Abstract
Lipopolysaccharide (LPS) produced by Gram-negative bacteria induces tolerance and suppresses inflammatory responses in vivo; however, the mechanisms are poorly understood. In this study we show that LPS induces apoptosis of bone marrow-derived dendritic cells (DCs) and modulates phenotypes of DCs. LPS treatment up-regulates expression of tolerance-associated molecules such as CD205 and galectin-1, but down-regulates expression of Gr-1 and B220 on CD11c+ DCs. Moreover, LPS treatment regulates the numbers of CD11c+CD8+, CD11c+CD11blow and CD11c+CD11bhi DCs, which perform different immune functions in vivo. Our data also demonstrated that intravenous transfer of LPS-treated DCs blocks experimental autoimmune encephalomyelitis (EAE) development and down-regulates expression of retinoic acid-related orphan receptor gamma t (ROR-γt), interleukin (IL)-17A, IL-17F, IL-21, IL-22 and interferon (IFN)-γ in myelin oligodendrocyte glycoprotein (MOG)-primed CD4+ T cells in the peripheral environment. These results suggest that LPS-induced apoptotic DCs may lead to generation of tolerogenic DCs and suppress the activity of MOG-stimulated effector CD4+ T cells, thus inhibiting the development of EAE in vivo. Our results imply a potential mechanism of LPS-induced tolerance mediated by DCs and the possible use of LPS-induced apoptotic DCs to treat autoimmune diseases such as multiple sclerosis.
Keywords: CD4+ T cell, dendritic cell, EAE, immune tolerance, immunotherapy
Introduction
Lipopolysaccharide (LPS), an endotoxin produced by Gram-negative bacteria, not only induces inflammatory responses, but also plays an important role in tolerance. This implies complex mechanisms of LPS the in regulation of autoimmunity and tolerance. For example, Zheng et al. reported that LPS-treated plasmacytoid dendritic cells (DCs) inhibit experimental chronic kidney disease. LPS-treated DCs facilitate the development of regulatory T cells (Tregs) and suppress the production of inflammatory cytokines 1.
In addition, LPS facilitates inflammatory responses. For example, LPS binds to Toll-like receptor 4 (TLR-4) and activates nuclear factor-kappa light chain enhancer of activated B cell (NF-кB)-mediated signal transduction 2. LPS stimulation increases the production of inflammatory cytokines such as interleukin (IL)-6 and IL-12 by DCs and macrophages 3,4. This may elicit the development of effector T cells and T cell-mediated immune responses.
Apoptosis of DCs plays an important role in the induction of tolerance in vivo 5. LPS treatment leads not only to DC maturation 6, but also causes cellular apoptosis 7. DCs engulf apoptotic cells via the CD205-mediated pathway and present self-antigen on the surface of DCs 8. Uptake of apoptotic cells leads to generation of tolerogenic DCs via unknown mechanisms, and these tolerogenic DCs can facilitate the development of regulatory T cells in vivo 9. However, it is not known whether or not apoptotic cell-induced tolerogenic DCs can affect the activity of effector CD4+ T cells such as T helper 17 (Th17) cells, which are necessary for the induction of autoimmune diseases.
DCs regulate immune responses in autoimmunity and immune tolerance 10,11. DC-based immunotherapy has been used to treat human diseases such as cancer and autoimmune diseases. However, the regulatory mechanisms of DC-mediated immune responses are still obscure. Determining these regulatory mechanisms might lead to the development of immunotherapy to treat cancer and autoimmune diseases such as multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE).
An indirect effect of LPS on DC-mediated development and differentiation of CD4+ T cells, which play an important role in induction of autoimmune diseases, was observed in this project. In addition, recent research has shown that LPS-treated DCs can secrete IL-10, an anti-inflammatory cytokine, to suppress immune responses and lead to tolerance 12,13.
EAE is an autoimmune disease in animal models of MS 14. Th17 cells are pathogenic T cells for EAE/MS development 15–17; however, it is unclear whether or not LPS-treated DCs can affect Th17 activity and block EAE/MS development. We hypothesized that LPS might modulate the phenotype of DCs and generate tolerogenic DCs to block CD4+ T cell activity that is necessary for induction of autoimmune diseases such as EAE/MS. To test this hypothesis, bone marrow-derived DCs were treated with LPS and transferred into EAE mice. Our results show that LPS treatment modulates the phenotype of DCs and that intravenous (i.v.) injection of LPS-treated DCs inhibits EAE development. Moreover, LPS-treated DCs can suppress CD4+ T cell activity in vitro and in vivo, suggesting that this mechanism underlies the effect of LPS-induced tolerant DCs on blocking EAE development.
Materials and methods
Mice
Wild-type C57 BL/6J female mice (8–12 weeks) were provided by the Jackson Laboratory (Bar Harbor, ME, USA). All mice were bred in Thomas Jefferson Animal Care facilities and all experimental procedures were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University.
Immunogen and peptide
Mouse MOG35–55 peptide (MEVGWYRSPFSRVVHLYRNGK), a part of myelin oligodendrocyte glycoprotein (MOG), was obtained from Invitrogen (Carlsbad, CA, USA).
Isolation of bone marrow
As described previously 18,19, femurs and tibiae were isolated from muscle tissue of mice. The intact bones were then sterilized with 70% ethanol for 5 min and washed with phosphate-buffered saline (PBS). Bone ends were cut and the bone marrow was flushed with PBS. Cellular clusters within the bone marrow suspension were disintegrated and washed with PBS.
Bone marrow-derived DC culture
As described previously 18,19, leucocytes from bone marrow were fed in bacteriological 100-mm Petri dishes (Falcon, Becton Dickinson, Heidelberg, Germany) at 2 × 106 cells per dish. Cells were cultured in RPMI-1640 complete medium (Gibco-BRL, Eggenstein, Germany), including penicillin (100 U/ml; Sigma, St Louis, MO, USA), streptomycin (100 U/ml; Sigma), L-glutamine (2 mM; Sigma), 2-mercaptoethanol (2-ME, 50 μM; Sigma), 10% heat-inactivated and filtered (0·22 μm; Millipore, Inc., Bedford, MA, USA) fetal calf serum (FCS; Sigma) and granulocyte–macrophage colony-stimulating factor (GM-CSF; PeproTech, Rocky Hill, NJ, USA) at 20 ng/ml at day 0 (10 ml medium per dish).
At day 3, 10 ml fresh medium with GM-CSF (20 ng/ml) was added to each dish and at day 6, half the medium (about 10 ml supernatant) was collected and centrifuged at 300 g for 5 min. Subsequently, cells were resuspended in 10 ml fresh medium with GM-CSF (20 ng/ml) and were then refed into the original dish. Only unadherent cells (DCs) were harvested and seeded in a fresh dish; 10 ml fresh medium including GM-CSF (20 ng/ml) was added at day 8.
Cells were also treated with lipopolysaccharide (LPS; Sigma) for 24 h at 1 μg/ml. LPS was isolated from Klebsiella pneumoniae. DCs or LPS-treated DCs were pulsed with MOG peptide for 30 min and then washed twice with PBS at 300 g for 5 min before i.v. transfer to EAE mice. Fresh unadherent DCs were then collected and washed with PBS at 300 g for 5 min and characterized by flow cytometry or conducted by i.v. transfer to EAE mice. More than 90% of cells express DC marker CD11c 18.
Apoptotic assay of DCs
Bone marrow-derived DCs, 1 × 106, were washed with cell staining buffer (Biolegend, San Diego, CA, USA) twice at 300 g for 5 min; 100 μl of cell suspension was transferred into a 5-ml test tube. Cell suspension was incubated with 5 μl of annexin V and 10 μl of propidium iodide (PI) solution (Biolegend) for 15 min at room temperature in the dark. Cells were then resuspended in 400 μl of annexin V binding buffer (Biolegend) and analysed immediately by flow cytometry.
Flow cytometry
Cultured DCs were incubated with anti-mouse CD8-α [phycoerythrin-cyanin 7 (PE-Cy7)], CD11b (PE), CD11c [allophycocyanin (APC)], B220 (APC-Cy7), CD205 (Pacific blue), Ly-6G/Ly-6C (Gr-1) [fluorescein isothiocyanate (FITC)] and galectin-1 [peridinin chlorophyll (PerCP)-Cy5·5] antibodies for 24 h at 4°C (Biolegend). MOG-primed T lymphocytes were isolated from EAE mice and incubated with anti-mouse CD4 antibodies. Cells were washed twice with 5% FCS in PBS at 300 g for 5 min, fixed with 5% formalin in PBS at 4°C for 2 h and then permeated for intracellular staining 18–20.
For intracellular staining, spleen cells were stimulated by leucocyte activator (BD) for 6 h. Splenocytes were then washed twice with 5% FCS in PBS at 300 g for 5 min and fixed with 5% formalin (Sigma) in PBS at 4°C for 2 h. After cells were washed twice with permeabilization buffer (Biolegend) at 300 g for 10 min, anti-mouse retinoic acid-related orphan receptor (ROR) gamma t (ROR-γt) (APC), interferon (IFN)-γ (PE-Cy-7), IL-17A (Pacific blue), IL-17F (FITC), IL-21 (PerCP-Cy5·5) and IL-22 (PE) antibodies (Biolegend) were incubated with cells at 4°C for 24 h. Cells were then washed twice with permeabilization buffer at 300 g for 5 min, resuspended in 0·5 ml cell staining buffer (Biolegend), and tested in a fluorescence activated cell sorter (FACS)Aria (BD Biosciences, San Jose, CA, USA). Data were analysed using FlowJo software (Treestar, Ashland, OR, USA) 18–20.
Enzyme-linked immunosorbent assay (ELISA)
Anti-mouse ELISA kits, including IFN-γ, IL-22, IL-10 and IL-17A, were purchased from R&D Systems. Assays were conducted according to the manufacturer's instructions. Plates were read out in Labsystems Multiskan MCC/340 (Fisher Scientific, Suwanee, GA, USA) and data were analysed using DSJV ELISA software (Fisher Scientific) 18–20.
Generation of effector T cells in vitro
C57 BL/6J mice were immunized with peptide35–55 (Invitrogen) 200 μg, QuilA (Sigma) 20 μg and keyhole limpet haemocyanin (KLH; Sigma) 20 μg per mouse at day 1. Spleen cells were then isolated at day 10 after immunization. T lymphocytes were purified with the mouse CD4+ T cell subset column kit (R&D Systems). CD4+ T cells (1 × 106 cells/per well) were co-cultured with DCs at 5:1 (T cells : DCs) and pulsed with MOG35–55 peptide at 0·1 μM in complete medium with mouse IL-2 at 1 ng/ml for 3 days. Cells were harvested and MOG-primed CD4+ T cells were gated and analysed by flow cytometry 19.
EAE induction and treatment
C57BL/6J mice (female, aged 8–12 weeks) were immunized with MOG35–55 peptide/complete Freund's adjuvant (CFA; Sigma) at 200 μg/200 μl/per mouse [subcutaneous (s.c.) injection]. Pertussis toxin (PT; Sigma) was injected simultaneously at 200 ng/per mouse [intraperitoneal (i.p.) injection] and the second PT injection was conducted after 48 h. EAE was assessed following standard clinical scores: 0·5, paralysis of half the tail; 1, paralysis of whole tail; 2, paralysis of tail and one leg; 3, paralysis of tail and two legs; 4, moribund; and 5, death 19,20.
Mice were divided into three groups. DCs were washed twice with PBS and were injected immediately via the tail vein (3 × 105 cells/per mouse/per time) on days 11, 14 and 17 post-immunization (p.i.): (i) injected with unpulsed DCs; (ii) injected with DCs pulsed with MOG peptide; and (iii) injected with LPS-treated DCs pulsed with MOG peptide 19,20.
At day 24 p.i., splenocytes were isolated and stimulated with MOG peptide (0·1 μM) and mouse IL-2 (1 ng/ml) for 3 days. Cells were then harvested for flow cytometry and the supernatant was assayed by ELISA 19,20.
Statistical analysis
Experimental data were analysed using Prism software (GraphPad, La Jolla, CA, USA). A two-way analysis of variance (anova) test was performed for analysis of clinical score of EAE; t-tests were conducted for analysis of flow cytometry and ELISA data. Data represent the mean and standard deviation (s.d.) or standard error of the arithmetic mean (s.e.m.). Results were regarded as showing a significant difference if the P-value was less than 0·05 18–20.
Results
LPS treatment facilitates the apoptosis of DCs in vitro
To investigate whether or not LPS affects the apoptosis of DCs in vitro, bone marrow-derived DCs were incubated with LPS (1 μg/ml) or without LPS treatment for 24 h. Our results showed that the percentage of apoptotic DCs (annexin V+ cells) is increased significantly after LPS treatment (Fig. 1). It can thus be concluded that LPS treatment (1 μg/ml) facilitates the apoptosis of DCs in vitro.
Fig. 1.
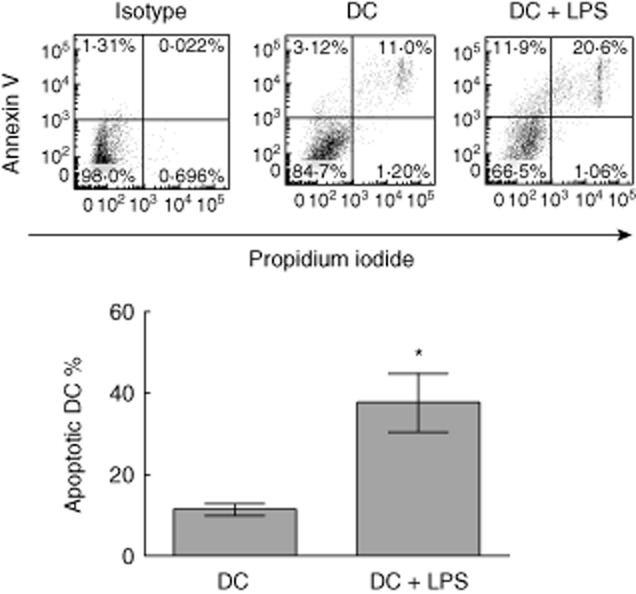
Lipopolysaccharide (LPS) treatment elicits the apoptosis of dendritic cells (DCs). Bone marrow-derived DCs were incubated with LPS at 1 μg/ml for 24 h, or without LPS stimulation. Cells were harvested and incubated with annexin V and propidium iodide (PI). The percentage of early stage (annexin V+PI−) and late stage (annexin V+PI+) of apoptotic DCs is shown. Error bars represent mean and standard deviation of percentage of apoptotic DCs (annexin V+ DCs) in three independent experiments (n = 3, t-test, *P < 0·05).
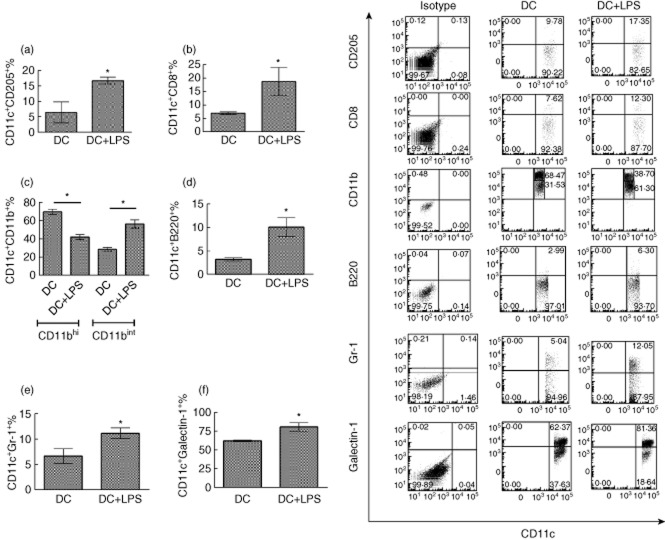
LPS treatment modulates the phenotype of DCs in vitro
To determine whether or not LPS treatment can affect the expression of CD205, CD8, CD11b, B220, Gr-1 and galectin-1 on DCs, DCs were treated with or without LPS stimulation for 24 h. Protein expression of CD205, CD8, CD11b, B220, Gr-1 and galectin-1 was detected on CD11c+ DCs (Fig. 2a–f). The results showed that LPS treatment up-regulates expression of tolerance-associated molecules, including CD205 and galectin-1, on CD11c+ DCs. However, Gr-1 and B220 expression on CD11c+ cells is down-regulated after LPS treatment. These results suggest that LPS may regulate DC-mediated immune tolerance through facilitating expression of tolerance-associated molecules on DCs.
Fig. 2.
Lipopolysaccharide (LPS) treatment modulates the phenotype of dendritic cells (DCs) in vitro. Bone marrow-derived DCs were isolated and cultured in granulocyte–macrophage colony-stimulating factor (GM-CSF) (20 ng/ml) medium for 8 days. DCs were treated with or without LPS stimulation at 1 μg/ml for 24 h. DCs were harvested and flow cytometry was conducted. Expression of CD205 (a), CD8 (b), CD11b (c), B220 (d), Gr-1 (e) and galectin-1 (f) was detected on CD11c+ DCs. CD11c+CD11blow and CD11c+CD11bhigh were gated separately in (c). Error bars represent the mean and standard deviation of triplicate determinations of percentage of CD11c+ cells in three independent experiments (n = 3, t-test, *P < 0·05).
In addition, the number of CD11c+CD8+ DCs was also increased after LPS treatment, suggesting that LPS may facilitate CD11c+CD8+-mediated immune responses in vitro (Fig. 2b).
Our results indicate that there are two subpopulations of CD11c+CD11b+ DCs, including CD11c+CD11blow and CD11c+CD11bhigh DCs. LPS treatment down-regulates the number of CD11c+CD11blow, but up-regulates that of CD11c+CD11bhigh DCs (Fig. 2c). These results imply that LPS may inhibit CD11c+CD11blow-mediated immune responses but facilitate CD11c+CD11bhigh DC-mediated immune responses in vitro.
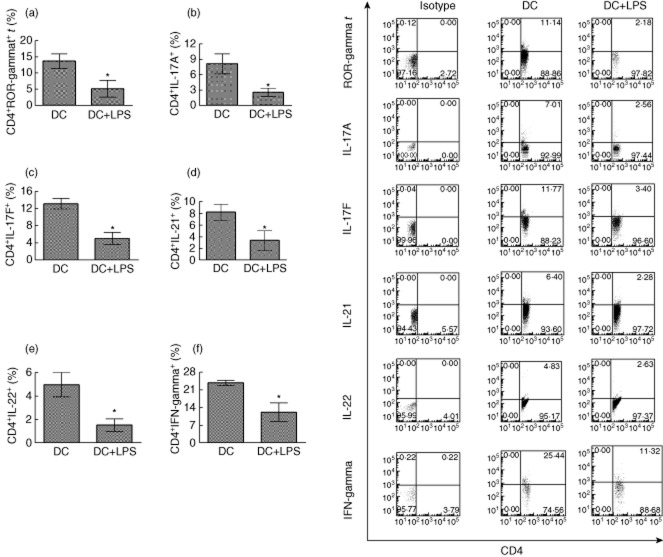
LPS-treated DCs down-regulate expression of multiple cytokines in CD4+ T cells in vitro
To determine whether or not LPS-treated DCs can affect CD4+ T cell activity, DCs were treated with LPS (1 μg/ml), or not, and co-cultured with MOG-primed CD4+ T cells. Flow cytometry analysis showed that LPS-treated DCs down-regulate expression of ROR-γt in CD4+ T cells. Moreover, expression of IL-17A, IL-17F, IL-21, IL-22 and IFN-γ is suppressed, compared to that of the LPS-untreated group (Fig. 3). These results suggest that LPS-treated DCs inhibit Th17 cell activity by down-regulating expression of ROR-γt and the production of multiple cytokines that are necessary for Th17 activity in vitro.
Fig. 3.
Lipopolysaccharide (LPS)-treated dendritic cells (DCs) down-regulate expression of retinoic acid-related orphan receptor gamma t (ROR-γt) and multiple cytokines in CD4+ T cells in vitro. Bone marrow DCs pulsed with myelin oligodendrocyte glycoprotein (MOG) peptide at 0·1 μM were treated with or without LPS stimulation at 1 μg/ml for 24 h. DCs were co-cultured with MOG-primed CD4+ T cells for 3 days. CD4+ T cells were harvested and gated. Expression of ROR-γt (a), interleukin (IL)-17A (b), IL-17F (c), IL-21 (d), IL-22 (e) and interferon (IFN)-γ (f) on CD4+ cells was detected. Error bars represent the mean and standard deviation of triplicate determinations of percentage of CD4+ T cells in three independent experiments (n = 3, t-test, *P < 0·05).
LPS-treated DCs block EAE development induced by MOG/CFA immunization
To investigate whether or not LPS-treated DCs also affect EAE development in vivo, C57 BL/6J mice were immunized with MOG/CFA to induce EAE in vivo. Bone marrow-derived DCs and DCs treated with LPS were pulsed with MOG peptide and transferred i.v. into EAE mice. Mice treated with unpulsed DCs served as a control. The results showed that LPS-treated DCs pulsed with MOG peptide can significantly block EAE development compared with the group transferred i.v. with LPS-untreated DCs pulsed with MOG peptide. EAE development in mice treated with DCs without loading MOG peptide is similar to that in mice transferred i.v. with DCs pulsed MOG peptide, but without LPS treatment (Fig. 4). These results imply that LPS treatment facilitates DC-mediated immune tolerance in mice with EAE. However, MOG stimulation does not significantly affect EAE development if LPS is not treated.
Fig. 4.
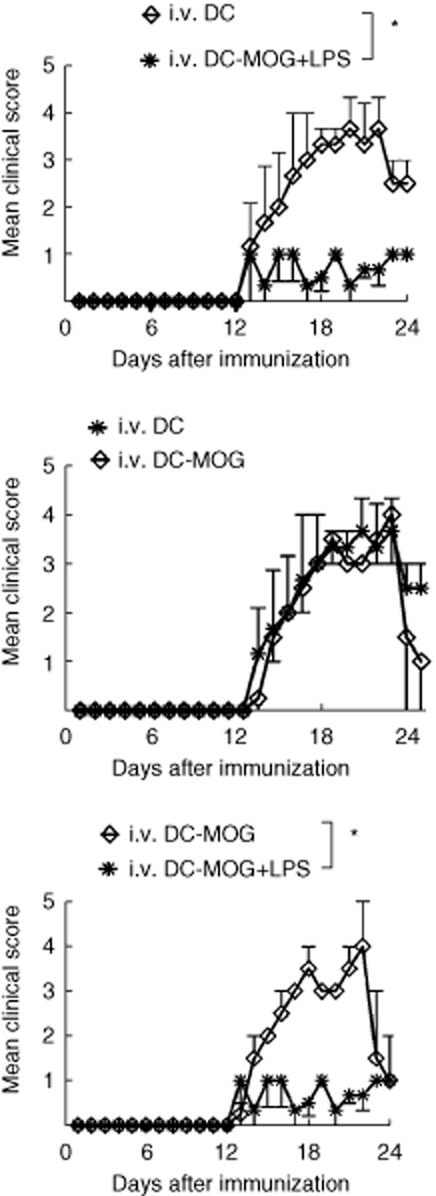
Lipopolysaccharide (LPS)-treated dendritic cells (DCs) inhibited experimental autoimmune encephalomyelitis (EAE) development. C57BL/6J mice were immunized subcutaneously (s.c.) with myelin oligodendrocyte glycoprotein (MOG)/complete Freund's adjuvant (CFA) at day 0. Pertussis toxin (PT) was injected intraperitoneally (i.p.) at days 0 and 2. DCs treated with LPS at 1 μg/ml for 24 h, or without LPS stimulation, were injected intravenously (i.v.) into EAE mice at days 11, 14 and 17 (3 × 105 cells/per mouse/per time). EAE development with clinical score from days 0 to 24 post-immunization (p.i.) is shown. All DCs treated not in with LPS or this experiment were incubated with MOG peptide at 0·1 μM for 30 min or without MOG peptide loading as a control. Cells were then washed with phosphate-buffered saline (PBS) twice at 300 g for 5 min immediately before i.v. transfer to mice. This experiment was repeated twice with similar results. Error bars represent the mean and standard error of the mean of EAE clinical score (n = 6–8, two-way analysis of variance (anova) test, *P < 0·05).
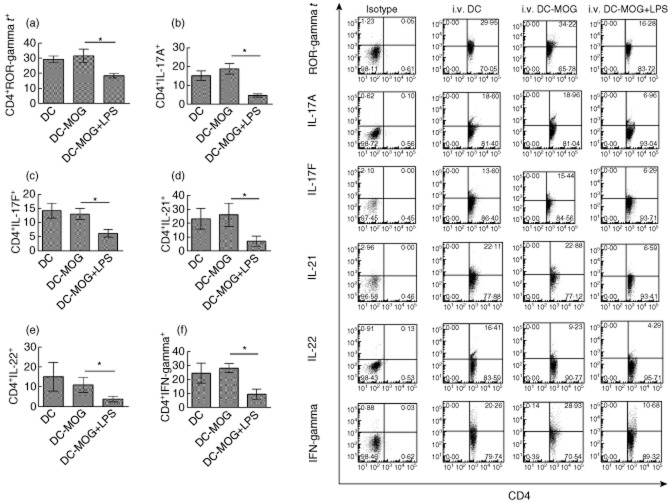
Intravenous transfer of LPS-treated DC down-regulates protein expression of multiple cytokines in CD4+ T cells in vivo
To investigate whether or not LPS-treated DCs can also affect protein expression of ROR-γt and cytokines in vivo, the expression of ROR-γt, IL-17A, IL-17F, IL-21, IL-22 and IFN-γ was detected using flow cytometry (Fig. 5). The results demonstrated that i.v. transfer of LPS-treated DCs pulsed with MOG peptide inhibits expression of ROR-γt, IL-17A, IL-17F, IL-21, IL-22 and IFN-γ in CD4+ T cells compared with that of i.v. DCs pulsed with MOG peptide but without LPS treatment groups. In contrast, expression of ROR-γt and cytokines in CD4+ T cells derived from mice treated with DCs without loading MOG peptide is similar to that in CD4+ T cells isolated from mice treated with DCs pulsed with MOG peptide, but without LPS treatment (Fig. 5). Our results suggest that LPS-treated DCs down-regulate cytokine production in Th17 cells and inhibit the activity of inflammatory Th17 cells in EAE mice.
Fig. 5.
Lipopolysaccharide (LPS)-treated dendritic cell (DC) transfer down-regulates expression of retinoic acid-related orphan receptor gamma t (ROR-γt) and multiple cytokines in CD4+ T cells in vivo. Spleen cells were isolated from experimental autoimmune encephalomyelitis (EAE) mice treated intravenously (i.v.) with dendritic cells (DCs), DCs-myelin oligodendrocyte glycoprotein (MOG) and DCs-MOG treated with LPS are shown in Fig. 4. CD4+ T cells were gated. Protein expression of ROR-γt (a), interleukin (IL)-17A (b), IL-17F (c), IL-21 (d), IL-22 (e) and interferon (IFN)-γ (f) in CD4+ T cells is indicated. Error bars represent the mean and standard deviation of triplicate determinations of percentage of CD4+ T cells in three independent experiments (n = 3, t-test, *P < 0·05).
In addition, an ELISA assay was conducted to investigate whether or not LPS-treated DC transfer can modulate production of these cytokines ex vivo. Our results showed that the amount of IL-10 produced is elevated after i.v. transfer of DCs treated with LPS and MOG peptide. In contrast, the amounts of IL-17A, IFN-γ and IL-22 are down-regulated after i.v. transfer of LPS-treated DCs pulsed with MOG peptide. Cytokine production in mice treated with DCs without loading MOG peptide is similar to that in mice treated with DCs pulsed with MOG peptide, but without LPS treatment (Fig. 6). These results suggest that LPS-treated DCs may not only affect Th17 activity, but also modulate the activity of other subsets of MOG-primed T lymphocytes such as Th22, Tregs and Th1.
Fig. 6.
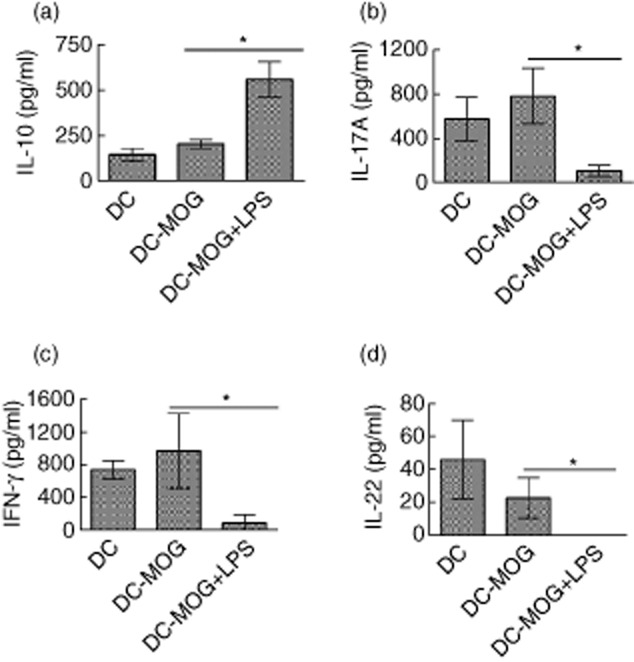
Lipopolysaccharide (LPS)-treated dendritic cells (DCs) modulate cytokine production ex vivo. Spleen cells were isolated from mice shown in Fig. 4. These cells were then restimulated by myelin oligodendrocyte glycoprotein (MOG) peptide (0·1 μM) for 3 days in complete medium with mouse interleukin (IL)-2 at 1 ng/ml. The supernatant was collected and an enzyme-linked immunosorbent assay (ELISA) assay was conducted for detecting production of IL-10 (a), IL-17A (b), interferon (IFN)-γ (c) and IL-22 (d). All experiments shown in this figure were repeated twice with similar results. Error bars represent the mean and standard deviation of triplicate determinations of cytokine concentration from one experiment (n = 3, t-test, *P < 0·05).
In addition, LPS treatment leads to maturation of DCs. For example, LPS treatment increases expression of CD40, CD80, CD86 and major histocompatibility complex (MHC) class II on DCs (Supporting information, Fig. S2). Gate scheme of DCs and CD4+ T cells is shown in Supporting information, Figs S1 and S3.
To investigate whether or not LPS-treated DC-induced immune tolerance is specific to MOG peptide, C57 mice were immunized with MOG/CFA to induce EAE. Bone marrow-derived DCs were stimulated by LPS (1 μg/ml) for 24 h and were pulsed with MOG peptide or without loading MOG peptide. LPS-stimulated DCs or PBS as a control were then transferred i.v. into EAE mice. Experimental data indicated that i.v. transfer of LPS-stimulated DCs pulsed with MOG peptide can significantly inhibit development of EAE. In contrast, i.v. transfer of LPS-treated DCs without loading MOG peptide does not affect EAE induction compared with results of the EAE control group treated with i.v PBS. Only i.v. transfer of LPS-stimulated DCs pulsed with MOG peptide can specifically block development of IL-17 producing CD4+ T cells (Supporting information, Fig. S4). It can be concluded that immune tolerance induced by LPS-treated DCs is specific to MOG peptide.
Discussion
LPS stimulation leads not only to DC maturation 21, but also causes cellular apoptosis 7. Apoptosis of DCs is an important factor for induction of immune tolerance in vivo 22; however, the molecular mechanisms of apoptotic DC-mediated immune tolerance have not been fully elucidated.
DC phagocytosis of apoptotic cells leads to generation of tolerogenic DCs 23. DCs engulf apoptotic cells through the CD205-mediated pathway and present epitopes of self-antigen on the surface 8. This leads to depletion of autoreactive T cells and induction of immune tolerance. LPS treatment increases CD205 expression on DCs. This may facilitate CD205-mediated phagocytosis to target apoptotic DCs induced by LPS and cause generation of tolerogenic DCs.
CD8+ DCs play an important role in immune tolerance 24, given that they are necessary for the development of Tregs 24,25. CD8+ DCs also facilitate the apoptosis of pathogenic CD4+ T cells and inhibit CD4+ T cell-mediated inflammatory responses 26. LPS treatment increases the number of CD11c+CD8+ DCs. This may elicit CD8+ DC-mediated immune tolerance in mice with EAE after DC transfer.
CD11c+CD11b+ DCs also play an important role in inhibition of EAE development 27. CD11c+CD11b+ DCs produce IL-10 to inhibit inflammatory responses in vivo 28. However, there are also data showing that CD11c+CD11b+ DCs are inflammatory DCs and facilitate T cell-mediated immune responses 29,30. The reason for the great difference in immune functions of CD11c+CD11b+ may be that there are diverse subpopulations of CD11c+CD11b+ DCs that perform different immune functions in vivo. For instance, our results indicated that there are two subpopulations of CD11c+CD11b+ DCs: CD11c+CD11bhigh and CD11c+CD11blow DCs. LPS treatment facilitates development of CD11c+CD11bhigh DCs, but down-regulates the number of CD11c+CD11blow DCs. It is known that CD11c+CD11bhigh DCs can inhibit T cell-mediated immune responses 31. Our results suggest that LPS modulates the balance between inflammatory CD11c+CD11blow and tolerogenic CD11c+CD11bhigh DCs. LPS treatment may elicit CD11c+CD11bhigh-mediated tolerance in EAE mice transferred with LPS-treated DCs.
It has been known that there are at least two populations of CD11c+ DCs: CD11clow and CD11chigh DCs. Zaft et al. reported that CD11chigh DCs are necessary for proliferation of CD8+ memory T cells 32. However, it is unclear whether or not CD11chigh DCs also regulate CD4+ T cell-mediated immune responses. This will be tested in further studies.
Recent data indicate that Gr-1 is expressed on inflammatory DCs. For example, CD11c+Gr-1+ inflammatory DCs facilitate CD8+ T cell-mediated anti-tumour immunity 33. LPS treatment down-regulates the number of CD11c+Gr-1+ DCs, suggesting that LPS-induced apoptotic DCs may inhibit the development of inflammatory DCs and block inflammatory CD11c+Gr-1+ DC-mediated T cell responses. Given that Gr-1 is also expressed on progenitors of other typical cells derived from bone marrow cells, such as granulocytes, it would be interesting to investigate whether or not LPS also regulates expression of Gr-1 on granulocytes.
It has been reported that CD11c+B220+ DCs play an important role in xenograft rejection, and this may be via activating effector T cells 34. LPS treatment suppresses the development of CD11c+B220+ DCs, which may block inflammatory responses mediated by these DCs.
Galectin-1 is another important tolerance-associated molecule that is expressed on DCs 18,35–37. Galectin-1-mediated signalling is necessary for the development of Tregs and leads to apoptosis of pathogenic T cells 38,39. Tolerogenic DCs induced by galectin-1 block the development of EAE in vivo 35. LPS treatment increases expression of galectin-1 on DCs. This may be one of the reasons why i.v. transfer of LPS-treated DCs can inhibit EAE development efficiently in vivo. LPS-induced apoptotic DCs might be digested by living DCs, which leads to generation of tolerogenic DCs with high expression of galectin-1, thus inhibiting the development of EAE.
In addition, given that the structure and composition of LPS derived from different bacterial sources are diverse, the functions of LPS isolated from different sources may be divergent 40,41. For example, our results indicate that LPS derived from K. pneumoniae facilitates galectin-1 expression on DCs. In contrast, LPS isolated from Escherichia coli down-regulates expression of galectin-1 on DCs 35. Moreover, Voigtlander et al. reported that treatment of LPS isolated from E. coli leads to generation of inflammatory DCs 42. These results imply the diversity of effects of LPS isolated from different bacterial sources on DC-mediated immune functions.
LPS is a product of Gram-negative bacteria that leads to tolerance in vivo 43,44, but the molecular mechanisms of LPS-induced tolerance remain obscure. It has been shown that LPS treatment of DCs results in activation of Th2 cells and Tregs and inhibition of inflammatory responses in EAE 13,45,46; however, it is not known whether or not LPS-treated DCs can affect the activity of Th17 cells, which are necessary for EAE induction. Our results showed that LPS-treated DCs modulate cytokine expression by Th17 cells and that i.v. transfer of LPS-treated DCs blocks EAE development. These findings suggest that LPS-induced tolerogenic DCs may affect the activity of Th17 cells via modulation of cytokine secretion by Th17 cells.
Th17 cells are pathogenic IL-17-producing CD4+ T cells that are necessary for the induction of autoimmune diseases such as EAE 47. ROR-γt is a gene transcriptional factor that plays an important role in development of Th17 cells 48. LPS-treated DCs down-regulate expression of ROR-γt in CD4+ T cells, suggesting that tolerogenic DCs induced by LPS may inhibit the development of Th17 cells via blocking expression of ROR-γt in CD4+ T cells and affect EAE development in vivo.
CD4+ T cell-mediated immune functions are dependent upon the secretion of multiple inflammatory cytokines such as IL-17A and IL-17F. LPS-treated DC transfer results in decreased numbers of CD4+IL-17A+ and CD4+IL-17F+ T cells, thus affecting EAE development. Given that LPS-treated DCs down-regulate the number of CD4+IL-17A+ and CD4+IL-17F+ T cells, our data suggest that LPS-induced apoptotic DCs may cause generation of tolerogenic DCs and inhibit EAE development via blocking MOG-primed CD4+ T cell activation in vivo.
Similarly, IL-21 is also a Th17 cell-produced proinflammatory cytokine 49 that is necessary for EAE development 50,51. Our results showed that LPS-treated DCs down-regulate IL-21 production in MOG-primed CD4+ T cells, consistent with their effect on suppressing EAE development. These data suggest that LPS-induced tolerant DCs down-regulate IL-21 secretion in CD4+ T cells, thus inhibiting IL-21-mediated immune responses in EAE development.
IL-22 is a cytokine that can be produced by Th22 and Th17 cells 52–55. The immune significance of IL-22 produced by Th17 cells is unknown. We hypothesized that IL-22 produced by CD4+ T cells is a regulator of Th17 activity. A decrease in IL-22 expression in MOG-stimulated CD4+ T cells inhibited IL-22-mediated immune responses. Thus, modulation of IL-22 expression by LPS-treated DCs may affect IL-22-mediated inflammatory responses in EAE mice.
IFN-γ plays an important role in regulating immune responses 56. IFN-γ not only facilitates inflammatory responses in autoimmunity, but also modulates induction of immune tolerance 56–58. IFN-γ is also produced by Th17 cells 59. Although IFN-γ may play a role in eliciting Th17-mediated inflammatory responses in EAE, the mechanism of IFN-γ in regulating Th17 cell activity is unclear. LPS-treated DCs down-regulate the amount of IFN-γ in MOG-primed CD4+ T cells. This effect may block IFN-γ-mediated Th17 immune responses in EAE mice and inhibit EAE development. Moreover, IFN-γ is produced by Th1 cells, which also play an important role in EAE induction 60. Our ELISA data demonstrated that LPS-treated DCs down-regulate the amount of IFN-γ, suggesting that LPS-treated DCs may also affect the activity of Th1 cells via down-regulation of IFN-γ production.
IL-10 is an anti-inflammatory cytokine that is also produced by Th17 cells 59; however, the immune significance of IL-10 produced by Th17 cells has not been elucidated fully. It is not known whether or not IL-10 produced by Th17 cells is a negative regulator for Th17 activity. Our ELISA data showed that LPS-treated DCs up-regulate the amount of IL-10. Because IL-10 is also produced by DCs and other subsets of CD4+ T cells such as Tregs 12,13, our results imply that LPS-treated DCs may also regulate the secretion of IL-10 in other T helper cells such as Tregs, with different effects and other innate cells such as DCs. This hypothesis will be investigated in future studies.
Molecular mechanisms of DC-mediated immune tolerance are complex and have not been elucidated fully. A tolerant experimental model induced by apoptotic cell-treated DCs has been established in our project. Our results imply that tolerogenic DCs might be generated after digestion of apoptotic cells. Expression of tolerance-associated molecules such as galectin-1 and CD205 is increased after incubation with apoptotic cells. Specific typical tolerogenic DCs such as CD11c+CD205+ or CD11c+galectin-1+ DCs may be generated after digestion of apoptotic cells. In addition, tolerogenic DCs may also provoke other regulatory networks in vivo – for example, modulation of cytokine production in CD4+ T cells – to block EAE development. Further studies to investigate how tolerogenic DCs regulate antigen-specific T cells and inhibit EAE development, particularly in adoptively transferred EAE, will be carried out in the near future.
It has been reported that Th2 cells play a protective role in development of EAE 61. Our previous data indicated that LPS-treated bone marrow-derived DCs facilitate differentiation of Th2 cells 46. Moreover, Power et al. reported that LPS-treated DCs block development of EAE through eliciting expansion of CD4+forkead box protein 3 (FoxP3+) Tregs 62. These results imply that LPS-induced tolerogenic DCs inhibit the development of autoimmunity via multiple pathways.
Zheng et al. reported that LPS-treated plasmacytoid DCs induce tolerance through facilitation of Treg development and inhibition of inflammatory cytokine production 1. Our data suggest that LPS treatment also elicits myeloid DC-mediated immune tolerance via blocking Th17 activity due to induction and presentation of apoptotic cells. Our results indicate diversity of mechanisms of LPS-treated DC-mediated immune tolerance in vivo.
There are many factors which affect effect of immunotherapy mediated by DCs. For example, Alderuccio et al. reported that immature DCs expressing MOG can delay upset of EAE 63. Our previously published data indicated that immature DCs delay development of EAE after day 25 (p.i.) if DCs are transferred i.v. into mice at an early stage (0–12 day p.i.) before EAE is induced 46. However, DCs pulsed with MOG peptide were transferred i.v. at a late stage (days 11, 14 and 17) after EAE is induced in our current research and results of clinical score were recorded from days 0–24 p.i. Our results indicated that DC-pulsed MOG do not efficiently inhibit EAE development. It may be too short to observe the effect of MOG-pulsed immature DCs on the development of EAE. Our data imply that the time–course of i.v. transfer of DC into EAE mice may be an important factor which affects the efficiency of immunotherapy mediated by DCs.
Previous research showed that MOG-pulsed DC-induced immune tolerance in EAE mice is antigen (MOG)-specific. Moreover, Lutz's data indicated that tumour necrosis factor (TNF)-α-treated DCs also block EAE development in vivo 64. As TNF-α is also able to induce apoptosis of DCs, TNF-α treatment may also lead to generation of tolerogenic DCs such as LPS. LPS and TNF-α may play a double role in modulation of autoimmunity and tolerance through regulating the balance of mature and tolerogenic DCs. This hypothesis should be investigated in the future.
In addition, some cytokines in the cell culture system may also modulate function of DCs. For example, Wells et al. reported that IL-4 modulates phenotype and function of bone marrow-derived DCs under serum-free conditions. However, IL-4 does not affect the phenotype and function of bone marrow-derived DCs after FCS is presented 65. Moreover, Yao et al. found that IL-4 treatment blocks production of IL-10 and enhances IL-12 secretion by DCs which were treated by E. coli-produced LPS 66. These results imply that mechanisms of DC-mediated immune tolerance or autoimmunity are extremely complicated. There are still many details which have not been elucidated.
In conclusion, an immunotherapy was designed using LPS-treated DCs to block EAE development in vivo. LPS treatment up-regulates the number of apoptotic DCs and modulates the phenotype of DCs. LPS treatment also improves the expression of tolerance-associated molecules such as CD205 and galectin-1 on DCs. Both in-vitro and ex-vivo data showed that DCs treated with LPS and MOG peptide affect the expression of multiple cytokines in MOG-stimulated CD4+ T cells. These effects may lead to inhibition of EAE development in vivo. Our results suggest that LPS-induced tolerogenic DCs may provide a potential immunotherapy for treatment of MS.
Acknowledgments
This study was supported by grants from the NIH and the National Multiple Sclerosis Society. We thank Katherine Regan for editorial assistance.
Disclosure
The authors declare no conflicts of interest.
Supporting Information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Fig. S1. Gate scheme of dendritic cells (DCs). Bone marrow-derived DCs were cultured in complete medium with granulocyte–macrophage colony-stimulating factor (GM-CSF) (20 ng/ml).
Fig. S2. Maturation status of dendritic cells (DCs) induced by lipopolysaccharide (LPS) stimulation.
Fig. S3. Gate strategy of CD4+ T cells.
Fig. S4. Lipopolysaccharide (LPS)-stimulated dendritic cells (DCs)-induced immune tolerance is specific to myelin oligodendrocyte glycoprotein (MOG) peptide.
References
- 1.Zheng D, Cao Q, Lee VW, et al. Lipopolysaccharide-pretreated plasmacytoid dendritic cells ameliorate experimental chronic kidney disease. Kidney Int. 2012;81:892–902. doi: 10.1038/ki.2011.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campo GM, Avenoso A, Campo S, D'Ascola A, Nastasi G, Calatroni A. Molecular size hyaluronan differently modulates toll-like receptor-4 in LPS-induced inflammation in mouse chondrocytes. Biochimie. 2010;92:204–215. doi: 10.1016/j.biochi.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Saito Y, Yanagawa Y, Kikuchi K, Iijima N, Iwabuchi K, Onoe K. Low-dose lipopolysaccharide modifies the production of IL-12 by dendritic cells in response to various cytokines. J Clin Exp Hematop. 2006;46:31–36. doi: 10.3960/jslrt.46.31. [DOI] [PubMed] [Google Scholar]
- 4.Rego D, Kumar A, Nilchi L, Wright K, Huang S, Kozlowski M. IL-6 production is positively regulated by two distinct Src homology domain 2-containing tyrosine phosphatase-1 (SHP-1)-dependent CCAAT/enhancer-binding protein beta and NF-kappaB pathways and an SHP-1-independent NF-kappaB pathway in lipopolysaccharide-stimulated bone marrow-derived macrophages. J Immunol. 2011;186:5443–5456. doi: 10.4049/jimmunol.1003551. [DOI] [PubMed] [Google Scholar]
- 5.Kushwah R, Hu J. Dendritic cell apoptosis: regulation of tolerance versus immunity. J Immunol. 2010;185:795–802. doi: 10.4049/jimmunol.1000325. [DOI] [PubMed] [Google Scholar]
- 6.Fedele G, Celestino I, Spensieri F, et al. Lipooligosaccharide from Bordetella pertussis induces mature human monocyte-derived dendritic cells and drives a Th2 biased response. Microbes Infect. 2007;9:855–863. doi: 10.1016/j.micinf.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Y, Cui G, Zhang N, Liu Z, Sun W, Peng Q. Lipopolysaccharide induces endothelial cell apoptosis via activation of Na(+)/H(+) exchanger 1 and calpain-dependent degradation of Bcl-2. Biochem Biophys Res Commun. 2012;427:125–132. doi: 10.1016/j.bbrc.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Shrimpton RE, Butler M, Morel AS, Eren E, Hue SS, Ritter MA. CD205 (DEC-205): a recognition receptor for apoptotic and necrotic self. Mol Immunol. 2009;46:1229–1239. doi: 10.1016/j.molimm.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kushwah R, Wu J, Oliver JR, et al. Uptake of apoptotic DC converts immature DC into tolerogenic DC that induce differentiation of Foxp3+ Treg. Eur J Immunol. 2010;40:1022–1035. doi: 10.1002/eji.200939782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lutz MB, Kurts C. Induction of peripheral CD4+ T-cell tolerance and CD8+ T-cell cross-tolerance by dendritic cells. Eur J Immunol. 2009;39:2325–2330. doi: 10.1002/eji.200939548. [DOI] [PubMed] [Google Scholar]
- 11.Langlois RA, Legge KL. Respiratory dendritic cells: mediators of tolerance and immunity. Immunol Res. 2007;39:128–145. doi: 10.1007/s12026-007-0077-0. [DOI] [PubMed] [Google Scholar]
- 12.Kwan WH, Boix C, Gougelet N, Fridman WH, Mueller CG. LPS induces rapid IL-10 release by M-CSF-conditioned tolerogenic dendritic cell precursors. J Leukoc Biol. 2007;82:133–141. doi: 10.1189/jlb.0406267. [DOI] [PubMed] [Google Scholar]
- 13.den Haan JM, Kraal G, Bevan MJ. Cutting edge: lipopolysaccharide induces IL-10-producing regulatory CD4+ T cells that suppress the CD8+ T cell response. J Immunol. 2007;178:5429–5433. doi: 10.4049/jimmunol.178.9.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furlan R, Cuomo C, Martino G. Animal models of multiple sclerosis. Methods Mol Biol. 2009;549:157–173. doi: 10.1007/978-1-60327-931-4_11. [DOI] [PubMed] [Google Scholar]
- 15.Hofstetter H, Gold R, Hartung HP. Th17 cells in MS and experimental autoimmune encephalomyelitis. Int MS J. 2009;16:12–18. [PubMed] [Google Scholar]
- 16.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Brereton CF, Sutton CE, Lalor SJ, Lavelle EC, Mills KH. Inhibition of ERK MAPK suppresses IL-23- and IL-1-driven IL-17 production and attenuates autoimmune disease. J Immunol. 2009;183:1715–1723. doi: 10.4049/jimmunol.0803851. [DOI] [PubMed] [Google Scholar]
- 18.Zhou F, Ciric B, Li H, et al. IL-10 deficiency blocks the ability of LPS to regulate expression of tolerance-related molecules on dendritic cells. Eur J Immunol. 2012;42:1449–1458. doi: 10.1002/eji.201141733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou F, Ciric B, Zhang GX, Rostami A. Immune tolerance induced by intravenous transfer of immature dendritic cells via up-regulating numbers of suppressive IL-10(+) IFN-gamma(+)-producing CD4(+) T cells. Immunol Res. 2013;56:1–8. doi: 10.1007/s12026-012-8382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F, Lauretti E, di Meco A, et al. Intravenous transfer of apoptotic cell-treated dendritic cells leads to immune tolerance by blocking Th17 cell activity. Immunobiology. 2013;218:1069–1076. doi: 10.1016/j.imbio.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HS, Chung SH, Song MY, et al. Effects of bee venom on the maturation of murine dendritic cells stimulated by LPS. J Ethnopharmacol. 2008;120:215–219. doi: 10.1016/j.jep.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Kushwah R, Oliver JR, Zhang J, Siminovitch KA, Hu J. Apoptotic dendritic cells induce tolerance in mice through suppression of dendritic cell maturation and induction of antigen-specific regulatory T cells. J Immunol. 2009;183:7104–7118. doi: 10.4049/jimmunol.0900824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gleisner MA, Rosemblatt M, Fierro JA, Bono MR. Delivery of alloantigens via apoptotic cells generates dendritic cells with an immature tolerogenic phenotype. Transplant Proc. 2011;43:2325–2333. doi: 10.1016/j.transproceed.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Lombardi V, Speak AO, Kerzerho J, Szely N, Akbari O. CD8alpha(+)beta(−) and CD8alpha(+)beta(+) plasmacytoid dendritic cells induce Foxp3(+) regulatory T cells and prevent the induction of airway hyper-reactivity. Mucosal Immunol. 2012;5:432–443. doi: 10.1038/mi.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamazaki S, Dudziak D, Heidkamp GF, et al. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J, Zhou C, Robertson J, et al. Identification of a bone marrow-derived CD8alphaalpha+ dendritic cell-like population in inflamed autoimmune target tissue with capability of inducing T cell apoptosis. J Leukoc Biol. 2010;88:849–861. doi: 10.1189/jlb.0310133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Zhang GX, Chen Y, et al. CD11c+ CD11b+ dendritic cells play an important role in intravenous tolerance and the suppression of experimental autoimmune encephalomyelitis. J Immunol. 2008;181:2483–2493. doi: 10.4049/jimmunol.181.4.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohtani M, Hoshii T, Fujii H, Koyasu S, Hirao A, Matsuda S. Cutting edge: mTORC1 in intestinal CD11c+ CD11b+ dendritic cells regulates intestinal homeostasis by promoting IL-10 production. J Immunol. 2012;188:4736–4740. doi: 10.4049/jimmunol.1200069. [DOI] [PubMed] [Google Scholar]
- 29.Trifilo MJ, Lane TE. The CC chemokine ligand 3 regulates CD11c+ CD11b+ CD8alpha− dendritic cell maturation and activation following viral infection of the central nervous system: implications for a role in T cell activation. Virology. 2004;327:8–15. doi: 10.1016/j.virol.2004.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundquist M, Wick MJ. Salmonella induces death of CD8alpha(+) dendritic cells but not CD11c(int)CD11b(+) inflammatory cells in vivo via MyD88 and TNFR1. J Leukoc Biol. 2009;85:225–234. doi: 10.1189/jlb.0708413. [DOI] [PubMed] [Google Scholar]
- 31.Liu Q, Zhang C, Sun A, Zheng Y, Wang L, Cao X. Tumor-educated CD11bhighIalow regulatory dendritic cells suppress T cell response through arginase I. J Immunol. 2009;182:6207–6216. doi: 10.4049/jimmunol.0803926. [DOI] [PubMed] [Google Scholar]
- 32.Zaft T, Sapoznikov A, Krauthgamer R, Littman DR, Jung S. CD11chigh dendritic cell ablation impairs lymphopenia-driven proliferation of naive and memory CD8+ T cells. J Immunol. 2005;175:6428–6435. doi: 10.4049/jimmunol.175.10.6428. [DOI] [PubMed] [Google Scholar]
- 33.Rich FJ, Kuhn S, Hyde EJ, Harper JL, Ronchese F, Kirman JR. Induction of T cell responses and recruitment of an inflammatory dendritic cell subset following tumor immunotherapy with Mycobacterium smegmatis. Cancer Immunol Immunother. 2012;61:2333–2342. doi: 10.1007/s00262-012-1291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito R, Katano I, Ida-Tanaka M, et al. Efficient xenoengraftment in severe immunodeficient NOD/Shi-SCID IL2rgammanull mice is attributed to a lack of CD11c+ B220+ CD122+ cells. J Immunol. 2012;189:4313–4320. doi: 10.4049/jimmunol.1200820. [DOI] [PubMed] [Google Scholar]
- 35.Ilarregui JM, Croci DO, Bianco GA, et al. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol. 2009;10:981–991. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- 36.Blois SM, Ilarregui JM, Tometten M, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13:1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- 37.Perone MJ, Bertera S, Tawadrous ZS, et al. Dendritic cells expressing transgenic galectin-1 delay onset of autoimmune diabetes in mice. J Immunol. 2006;177:5278–5289. doi: 10.4049/jimmunol.177.8.5278. [DOI] [PubMed] [Google Scholar]
- 38.Garin MI, Chu CC, Golshayan D, Cernuda-Morollon E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4+ CD25+ T cells. Blood. 2007;109:2058–2065. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- 39.Kopcow HD, Rosetti F, Leung Y, Allan DS, Kutok JL, Strominger JL. T cell apoptosis at the maternal–fetal interface in early human pregnancy, involvement of galectin-1. Proc Natl Acad Sci USA. 2008;105:18472–18477. doi: 10.1073/pnas.0809233105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathiak G, Kabir K, Grass G, et al. Lipopolysaccharides from different bacterial sources elicit disparate cytokine responses in whole blood assays. Int J Mol Med. 2003;11:41–44. [PubMed] [Google Scholar]
- 41.Agarwal S, Piesco NP, Johns LP, Riccelli AE. Differential expression of IL-1 beta, TNF-alpha, IL-6, and IL-8 in human monocytes in response to lipopolysaccharides from different microbes. J Dent Res. 1995;74:1057–1065. doi: 10.1177/00220345950740040501. [DOI] [PubMed] [Google Scholar]
- 42.Voigtlander C, Rossner S, Cierpka E, et al. Dendritic cells matured with TNF can be further activated in vitro and after subcutaneous injection in vivo which converts their tolerogenicity into immunogenicity. J Immunother. 2006;29:407–415. doi: 10.1097/01.cji.0000210081.60178.b4. [DOI] [PubMed] [Google Scholar]
- 43.Lutz MB, Kukutsch NA, Menges M, Rossner S, Schuler G. Culture of bone marrow cells in GM-CSF plus high doses of lipopolysaccharide generates exclusively immature dendritic cells which induce alloantigen-specific CD4 T cell anergy in vitro. Eur J Immunol. 2000;30:1048–1052. doi: 10.1002/(SICI)1521-4141(200004)30:4<1048::AID-IMMU1048>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 44.Ishiyama K, Ohdan H, Tokita D, et al. Induction of endotoxin tolerance inhibits alloimmune responses. Transpl Immunol. 2006;16:158–165. doi: 10.1016/j.trim.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Lau AW, Biester S, Cornall RJ, Forrester JV. Lipopolysaccharide-activated IL-10-secreting dendritic cells suppress experimental autoimmune uveoretinitis by MHCII-dependent activation of CD62L-expressing regulatory T cells. J Immunol. 2008;180:3889–3899. doi: 10.4049/jimmunol.180.6.3889. [DOI] [PubMed] [Google Scholar]
- 46.Zhang GX, Kishi M, Xu H, Rostami A. Mature bone marrow-derived dendritic cells polarize Th2 response and suppress experimental autoimmune encephalomyelitis. Mult Scler. 2002;8:463–468. doi: 10.1191/1352458502ms857oa. [DOI] [PubMed] [Google Scholar]
- 47.Peters A, Pitcher LA, Sullivan JM, et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity. 2011;35:986–996. doi: 10.1016/j.immuni.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgamma t directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 49.Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu R, Bai Y, Vollmer TL, et al. IL-21 receptor expression determines the temporal phases of experimental autoimmune encephalomyelitis. Exp Neurol. 2008;211:14–24. doi: 10.1016/j.expneurol.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Piao WH, Jee YH, Liu RL, et al. IL-21 modulates CD4+ CD25+ regulatory T-cell homeostasis in experimental autoimmune encephalomyelitis. Scand J Immunol. 2008;67:37–46. doi: 10.1111/j.1365-3083.2007.02035.x. [DOI] [PubMed] [Google Scholar]
- 52.Annunziato F, Romagnani S. Heterogeneity of human effector CD4+ T cells. Arthritis Res Ther. 2009;11:257–274. doi: 10.1186/ar2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujita H, Nograles KE, Kikuchi T, Gonzalez J, Carucci JA, Krueger JG. Human Langerhans cells induce distinct IL-22-producing CD4+ T cells lacking IL-17 production. Proc Natl Acad Sci USA. 2009;106:21795–21800. doi: 10.1073/pnas.0911472106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kreymborg K, Etzensperger R, Dumoutier L, et al. IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J Immunol. 2007;179:8098–8104. doi: 10.4049/jimmunol.179.12.8098. [DOI] [PubMed] [Google Scholar]
- 56.Weyand CM, Younge BR, Goronzy JJ. IFN-gamma and IL-17: the two faces of T-cell pathology in giant cell arteritis. Curr Opin Rheumatol. 2011;23:43–49. doi: 10.1097/BOR.0b013e32833ee946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malmberg KJ, Levitsky V, Norell H, et al. IFN-gamma protects short-term ovarian carcinoma cell lines from CTL lysis via a CD94/NKG2A-dependent mechanism. J Clin Invest. 2002;110:1515–1523. doi: 10.1172/JCI15564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou F. Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. Int Rev Immunol. 2009;28:239–260. doi: 10.1080/08830180902978120. [DOI] [PubMed] [Google Scholar]
- 59.Zielinski CE, Mele F, Aschenbrenner D, et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 60.Yura M, Takahashi I, Serada M, et al. Role of MOG-stimulated Th1 type ‘light up’ (GFP+) CD4+ T cells for the development of experimental autoimmune encephalomyelitis (EAE) J Autoimmun. 2001;17:17–25. doi: 10.1006/jaut.2001.0520. [DOI] [PubMed] [Google Scholar]
- 61.Falcone M, Bloom BR. A T helper cell 2 (Th2) immune response against non-self antigens modifies the cytokine profile of autoimmune T cells and protects against experimental allergic encephalomyelitis. J Exp Med. 1997;185:901–907. doi: 10.1084/jem.185.5.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ellestad KK, Tsutsui S, Noorbakhsh F, et al. Early life exposure to lipopolysaccharide suppresses experimental autoimmune encephalomyelitis by promoting tolerogenic dendritic cells and regulatory T cells. J Immunol. 2009;183:298–309. doi: 10.4049/jimmunol.0803576. [DOI] [PubMed] [Google Scholar]
- 63.Ko HJ, Chung JY, Nasa Z, et al. Targeting MOG expression to dendritic cells delays onset of experimental autoimmune disease. Autoimmunity. 2011;44:177–187. doi: 10.3109/08916934.2010.515274. [DOI] [PubMed] [Google Scholar]
- 64.Menges M, Rossner S, Voigtlander C, et al. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J Exp Med. 2002;195:15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wells JW, Darling D, Farzaneh F, Galea-Lauri J. Influence of interleukin-4 on the phenotype and function of bone marrow-derived murine dendritic cells generated under serum-free conditions. Scand J Immunol. 2005;61:251–259. doi: 10.1111/j.1365-3083.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- 66.Yao Y, Li W, Kaplan MH, Chang CH. Interleukin (IL)-4 inhibits IL-10 to promote IL-12 production by dendritic cells. J Exp Med. 2005;201:1899–1903. doi: 10.1084/jem.20050324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Gate scheme of dendritic cells (DCs). Bone marrow-derived DCs were cultured in complete medium with granulocyte–macrophage colony-stimulating factor (GM-CSF) (20 ng/ml).
Fig. S2. Maturation status of dendritic cells (DCs) induced by lipopolysaccharide (LPS) stimulation.
Fig. S3. Gate strategy of CD4+ T cells.
Fig. S4. Lipopolysaccharide (LPS)-stimulated dendritic cells (DCs)-induced immune tolerance is specific to myelin oligodendrocyte glycoprotein (MOG) peptide.