day mortality (HR:2.70; 95%CI:1.08–6.76; P = 0.034) and 90-day mortality (HR:2.51; 95%CI:1.03–6.15; P = 0.044). Similarly, patients whose phosphorus level reached nadir after POD3 had higher PHI, major complications and mortality.
Conclusion
Elevated POD2 phosphorus levels >2.4 mg/dl and a delayed nadir in phosphorus beyond POD3 are associated with increased post-operative hepatic insufficiency, major complications and early mortality. Failure to develop hypophosphataemia within 72 h after a major hepatectomy may reflect insufficient liver remnant regeneration.
Introduction
Hypophosphataemia is a commonly observed phenomenon after a major hepatic resection. While small prior case series describing this occurrence have focused on the risk of complications associated with profound hypophosphataemia after a hepatectomy,1–4 a decrease in the circulating level of serum phosphorus is an expected and appropriate physiological sequela of a liver resection.5–7 Serum phosphorus levels after a major hepatic resection follow a characteristic trend, typically decreasing over the first 72 h to reach a nadir by postoperative day (POD) 2 or 3, before slowly increasing back to pre-operative levels by POD 5–7.1–3,5 While the regulatory mechanisms for this phenomenon are likely multi-factorial, hypophosphataemia after a hepatectomy is thought to partially reflect physiological regeneration of the remaining liver remnant.
The unique regenerative potential of hepatocytes and the compensatory capacity of the functional liver remnant post-hepatectomy allow for the resection of up to 75–80% of a non-diseased liver.8,9 Liver regeneration commences early and reaches its kinetic maximum over the first 72 h after a hepatectomy; the process is remarkably efficient, with functional compensation for the liver’s synthetic and enzymatic demands often complete by POD 5–7.10,11 The liver demonstrates significant early uptake of serum phosphorus, which peaks during the first few days post-hepatectomy, corresponding to the period of maximum liver regeneration and correlating with the decrease in free serum phosphorus levels commonly observed.12,13 The maximum metabolic demand on the regenerating liver typically occurs during the first 72 h, with the decrease in serum phosphorus levels post-hepatectomy mirroring this pattern.11,14–18
Patients who develop post-operative hepatic insufficiency (PHI) after a hepatectomy do not exhibit the same appropriate regenerative response, and may demonstrate insufficient functional compensation as early as POD 1 or 2 compared with those with appropriate liver remnant regeneration.10 As a result of inadequate or delayed hepatic regeneration, patients at risk for PHI may exhibit early derangements in normal metabolic responses, such as a failure to appropriately utilize phosphorus. Thus, early signs of inadequate liver regeneration may include failure to develop hypophosphataemia or a delayed decrease in serum phosphorus levels that normally occurs during the first 72 h.
It was hypothesized that after a major hepatectomy, absence of expected post-operative hypophosphataemia or delayed development of hypophosphataemia may be associated with poor liver remnant regeneration and an increased risk of PHI, complications and early mortality.
Patients and methods
This study protocol was conducted with the approval of the Institution Review Board and in accordance with the Health Insurance Portability and Accountability Act of 1996. From a prospectively maintained institutional database, all patients who underwent a major hepatectomy, defined as resection of three or more hepatic segments, between January 2000 and July 2012 were identified.
From a thorough retrospective chart review of all patients’ medical records, pre-operative demographics and peri-operative clinicopathological variables were collected. All available post-operative serum phosphorus levels were collected from the first seven post-operative days; the day on which each patient’s postoperative phosphorus nadir (i.e. the lowest absolute level recorded) was reached during these first 7 days was also determined. The institutional laboratory reference range for normal serum phosphorus levels is 2.4–4.7 mg/dl. Failure to develop or delayed development of post-hepatectomy hypophosphataemia was evaluated on the basis of two distinct variables: the absolute serum phosphorus level, as well as the post-operative day on which patients reached their nadir phosphorus value. As most patients with appropriate liver regeneration were predicted to develop some degree of hypophosphataemia by POD2, POD2 phosphorus level was analysed as a dichotomous variable, with 2.4 mg/dl, the lower limit of normal reference range, selected as the cutoff. Similarly, patients with appropriate liver regeneration were predicted to reach their nadir phosphorus level within the first 72 h; thus patients were dichotomized into those who reached their phosphorus nadir during POD1 through POD3 compared with those who demonstrated a delayed phosphorus nadir after POD3.
Serum phosphorus levels on POD2 were also analysed according to the definitions proposed by George and Shiu:2 normal phosphorus (>2.5 mg/dl), moderate hypophosphataemia (1.6–2.5 mg/dl), severe hypophosphataemia (1.1–1.5 mg/dl), or profound hypophosphataemia (<1.1 mg/dl).
The total amount of post-operative phosphorus repletion over the first 72 h post-hepatectomy, accounting for both maintenance intravenous (i.v.) fluids as well as any bolus i.v. or oral repletion doses, was quantified (in millimoles) based on a thorough review of the medical administration record. Phosphorus repletion was at the discretion of the attending surgeon and did not follow a standardized protocol or regimen.
Primary outcomes assessed were post-operative hepatic insufficiency, defined as a peak post-operative serum bilirubin >7.0 mg/dl as first described by Mullen et al.19 major complications, defined as Grade III-V complications according to the Clavien–Dindo classification system,20 and 30-day and 90-day mortality.
Univariate and multivariate (MV) logistic regression analysis were used to evaluate the association of all pertinent pre- and peri-operative variables with each endpoint. All variables with a P-value ≤0.05 on univariate analysis were included into the multivariate model. Separate distinct MV models were developed to independently assess the association of each endpoint with POD2 phosphorus >2.4 mg/dl, as well as a delayed post-operative phosphorus nadir day beyond POD3. Statistical significance was defined as a P-value <0.05. All statistical analyses were conducted with SPSS 21.0 software (IBM, Inc., Armonk, NY, USA).
Results
Of the 749 patients who underwent a major hepatectomy from 2000–2012, 719 (96%) had post-operative serum phosphorus levels available for analysis. Clinicopathological characteristics of this cohort are summarized in Table 1.
Table 1.
Demographic and clinicopathological characteristics of patients undergoing a major hepatectomy from 2000–2012 (n = 719)
| Variable | N (%) or median [range] |
|---|---|
| Gender, male | 298 (41%) |
| Race | |
| White | 467 (65%) |
| Black | 164 (23%) |
| Other | 88 (12%) |
| Age, median [range], years | 57 [18–88] |
| BMI, median [range], kg/m2 | 26.4 [16.6–57.8] |
| ASA Class | |
| 2 | 229 (32%) |
| 3 | 463 (64%) |
| 4 | 20 (3%) |
| Hepatitis B | 18 (3%) |
| Hepatitis C | 20 (3%) |
| Smoking history, >10 pack-years | 217 (30%) |
| Alcohol abuse | 44 (6%) |
| Cancer diagnosis (indication for hepatectomy) | 519 (72%) |
| Platelet count, median [range], × 103/ml | 241 [69–900] |
| Albumin, median [range], gm/dl | 3.6 [1.3–4.7] |
| Total bilirubin, median [range], mg/dl | 0.6 [0.1–24.6] |
| INR, median [range] | 1.00 [0.81–2.61] |
| Portal vein embolization | 30 (4%) |
| Resection type | |
| Extended left | 41 (6%) |
| Extended right | 143 (20%) |
| Left hemihepatectomy | 163 (23%) |
| Right hemihepatectomy | 279 (39%) |
| Central hepatectomy | 17 (2%) |
| Non-anatomic | 76 (10%) |
| Bile duct resection | 143 (20%) |
| EBL, median [range], ml | 400 [50–5900] |
| Intra-operative transfusion | 184 (26%) |
| Pathology | |
| Metastatic colorectal cancer | 229 (32%) |
| Hepatocellular carcinoma | 69 (9%) |
| Cholangiocarcinoma | 88 (12%) |
| Metastatic NET | 34 (5%) |
| Other | 299 (42%) |
| Cirrhosis | 26 (4%) |
| Steatosis | 207 (29%) |
| Any complication | 443 (61.6%) |
| Major complications (Grade III–V) | 169 (23.5%) |
| Post-operative hepatic insufficiency (PHI) | 63 (8.8%) |
| 30-day mortality | 29 (4.0%) |
| 90-day mortality | 39 (5.4%) |
ASA, American Society of Anesthesiology; BMI, body mass index; EBL, estimated blood loss; INR, international normalized ratio; NET, neuroendocrine tumour.
The median serum phosphorus levels for the cohort are depicted in Fig. 1a. The overall trajectory of a characteristic initial steep decline and subsequent slow, incremental recovery was similar for all patients undergoing a major hepatectomy, regardless of the extent of resection (Fig. 1b). The median phosphorus level on POD2 was 2.2 mg/dl (interquartile range, 1.7–2.8) and 231 patients (32.1%) had a POD2 phosphorus level that remained >2.4 mg/dl, the lower limit of the normal reference range. In 589 (81.9%) patients, the phosphorus nadir occurred within the first 3 days post-hepatectomy; thus 130 patients (18.1%) had a delayed post-operative phosphorus nadir after POD3.
Figure 1.
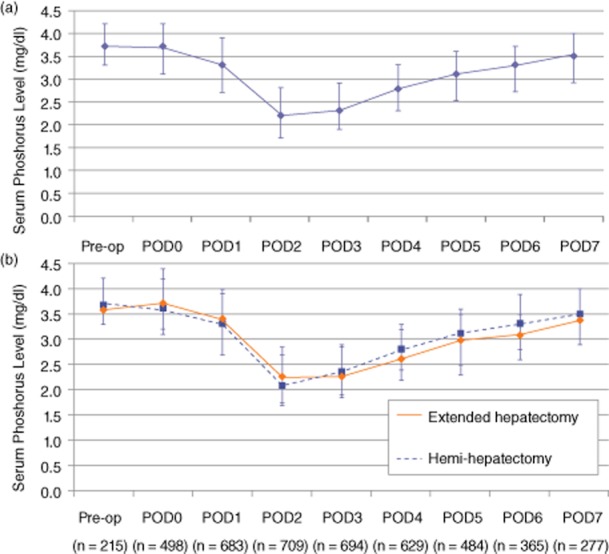
(a) Median serum phosphorus levels [with interquartile (25–75%) range] after a major hepatectomy. (b) Median serum phosphorus levels [with interquartile (25–75%) range] stratified by the extent of major hepatectomy
Morbidity and mortality rates by degree of hypophosphataemia, using the definitions proposed by George and Shiu,2 are shown in Table 2; patients with ‘normal’ phosphorus levels (>2.5 mg/dl), that is, those who failed to develop hypophosphataemia, had the highest rates of PHI (12.3%), major complications (30.3%), 30-day mortality (6.5%) and 90-day mortality (7.1%) among the four subgroups.
Table 2.
Relative frequency of post-operative hepatic insufficiency (PHI), major complications, 30- and 90-day mortality, stratified by post-operative day 2 (POD2) phosphorus levels (as defined by George and Shiu2)
| PHI | Major complications | 30-day mortality | 90-day mortality | |
|---|---|---|---|---|
| ‘Normal’ phosphorus level (>2.5 mg/dl) | 12.3% | 30.3% | 6.5% | 7.1% |
| ‘Moderate’ hypophosphataemia (1.6–2.5 mg/dl) | 8.0% | 20.1% | 3.8% | 5.3% |
| ‘Severe’ hypophosphataemia (1.1–1.5 mg/dl) | 8.5% | 19.5% | 2.8% | 3.8% |
| ‘Profound’ Hypophosphataemia (<1.1 mg/dl) | 3.4% | 16.7% | 0 | 3.4% |
| P = 0.008 | P = 0.037 | P = 0.010 | P = 0.166 |
Using the institutional cutoff for hypophosphataemia of 2.4 mg/dl, on univariate analysis, patients with a POD2 phosphorus level >2.4 mg/dl demonstrated a significantly increased risk of PHI [12.6% (29/231) vs. 6.9% (34/488); P = 0.020], major complications [28.1% (65/231) vs. 21.3% (104/488); P = 0.031], 30-day mortality [7.8% (18/231) vs. 2.3% (11/488); P = 0.001] and 90-day mortality [8.7% (20/231) vs. 3.9% (19/488); P = 0.018].
Multivariate regression analyses of risk factors for PHI and major complications are presented in Table 3a and for 30-day and 90-day mortality in Table 3b. On MV analysis, POD2 phosphorus >2.4 mg/dl remained independently associated with a significantly increased risk of PHI [hazard ratio (HR) 1.78; 95% confidence interval (CI): 1.02–3.17; P = 0.048; Table 3a], major complications (HR 1.57; 95% CI: 1.02–2.47; P = 0.049; Table 3a), 30-day mortality (HR 2.70; 95% CI: 1.08–6.76; P = 0.031; Table 3b) and 90-day mortality (HR 2.51; 95% CI: 1.03–6.15; P = 0.044; Table 3b).
Table 3a.
Multivariate analysis of risk factors for post-operative hepatic insufficiency (PHI) and major complications after major hepatectomy
| Variable | PHI | Major complications | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Male gender | 1.15 | 0.63–2.11 | 0.637 | 1.21 | 0.79–1.86 | 0.366 |
| ASA class | 1.93 | 1.05–3.54 | 0.040 | 1.66 | 1.07–2.55 | 0.024 |
| Pre-op albumin | 0.65 | 0.38–1.08 | 0.103 | 0.61 | 0.40–0.93 | 0.019 |
| Pre-op INR | 3.88 | 0.90–16.68 | 0.068 | 2.50 | 0.47–12.50 | 0.290 |
| Pre-op total bilirubin | 1.24 | 1.06–1.45 | 0.006 | 1.02 | 0.87–1.11 | 0.755 |
| Portal vein embolization | 2.12 | 0.75–5.93 | 0.159 | 1.19 | 0.49–2.87 | 0.712 |
| Bile duct resection | 1.26 | 0.63–2.54 | 0.524 | 1.22 | 0.73–2.03 | 0.461 |
| EBL | 1.00 | 1.00–1.001 | 0.141 | 1.01 | 1.00–1.02 | 0.198 |
| Intra-op transfusion | 1.09 | 0.52–2.31 | 0.818 | 1.20 | 0.68–2.13 | 0.533 |
| Cancer diagnosis | 1.43 | 0.67–3.07 | 0.356 | 1.96 | 1.13–3.39 | 0.022 |
| Cirrhosis | 1.76 | 0.56–5.55 | 0.363 | 2.63 | 1.01–6.82 | 0.047 |
| POD2 phosphorus > 2.4 | 1.78 | 1.02–3.17 | 0.048 | 1.57 | 1.02–2.47 | 0.049 |
Statistically significant variables (P < 0.05) indicated in bold. HR, hazard ratio; CI, confidence interval; ASA, American Society of Anesthesiology; EBL, estimated blood loss; POD, post-operative-day 2.
Table 3b.
Multivariate analysis of risk factors for 30- and 90-day mortality after a major hepatectomy
| Variable | 30-day mortality | 90-day mortality | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Male gender | 2.05 | 0.87–4.81 | 0.097 | 1.21 | 0.59–2.49 | 0.596 |
| ASA class | 4.25 | 1.61–11.24 | 0.014 | 2.60 | 1.09–6.24 | 0.031 |
| Pre-op albumin | 0.68 | 0.31–1.50 | 0.330 | 0.53 | 0.27–1.04 | 0.069 |
| Pre-op INR | 1.75 | 0.03–10.61 | 0.712 | 1.02 | 0.11–9.13 | 0.983 |
| Pre-op total bilirubin | 1.02 | 0.83–1.22 | 0.896 | 1.03 | 0.80–1.19 | 0.778 |
| Portal vein embolization | 1.04 | 0.15–3.94 | 0.873 | 1.07 | 0.27–4.24 | 0.920 |
| Bile duct resection | 1.42 | 0.52–3.86 | 0.394 | 1.62 | 0.71–3.70 | 0.245 |
| EBL | 1.00 | 1.00–1.001 | 0.302 | 1.00 | 1.00–1.001 | 0.182 |
| Intra-op transfusion | 2.14 | 0.76–6.07 | 0.145 | 1.95 | 0.81–4.72 | 0.144 |
| Cancer diagnosis | 9.16 | 1.50–56.01 | 0.018 | 9.47 | 2.03–44.86 | 0.004 |
| Cirrhosis | 10.46 | 2.88–37.99 | <0.001 | 8.68 | 2.71–27.79 | <0.001 |
| POD2 phosphorus > 2.4 | 2.70 | 1.08–6.76 | 0.031 | 2.51 | 1.03–6.15 | 0.044 |
Statistically significant variables (P < 0.05) indicated in bold. ASA, American Society of Anesthesiology; CI, confidence interval; EBL, estimated blood loss; HR, hazard ratio; INR, international normalized ratio; PHI, post-operative hepatic insufficiency; POD2, post-operative day 2.
Patients who experienced a delayed post-operative phosphorus nadir beyond POD3 demonstrated a significantly increased risk of PHI [13.1% (17/130) vs. 7.8% (46/589); P = 0.037], major complications [37.6% (49/130) vs. 20.4% (120/589); P < 0.001], 30-day mortality [8.5% (11/130) vs. 3.1% (18/589); P = 0.009] and 90-day mortality [11.5% (15/130) vs. 4.1% (24/589); P = 0.004] on univariate analysis.
On MV analysis assessing delayed phosphorus nadir as a marker of inadequate liver regeneration, patients reaching their phosphorus nadir after POD3 remained at a significantly increased risk of PHI (HR 1.86; 95% CI: 1.02–3.39; P = 0.044; Table 3c). Major complications (HR 1.90; 95% CI: 1.09–3.31; P = 0.022; Table 3c) and 30-day mortality (HR 2.39; 95% CI: 1.09–5.72; P = 0.045; Table 3d) demonstrated a trend towards an increased risk of 90-day mortality (HR 2.11; 95% CI: 0.99–4.49; P = 0.054; Table 3d).
Table 3c.
Multivariate analysis of risk factors for post-operative hepatic insufficiency (PHI) and major complications after a major hepatectomy
| Variable | PHI | Major complications | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Gender | 1.22 | 0.67–2.21 | 0.515 | 1.13 | 0.69–1.85 | 0.174 |
| ASA class | 1.92 | 1.05–3.52 | 0.039 | 1.77 | 1.04–3.02 | 0.037 |
| Pre-op albumin | 0.92 | 0.53–1.61 | 0.784 | 0.54 | 0.33–0.88 | 0.014 |
| Pre-op INR | 2.39 | 0.52–10.89 | 0.260 | 1.04 | 0.18–5.88 | 0.955 |
| Pre-op total bilirubin | 1.22 | 1.06–1.40 | 0.008 | 1.01 | 0.89–1.14 | 0.890 |
| Portal vein embolization | 2.47 | 0.91–6.73 | 0.083 | 1.73 | 0.65–4.62 | 0.266 |
| Bile duct resection | 1.36 | 0.69–2.67 | 0.377 | 1.20 | 0.64–2.25 | 0.581 |
| EBL | 1.00 | 1.00–1.001 | 0.142 | 1.00 | 1.00–1.001 | 0.479 |
| Intra-op transfusion | 1.23 | 0.59–2.60 | 0.578 | 1.23 | 0.66–2.31 | 0.506 |
| Cancer diagnosis | 1.36 | 0.64–2.89 | 0.421 | 1.23 | 0.67–2.27 | 0.514 |
| Cirrhosis | 1.67 | 0.56–5.03 | 0.362 | 1.94 | 0.80–4.72 | 0.148 |
| Phos nadir after POD3 | 1.86 | 1.02–3.39 | 0.044 | 1.90 | 1.09–3.31 | 0.022 |
Statistically significant variables (P < 0.05) indicated in bold. HR, hazard ratio; CI, confidence interval; ASA, American Society of Anesthesiology; INR, international normalized ratio; EBL, estimated blood loss; POD3, post-operative day 3.
Table 3d.
Multivariate analysis of risk factors for 30- and 90-day mortality after a major hepatectomy
| Variable | 30-day mortality | 90-day mortality | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Gender | 1.88 | 0.78–4.52 | 0.158 | 1.08 | 0.52–2.24 | 0.849 |
| ASA class | 3.60 | 1.45–8.96 | 0.014 | 2.13 | 0.94–4.81 | 0.072 |
| Pre-op albumin | 0.94 | 0.45–1.95 | 0.861 | 0.66 | 0.33–1.30 | 0.233 |
| Pre-op INR | 1.21 | 0.11–6.64 | 0.379 | 1.56 | 0.06–7.28 | 0.720 |
| Pre-op total bilirubin | 1.05 | 0.89–1.23 | 0.590 | 1.05 | 0.75–1.19 | 0.628 |
| Portal vein embolization | 1.06 | 0.21–5.38 | 0.936 | 1.30 | 0.34–4.93 | 0.704 |
| Bile duct resection | 1.90 | 0.77–4.69 | 0.163 | 1.56 | 0.69–3.57 | 0.285 |
| EBL | 1.00 | 1.00–1.01 | 0.131 | 1.00 | 1.00–1.01 | 0.421 |
| Intra-op transfusion | 2.79 | 1.01–7.70 | 0.047 | 3.32 | 1.40–7.86 | 0.012 |
| Cancer diagnosis | 6.43 | 1.14–36.34 | 0.049 | 5.36 | 1.43–20.12 | 0.008 |
| Cirrhosis | 7.56 | 2.34–24.45 | 0.001 | 6.84 | 2.42–19.35 | <0.001 |
| Phos nadir after POD3 | 2.39 | 1.09–5.72 | 0.045 | 2.11 | 0.99–4.49 | 0.054 |
Statistically significant variables (P < 0.05) indicated in bold. ASA, American Society of Anesthesiology; CI, confidence interval; EBL, estimated blood loss; HR, hazard ratio; INR, international normalized ratio; POD3, postoperative day 3.
Four hundred ninety-six patients (69%) received phosphorus repletion during the first 72 h post-operatively; the median amount of repletion was 55 mmol (range, 10–170 mmol). Phosphorus repletion during the first 72 h post-hepatectomy was not associated with a risk of PHI [8.5% (42/496) vs. 9.4% (21/223); P = 0.802], major complications [22.4% (111/496) vs. 26.0% (58/223); P = 0.544], or early mortality [3.6% (18/496) vs. 4.9% (11/223); P = 0.408]. Neither the use of the Pringle manoeuver nor the duration of vascular inflow occlusion when used was significantly associated with the risk of PHI (P = 0.104), major complications (P = 0.986), 30- (P = 0.945) or 90-day mortality (P = 0.763). The use or duration of the Pringle manoeuver also did not correlate with post-operative serum phosphorus, the degree of hypophosphataemia, or the timing to reach phosphorus nadir (data not shown).
Discussion
In this large series of patients who underwent a major hepatectomy, failure to develop expected hypophosphataemia, whether defined as a POD2 serum phosphorus level >2.4 mg/dl or as a delayed nadir in serum phosphorus beyond POD3, was independently associated with a significantly increased risk of PHI, major complications and early mortality.
Previous series have been limited by small cohort sizes and the inclusion of both minor and major hepatic resections. Several of these small patient series over the past few decades examining hypophosphataemia after a hepatectomy or live donor hepatectomy have reported increased rates of post-operative complications.2,3,5,21 Other studies have failed to demonstrate an association between hypophosphataemia and the risk of complications.1,6,7 Yet few of these studies have adequately acknowledged that the development of some degree of postoperative hypophosphataemia, even extreme hypophosphataemia, is a normal, transient physiological sequela of hepatectomy; this appropriate response is observed to some extent in nearly all patients and corresponds to the increased metabolic demands of the regenerating liver remnant. In fact, failure to develop hypophosphataemia after a major hepatic resection may reflect a lack of or inadequate liver regeneration, indicating patients at a greater risk of PHI and complications. Based on the observation of hypophosphataemia as a common and appropriate post-operative consequence of a liver resection, it was hypothesized that absence of or delayed development of hypophosphataemia after a major resection may be associated with an increased risk of PHI, major morbidity and mortality. Failure to develop hypophosphataemia was defined as POD2 serum phosphorus >2.4 mg/dl, as this represented the lower limit of the normal institutional reference range. The timing of hypophosphataemia after a hepatectomy was also examined. Delayed development of appropriate hypophosphataemia was defined and independently analysed as a serum phosphorus nadir occurring after POD3, as previous studies have suggested that serum phosphorus decreases dramatically over the first 72 h post-hepatectomy before slowly returning to normal, preoperative levels. Indeed, >80% of patients in the current cohort reached their serum phosphorus nadir during POD 1–3 as expected.
Liver regeneration begins nearly immediately after a hepatectomy, and DNA synthesis and hepatocyte proliferation within the liver remnant are largely complete within 72 h.15,22,23 The increased rates of compensatory DNA replication and protein synthesis within the liver remnant after a hepatectomy are accompanied by dramatic increases in energy requirements, as evidenced by enhanced adenosine triphosphate (ATP) consumption and increased incorporation of radiolabelled phosphate by the liver.12,13,17 Changes in hepatocyte energy consumption and phosphorus metabolism are observed in other acute stress phases such as non-hepatic surgical intervention, but the magnitude of these changes is most profound after a hepatectomy.17 The liver remnant must support one or more rounds of hepatocyte mitosis while maintaining hepatic synthetic and excretory functions with a fraction of the original liver volume.14,15,17 This leads to dramatically increased ATP consumption over the first 72 h, followed by gradual restoration of the liver’s energy balance over the next days to weeks.13,16–18
Several groups have suggested that increased hepatic uptake of phosphorus by the regenerating liver remnant post-hepatectomy cannot fully explain the magnitude of hypophosphataemia typically observed, and have demonstrated that increased urinary excretion of phosphorus plays a significant role in this post-operative phenomenon.24–28 This transient hyperphosphaturia may be mediated by acute phase reactants or circulatory factors released by the injured, regenerating liver or by disruption of normal hepatorenal feedback mechanisms; these mechanisms are still under investigation.25,27,28 The present study did not focus on the molecular mechanisms underlying this phenomenon, but on the implications of failure to develop hypophosphataemia. The regulation of serum phosphorus after a hepatectomy is probably complex and multifactorial; the hypothesis regarding the absence of physiological hypophosphataemia and the implications of persistently elevated serum phosphorus as a marker of inadequate liver regeneration is independent of the exact mechanisms governing serum phosphorus levels. Regardless of renal involvement in the regulatory process, failure to develop hypophosphataemia after a major hepatectomy appears to suggest inadequate liver regeneration.
Contrary to several previous studies, a direct correlation between severe hypophosphataemia and an increased risk of post-operative complications was not observed.2,3,5 In fact, using the categories of serum phosphorus levels as defined by George and Shiu,2 patients with ‘normal’ phosphorus levels (>2.5 mg/dl) on POD2 demonstrated the greatest risk of PHI, major complications and early mortality, whereas those patients with ‘profound’ hypophosphataemia (<1.1 mg/dl) had the lowest risk of each of these respective endpoints. Rather than a harbinger of post-operative complications, profound hypophosphataemia may simply represent a more extensive hepatic resection and a greater synthetic and metabolic burden on the regenerating liver remnant. These findings support the hypothesis that hypophosphataemia after a major hepatectomy reflects appropriate liver regeneration and that failure to develop hypophosphataemia or delayed development of hypophosphataemia can identify patients at increased risk of PHI, major complications and early mortality.
Given the retrospective nature of this study, the relationships of increased risk of PHI, complications and early mortality with absent or delayed hypophosphataemia demonstrated in these analyses are only associative in nature; no definitive conclusions regarding causality can be drawn. An additional limitation of this study was the lack of standardization of phosphorus repletion. Previous retrospective studies have also struggled with this limitation. While 69% of patients received some degree of phosphorus repletion over the first 72 h post-operatively, the range of repletion quantities was large and did not follow a standard protocol. Repletion was typically reactionary, in response to hypophosphataemia, rather than empiric. Given the heterogeneity of repletion regimens, the role of phosphorus repletion was analysed qualitatively, not quantitatively; phosphorus repletion during the first 72 h was not associated with the risk of PHI, major complications, or early mortality. Iatrogenic repletion of patients with exogenous phosphorus after a hepatectomy may slightly dampen the initial decline in serum levels and hasten the return to normal preoperative levels, but neither the overall propensity or timeline for developing post-operative hypophosphataemia and the risk of associated complications appear affected by repletion. Further investigation of hypophosphataemia after a major hepatectomy in which phosphorus repletion is standardized post-operatively may help confirm these findings.
Serum phosphorus levels persistently >2.4 mg/dl on POD2 and a delayed nadir in phosphorus beyond POD3 are independently associated with a significantly increased risk of post-operative hepatic insufficiency, major complications and early mortality. Failure to develop hypophosphataemia within 72 h after a major hepatectomy may reflect insufficient liver remnant regeneration. Early detection of these patients at risk for PHI, secondary complications and even post-operative mortality after a hepatectomy may allow for earlier clinical intervention and potentially improved outcomes. Serum phosphorus levels, specifically the absence of or delayed development of physiologic hypophosphataemia after major hepatic resection, may provide early prognostic data suggesting inadequate liver regeneration and an increased risk of post-operative hepatic insufficiency and accompanying morbidity and mortality.
References
- Keushkerian S, Wade T. Hypophosphatemia after major hepatic resection. Curr Surg. 1984;41:12–14. [PubMed] [Google Scholar]
- George R, Shiu MH. Hypophosphatemia after major hepatic resection. Surgery. 1992;111:281–286. [PubMed] [Google Scholar]
- Smyrniotis V, Kostopanagiotou G, Katsarelias D, Theodoraki K, Hondros K, Kouskouni E. Changes of serum phosphorus levels in hepatic resections and implications on patients’ outcomes. Int Surg. 2003;88:100–104. [PubMed] [Google Scholar]
- Buell JF, Berger AC, Plotkin JS, Kuo PC, Johnson LB. The clinical implications of hypophosphatemia following major hepatic resection or cryosurgery. Arch Surg. 1998;133:757–761. doi: 10.1001/archsurg.133.7.757. [DOI] [PubMed] [Google Scholar]
- Giovannini I, Chiarla C, Nuzzo G. Pathophysiologic and clinical correlates of hypophosphatemia and the relationship with sepsis and outcome in postoperative patients after hepatectomy. Shock. 2002;18:111–115. doi: 10.1097/00024382-200208000-00003. [DOI] [PubMed] [Google Scholar]
- Lee HW, Suh KS, Kim J, Shin WY, Cho EH, Yi NJ, et al. Hypophosphatemia after live donor right hepatectomy. Surgery. 2008;144:448–453. doi: 10.1016/j.surg.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Tan HP, Madeb R, Kovach SJ, Orloff M, Mieles L, Johnson LA, et al. Hypophosphatemia after 95 right-lobe living-donor hepatectomies for liver transplantation is not a significant source of morbidity. Transplantation. 2003;76:1085–1088. doi: 10.1097/01.TP.0000085652.47821.8A. [DOI] [PubMed] [Google Scholar]
- Lee SG, Hwang S. How I do it: assessment of hepatic functional reserve for indication of hepatic resection. J Hepatobiliary Pancreat Surg. 2005;12:38–43. doi: 10.1007/s00534-004-0949-9. [DOI] [PubMed] [Google Scholar]
- Schneider PD. Preoperative assessment of liver function. Surg Clin North Am. 2004;84:355–373. doi: 10.1016/S0039-6109(03)00224-X. [DOI] [PubMed] [Google Scholar]
- Lock JF, Malinowski M, Seehofer D, Hoppe S, Rohl RI, Niehues SM, et al. Function and volume recovery after partial hepatectomy: influence of preoperative liver function, residual liver volume, and obesity. Langenbecks Arch Surg. 2012;397:1297–1304. doi: 10.1007/s00423-012-0972-2. [DOI] [PubMed] [Google Scholar]
- Tralhao JG, Abrantes AM, Hoti E, Oliveiros B, Cardoso D, Faitot F, et al. Hepatectomy and liver regeneration: from experimental research to clinical application. ANZ J Surg. 2013 doi: 10.1111/ans.12201. doi: 10.1111/ans.12201. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Fisher B, Szuch P, Fisher ER. Evaluation of a humoral factor in liver regeneration utilizing liver transplants. Cancer Res. 1971;31:322–331. [PubMed] [Google Scholar]
- Ove P, Takai SI, Umeda T, Lieberman I. Adenosine triphosphate in liver after partial hepatectomy and acute stress. J Biol Chem. 1967;242:4963–4971. [PubMed] [Google Scholar]
- Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- Kooby DA, Zakian KL, Challa SN, Matei C, Petrowsky H, Yoo HH, et al. Use of phosphorous-31 nuclear magnetic resonance spectroscopy to determine safe timing of chemotherapy after hepatic resection. Cancer Res. 2000;60:3800–3806. [PubMed] [Google Scholar]
- Mann DV, Lam WW, Hjelm NM, So NM, Yeung DK, Metreweli C, et al. Metabolic control patterns in acute phase and regenerating human liver determined in vivo by 31-phosphorus magnetic resonance spectroscopy. Ann Surg. 2002;235:408–416. doi: 10.1097/00000658-200203000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakian KL, Koutcher JA, Malhotra S, Thaler H, Jarnagin W, Schwartz L, et al. Liver regeneration in humans is characterized by significant changes in cellular phosphorus metabolism: assessment using proton-decoupled 31P-magnetic resonance spectroscopic imaging. Magn Reson Med. 2005;54:264–271. doi: 10.1002/mrm.20560. [DOI] [PubMed] [Google Scholar]
- Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. discussion 62-4. [DOI] [PubMed] [Google Scholar]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomposelli JJ, Pomfret EA, Burns DL, Lally A, Sorcini A, Gordon FD, et al. Life-threatening hypophosphatemia after right hepatic lobectomy for live donor adult liver transplantation. Liver Transpl. 2001;7:637–642. doi: 10.1053/jlts.2001.26287. [DOI] [PubMed] [Google Scholar]
- Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini I, Chiarla C, Giuliante F, Ardito F, Vellone M, Nuzzo G. Hepatic resection-related hypophosphatemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg. 2006;243:429. doi: 10.1097/01.sla.0000202002.17260.c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafidi O, Lapointe RW, Lepage R, Kumar R, D’Amour P. Mechanisms of renal phosphate loss in liver resection-associated hypophosphatemia. Ann Surg. 2009;249:824–827. doi: 10.1097/SLA.0b013e3181a3e562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafidi O, Lepage R, Lapointe RW, D’Amour P. Hepatic resection-related hypophosphatemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg. 2007;245:1000–1002. doi: 10.1097/SLA.0b013e31805d0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem RR, Tray K. Hepatic resection-related hypophosphatemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg. 2005;241:343–348. doi: 10.1097/01.sla.0000152093.43468.c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta HK, Malik M, Neely RD. Hepatic surgery-related hypophosphatemia. Clin Chim Acta. 2007;380:13–23. doi: 10.1016/j.cca.2007.01.027. [DOI] [PubMed] [Google Scholar]


