Abstract
We investigated the impact of rice prolamin extract (RPE) on lipopolysaccharide (LPS)-induced nuclear factor (NF)-κB signalling in intestinal epithelial cells and macrophages, and determined the therapeutic efficacy of RPE in acute murine colitis. The effect of RPE on LPS-induced NF-κB signalling and proinflammatory gene expression was evaluated by reverse transcription–polymerase chain reaction (RT–PCR), Western blotting, immunofluorescence and electrophoretic mobility shift assay (EMSA). The in-vivo efficacy of RPE was assessed in mice with 3% dextran sulphate sodium (DSS)-induced colitis. Apoptotic and cellular proliferative activities were evaluated by immunostaining with cleaved caspase-3 and proliferating cell nuclear antigen (PCNA) antibodies. RPE inhibited LPS-induced expression of monocyte chemotactic protein (MCP)-1, interleukin (IL)-6 and tumour necrosis factor (TNF)-alpha and LPS-induced NF-κB signalling in intestinal epithelial cells and macrophages. RPE-fed, DSS-exposed mice showed less weight loss, longer colon length and lower histological score compared to control diet-fed, DSS-exposed mice. Immunostaining analysis revealed a significant decrease of cleaved caspase-3 positive cells in RPE-fed, DSS-exposed mice compared to DSS-exposed mice. Also, the number of PCNA-positive cells within intact colonic crypts decreased significantly in RPE-fed, DSS-exposed mice compared to control diet-fed, DSS-exposed mice. DSS-induced NF-κB signalling was inhibited by RPE. RPE ameliorates intestinal inflammation by inhibiting NF-κB activation and modulating intestinal apoptosis and cell proliferation in an acute murine colitis.
Keywords: colon inflammation, dextran sulphate sodium, NF-κB, prolamin, rice
Introduction
Cereals are the most important food crops in the world. Three grains – maize, wheat and rice – account for more than 70% of the total world cereal production 1. Rice provides 35–60% of calories ingested by many people, especially in Asia 2. Proteins and starch are the two major components of rice. Rice proteins are nutritional for human health, with hypoallergenic properties and easier digestibility among cereal proteins 1,3,4, and consist of four important fractions, identified by differential solvent solubility: water-soluble albumin, salt-soluble globulin, alkali-soluble glutelin and alcohol-soluble prolamin 1,3,4.
Various naturally occurring dietary compounds have significant pharmacological and biological activities 5,6. Rice and rice fractions possess anti-inflammatory, anti-oxidative and anti-carcinogenic activities in vitro and in vivo 7, and have been shown to protect people from Helicobacter pylori-related gastric mucosal diseases, ethanol-induced gastric mucosal injury and experimental peptic ulceration 8–11. Fermented brown rice and rice bran may prevent chemical-induced gastric, colonic, bladder, oesophageal and hepatic carcinogenesis in rats 12–16. Therefore, it is extremely important to understand the basic physiochemical, functional properties of rice and rice fractions, and identify their mechanism for application in mainstream medicine.
Ulcerative colitis and Crohn's disease are two major forms of inflammatory bowel disease (IBD), a chronic, relapsing and destructive inflammatory disorder of the gastrointestinal tract 17. The pathogenesis of IBD involves impaired intestinal barrier function, dysregulated immune responses and altered microbiota composition. Most of these features are controlled by proteases and their inhibitors to maintain gut immune homeostasis. Unrestrained or excessive mucosal immune activation and over-production of proinflammatory cytokines can lead to pathological gastrointestinal conditions such as IBD 18–20. The nuclear factor kappa B (NF-κB) signalling cascade plays a critical role in the regulation of immune and inflammatory responses, and has been implicated in the pathogenesis of IBD and in experimental colitis models, including dextran sulphate sodium (DSS), trinitrobenzene sulphonic acid (TNBS)-induced colitis and interleukin (IL)-10 knock-out mice 21–26.
Rice and rice fractions ameliorate inflammation in a murine colitis model including DSS-induced colitis by inhibiting NF-κB activation 27–29. Beneficial health effects have been described for the rice protein prolamin, which include anti-oxidant activity and activation of the human anti-leukaemia immune response 30,31. However, there are no data about the impact of rice prolamin extract (RPE) on intestinal inflammation.
In the present study, we examined the impact of RPE on lipopolysaccharide (LPS)-induced NF-κB signalling in rat intestinal epithelial cells (RIE) and bone marrow-derived macrophage (BMM), and determined the therapeutic efficacy of RPE in DSS-induced experimental colitis.
Materials and methods
Materials
Rice (cultivar Dongjinchal) supplied from Dasan M&F (Changpyeong, Korea) was ground to pass through a 0·12 mm screen (Daehwa Precision, Cheonan, Korea).
Extraction of prolamins from rice flour
Prolamins were extracted from rice flour with 70% ethanol, as described previously 32 with minor modifications. Rice flour (100 g) was defatted with hexane and dried in a fume hood at room temperature for at least 24 h. The defatted flour was then extracted by stirring in 400 ml distilled water at 20°C for 4 h and centrifuged at 3000 g for 30 min to remove the albumin fraction (supernatant). The residue from this step was then similarly extracted with 400 ml of 5% NaCl at 20°C for 4 h to remove the globulin fraction. The residue after extraction of globulin was extracted with 300 ml of 70% ethanol at 20°C for 4 h to isolate the prolamin fraction. Each extraction step was repeated twice to remove most of the protein fraction. The prolamin fraction was then dialyzed against distilled water, lyophilized and then stored at 4°C. An aliquot of the freeze-dried extract was dissolved in 70% ethanol and its protein concentration was determined with a Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL, USA) and purified 13 kDa prolamin as the standard. The total solids in the prolamin fraction extracted from 100 g rice flour was 0·74 g and the protein content of the solids was 75·5%.
Solubilization of prolamins
Rice prolamins were solubilized for the cell culture study. The prolamin fraction obtained in the previous step was suspended in distilled water (4 mg/ml) and incubated at 55°C for 5 h with shaking. After incubation, the suspension was centrifuged at 3000 g for 30 min and the supernatant was lyophilized and stored at 4°C. The average total solid solubilized from 100 mg prolamin fraction was 30·1 mg and the protein content of the solids was 76·8%.
Cell culture and treatment
The rat intestinal epithelial cell line (RIE) was purchased from the American Type Culture Collection (Manassas, VA, USA). RIE was maintained in Dulbecco's modified Eagle's medium (DMEM) (glucose 4·5 g/l and 11 mg/l of sodium pyruvate; Hyclone, Loan, UT, USA) and supplemented with 10% heat-inactivated fetal bovine serum (FBS; Hyclone), 50 units/ml penicillin (Gibco, Grand Island, NY, USA) and 50 μg/ml streptomycin. RIE was incubated at 37°C in a 5% CO2 incubator. Bone marrow cells were isolated from 5–8-week-old C57/Bl/6 mice (Samtako Science, Daejeon, Korea), as described previously 33. Mice were killed by cervical dislocation. Femora and tibiae were removed aseptically and dissected free of adherent soft tissue. The bone ends were cut, and the marrow cavity was flushed out into a Petri dish by slowly injecting minimum essential medium-α (MEM-α) (Hyclone) at one end of the bone using a sterile 21-gauge needle. The bone marrow suspension was carefully agitated with a plastic Pasteur pipette to obtain a single-cell suspension. Bone marrow cells were washed and depleted of red blood cells (RBCs) by hypotonic lysis using RBC lysing buffer (Sigma-Aldrich, St Louis, MO, USA). After washing twice with phosphate-buffered saline (PBS), the cells were suspended in MEM-α medium supplemented with 10% FBS and 50 units/ml penicillin, 50 μg/ml streptomycin (Gibco). The number of viable cells was determined with Trypan blue (Gibco) and bone marrow cells were cultured on 10 cm2 tissue culture dishes (2 × 106 cells/dish). Mouse macrophage colony-stimulating factor (M-CSF; BioSource, Camarillo, CA, USA) 10 ng/ml was added to every 10 cm2 dish to differentiate BMM. On day 3, non-adherent cells were discarded and adherent cells (immature BMM) were suspended in fresh MEM-α with M-CSF and used in subsequent experiments. All cells were cultured at 37°C in a humidified atmosphere containing 5% CO2. Lipopolysaccharide (LPS) from Escherichia coli (serotype 0111;B4) purchased from Sigma-Aldrich was dissolved in sterile, pyogen-free PBS. NF-κB inhibitor (Bay 11-7082) was obtained from Calbiochem (San Diego, CA, USA) and dissolved in 0·05% dimethyl sulphoxide (DMSO; Sigma-Aldrich). Cells were pretreated with various concentrations of RPE (500–2000 μg/ml) and Bay 11-7082 (5–10 μM) after stimulation with LPS (0·5–10 μg/ml) for designated times. All animal protocols were approved by the Institutional Animal Care and Use Committee of the Chonnam National University Hospital, Gwangju, Korea.
Immunofluorescence
Cells were plated on an eight-chamber slide (Nunc, Rochester, NY, USA). The cells were exposed to LPS (1 μg/ml) for 30 min in the absence or presence of RPE pretreatment. Cells were rinsed in PBS and fixed by incubation with 4% formaldehyde in PBS for 15 min at room temperature. After a further wash with PBS, cells were permeabilized with PBS containing 0·25% Triton X-100. Cells were blocked in PBS containing 1% BSA and 10% goat serum. The cells were incubated with polyclonal anti-RelA antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) in primary antibody dilution buffer overnight at 4°C. After PBS washing, Alexa 488 (green)-coupled secondary antibody (Invitrogen, Carlsbad, CA, USA) was applied for 15 min at room temperature. Coverslips were mounted on the slides using ProLong Gold anti-fade reagent containing 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) and photographed using fluorescent microscopy.
Electrophoretic mobility shift assay (EMSA)
Cells were harvested in 1 × PBS and pelleted (1500 g × 10 s, 4°C). After incubating for 15 min on ice, the cellular suspension lysed in buffer [10 mM HEPES (pH 7·9), 0·5 mM KCl, 1·5 mM MgCl2, 0·5 mM dithiothreitol (DTT), 0·2 mM phenylmethylsulphonylfluoride (PMSF), 0·1% NP40] was centrifuged at 12 000 g for 5 min. The precipitant was centrifuged again after resuspending in high salt buffer [20 mM HEPES pH 7·9, 25% glycerol, 1·5 mM MgCl2, 0·8 M KCl, 0·2 mM ethylenediamine tetraacetic acid (EDTA), 0·5 mM dithiothreitol (DTT), 0·2 mM PMSF]. The protein concentration of nuclear extracts was determined by using a BSA protein assay (Thermo Scientific, Pittsburgh, PA, USA). The nuclear extracts were stored at −70°C. EMSA was performed using the LightShift™ Chemiluminescent EMSA kit (Pierce, Rockford, IL, USA), according to the manufacturer's instructions. Biotin-labelled double-stranded oligonucleotide probe (10 fmol) was added to the reaction mixture containing about 5 μg nuclear extracts for 20 min at room temperature. The sequence of NF-κB/p65 oligonucleotides used for EMSA was 5′-CAT CGG AAA TTT CCG GAA ATT TCC GGA AAT TTC CGG C-3′/5′-GCC GGA AAT TTC TGG AAA TTT CCG GAA ATT TCC AT G-3′. Protein/nucleic acid complexes were resolved using 5% non-denatured polyacrylamide gel and transferred to Biodyne™ B nylon membrane (Thermo Scientific) in 0·5 × Tris–borate–EDTA (TBE) buffer. The biotin-labelled NF-κB/p65 probe was exposed by using a streptavidin–horseradish peroxidase conjugate and chemiluminescence substrate.
Animals and induction of experimental colitis
Male C57/Bl/6 mice (10–12 weeks of age) were purchased from Jungang Lab Animal, Inc. (Seoul, Korea) and were housed in standard steel cages at Chonnam National University Hwasun Hospital Animal Research Resources Center. The mice were maintained at 22°C with a 12:12 h light/dark cycle and allowed to drink and feed ad libitum at all times. RPE was incorporated into standard laboratory chow AIN-76 in different amounts (weight-percentages: 0·05% RPE, 0·1% RPE, 0·2% RPE; Dae Han Biolink, Chungbuk, Korea). Mice (six per group) were prefed RPE-chow (0·05%, 0·1%, 0·2%) or control chow (AIN-76) for 3 days (loading period). After this time, mice were given 3% DSS (MP Biomedicals; Aurora, OH, USA) in drinking water for an additional 6 days. Control mice were given water. Water consumption was comparable between the different groups. Similarly, chow consumption (control AIN76 and BTE) was comparable between DSS and water control groups, both before and during the induction of colitis. This experiment was approved and performed in accordance with the guidelines of the institutional Animal Care and Use Committee of the Chonnam National University Hwasun Hospital, Gwangju, Korea.
Assessment of severity of colitis
Mice were monitored daily for weight loss as well as signs of rectal bleeding (Hemoccult; Beckmann Coulter, Fullerton, CA, USA). At the end of the experiments, mice were killed by cervical dislocation. The entire colon was dissected and flushed with ice-cold PBS. The whole colonic length was measured immediately after killing. Sections of caecum, proximal and distal colon were taken, fixed in 10% neutral-buffered formalin for 24 h at room temperature and embedded in paraffin to provide sections for histological evaluation. Histological sections of mouse colon cut from paraffin blocks were stained with haematoxylin and eosin. In blinded fashion, the histological lesions were scored by two independent observers using a validated scoring system as described previously 34,35.
Real-time reverse transcription–polymerase chain reaction (RT–PCR)
Total RNA was extracted by using TRIzol reagent (Invitrogen) following the standard protocol. Measuring the quantity and purity of total RNA were determined by using a Nanodrop reader (Nanodrop Technologies, Wilmington, DE, USA). Subsequently, 1 μg total RNA was converted to first-strand cDNA using Moloney murine leukaemia virus (MMLV) reverse transcriptase (Invitrogen) and RNAsin (Takara, Otsu, Shiga, Japan). cDNA was amplified using gene-specific primers and GoTaq® DNA polymerase (Promega, Madison, WI, USA). Primer sequences were: monocyte chemotactic protein 1 (MCP-1), 5′-GAAGACCTTAGGGCAGATGCAG-3′/5′-CTGTCATGCTTCTGGGCCTG-3′; IL-6, 5′-GGATACCACCCACAACAGACC-3′/5′-GGTCCTTAGCCACTCCTTCTG-3′; tumour necrosis factor (TNF)-α, 5′- GCACAGAAAGCATGATCCGCG-3′/5′-GACAGAAGAGCGTGGTGGCCC-3′; and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 5′-ACCACAGTCCATGCCATCAC-3′/5′-TCCACCACCCTGTTGCTGTA-3′. GAPDH was used as internal control.
Western blot analysis
The protein concentration of lysates was measured using a quantification assay (Bio-Rad Laboratories, Hercules, CA, USA). Proteins (20 μg) were separated using 10% (13% for caspase analysis) sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. The analysis used primary antibodies as described by the manufacturer of the antibodies; polyclonal anti-IκBα, phospho-IκBα (Cell Signaling Technology, Danvers, MA, USA), phospho-p65 (Santa Cruz Biotechnology) and β-actin (ICN, Costa Mesa, CA, USA). After final rinsing with Tris-buffered saline-Tween (TBST) each membrane was incubated with secondary horseradish peroxidase (HRP)-linked anti-rabbit immunoglobulin (Ig)G for 1 h. After washing, the immunoblots were visualized using chemiluminescence (ECL) HRP substrate (Millipore) and analysed by a Ras-4000 image analyser (Fujifilm, Tokyo, Japan).
Measurements of proinflammatory mediators
Serum were obtained from the mice and stored in liquid nitrogen for MCP-1, IL-6 and TNF-α measurements by avidin–biotin complex (ABC) enzyme-linked immunosorbent assay (ELISA) following the manufacturer's instructions. MCP-1, IL-6 and TNF-α contents were determined on the basis of the optical density value obtained using a model 550 microplate reader (Bio-Rad) and standard curve. The linear regression of the standard curve was 0·998. Each assay was repeated three times, with results expressed as the average.
Immunohistochemical assessment of apoptosis and cell proliferation
Immunohistochemistry for proliferating cell nuclear antigen (PCNA) and cleaved caspase-3-positive nuclei was performed on 4-μm-thick paraffin-embedded sections from the colon harvested from each mouse in each group. Paraffin tissue sections were deparaffinized, rehydrated and retrieved with retrieval buffer. The tissues were treated with a peroxidase-blocking solution (Dako, Carpinteria, CA, USA) to block the endogenous peroxidase activity and were incubated with monoclonal anti-PCNA antibody (Dako, Glostrup, Denmark) or polyclonal anti-cleaved caspase-3 antibody (R&D Systems, Minneapolis, MN, USA) in primary diluent solution (Invitrogen) overnight at 4°C. After washing in TBST, the tissues were stained using Dako Real™ Envision HRP/DAB detection system (Dako). Stained tissues were viewed and photographed under a light microscope. Distinct nuclear PCNA and cleaved caspase-3 immunoreactivity were considered positive. The PCNA and cleaved caspase-3 staining indices were expressed as the mean percentage of the area showing positive staining for PCNA and cleaved caspase-3 relative to the total area of tissue chosen. For each calculation, four representative areas in a section were selected under a light microscope. The number of cells showing a positive reaction for PCNA and cleaved caspase-3 staining was counted in a total of at least 200 cells in five different areas, and expressed as percentage [mean ± standard error of the mean (s.e.m.)].
Statistical analyses
Data are expressed as mean ± s.e.m. Groups of data were analysed using the Kruskal–Wallis non-parametric test, and if the result indicated statistical differences among groups, comparisons between groups were conducted using the Mann–Whitney U-test. In-vitro experiments were conducted in triplicate; representative results are shown. For in-vitro experiments, data were analysed by non-parametric t-test or Wilcoxon's rank sum test where appropriate. A P-value < 0·05 was considered statistically significant.
Results
RPE inhibits the expression of LPS-induced proinflammatory mediators in RIE and BMM
To investigate the impact of RPE on proinflammatory mediator production, we stimulated the RIE and BMM with 1 μg/ml of LPS and treated cells using various concentrations of RPE and measured the expression of proinflammatory mediators. LPS-induced MCP-1, IL-6 and TNF-α mRNA expressions were significantly reduced in the presence of RPE at 1 h in RIE and 4 h in BMM (Fig. 1a,b).
Fig. 1.
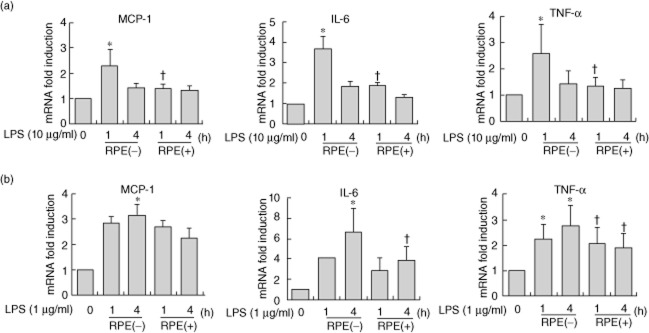
Rice prolamin extract (RPE) inhibits the expression of lipopolysaccharide (LPS)-induced proinflammatory mediators in rat intestinal epithelial cells (RIE) and bone marrow derived macrophage (BMM). RIE (a) and BMM (b) were pretreated with RPE (1000 μg/ml) for 1 h and then stimulated with LPS (10 μg/ml for RIE and 1 μg/ml for BMM) and harvested at 1 or 4 h. RNA was isolated using the TRIzol procedure, and 1 μg of total RNA was reverse-transcribed and amplified using specific primer for monocyte chemotactic protein (MCP)-1, interleukin (IL)-6, tumour necrosis factor (TNF)-α and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). RPE significantly reduced the LPS-induced MCP-1, IL-6, TNF-α mRNA accumulations at 1 h in RIE and 4 h in BMM (*P < 0·05, compared to LPS stimulation).
RPE inhibits LPS-induced NF-κB signalling in RIE and BMM
We investigated the impact of RPE on NF-κB signalling, regulating the production of proinflammatory mediators. Western blotting showed that the LPS-induced inhibitor of NF-κ B (IκBα) phosphorylation/degradation and phosphorylation of NF-κB/p65 were dramatically inhibited by RPE treatment in RIE and BMM (Fig. 2a,b). As the nuclear translocation of NF-κB/p65 follows IκBα phosphorylation/degradation, we tested if RPE perturbed the distribution of NF-κB/p65 as assessed by nuclear accumulation. As shown in Fig. 3, RPE treatment inhibited the nuclear translocation of NF-κB/p65 in all tested cells. Lastly, EMSA was performed on nuclear extracts of RIE and BMM using a consensus oligonucleotide for NF-κB binding. The pharmacological NF-κB inhibitor Bay11-7082 inhibited LPS-induced DNA-binding activity of NF-κB (Fig. 4a,b). RPE treatment inhibited the induction of specific NF-κB binding with NF-κB site by LPS-like Bay11-7082 (Fig. 4a,b). These results suggested that inhibition of LPS-induced proinflammatory mediator production by RPE occurred via the inhibition of NF-κB signalling.
Fig. 2.
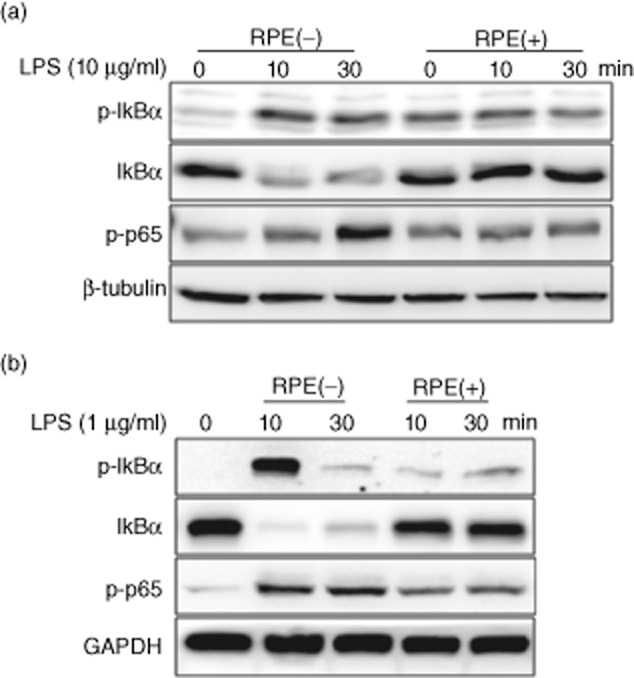
Rice prolamin extract (RPE) inhibits lipopolysaccharide (LPS)-induced IκBα phosphorylation/degradation and phosphorylation of nuclear factor (NF)-κB/p65 in rat intestinal epithelial cells (RIE) and bone marrow-derived macrophage (BMM). RIE (a) and BMM (b) were pretreated for 1 h with RPE (1000 μg/ml) and then stimulated with LPS (10 μg/ml for RIE and 1 μg/ml for BMM) for 10 and 30 min. IκBα phosphorylation/degradation and phosphorylation of NF-κB/p65 were evaluated by Western blotting. The LPS-induced IκBα phosphorylation/degradation and phosphorylation of NF-κB/p65 were inhibited by co-incubation of LPS with RPE.
Fig. 3.
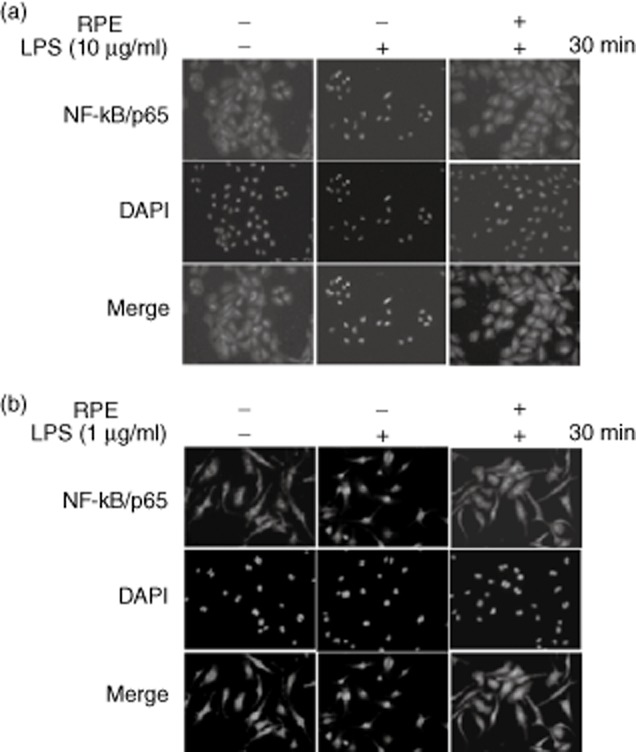
Rice prolamin extract (RPE) inhibits the nuclear translocation of nuclear factor (NF)-κB/p65 in RIE and BMM. RIE (a) and BMM (b) were pretreated for 1 h with RPE (1000 μg/ml) and then stimulated with lipopolysaccharide (LPS) (10 μg/ml for RIE and 1 μg/ml for BMM) for 30 min. Nuclear translocation of NF-κB/p65 was visualized by immunofluorescence. Co-incubation of LPS with RPE inhibited the nuclear translocation of NF-κB/p65. RPE, rice prolamin extract; RIE, rat intestinal epithelial cell; BMM, bone marrow-derived macrophage.
Fig. 4.
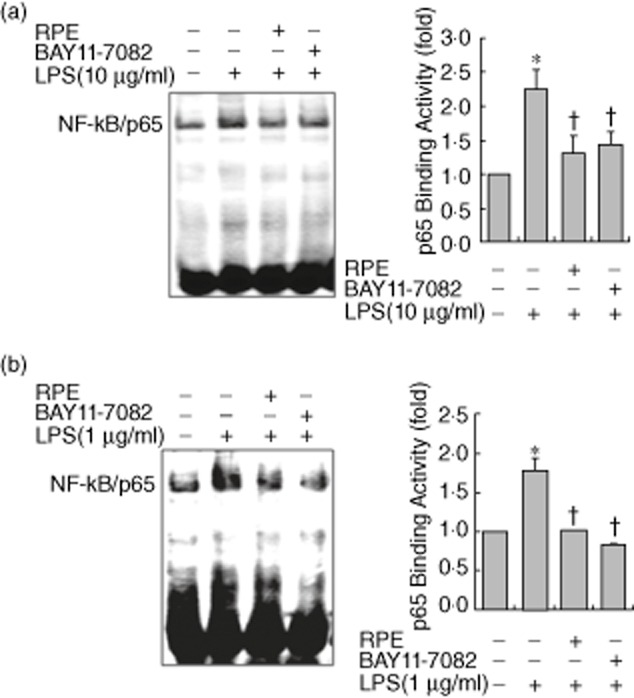
Rice prolamin extract (RPE) inhibits LPS-induced DNA-binding activity of nuclear factor (NF)-κB/p65 in RIE and BMM. RIE (a) and BMM (b) were pretreated for 1 h with RPE (1000 μg/ml) or Bay11-7082 (5 μM) and then stimulated with lipopolysaccharide (LPS) (10 μg/ml for RIE and 1 μg/ml for BMM) for 30 min. The DNA binding activity of NF-κB was evaluated by electrophoretic mobility shift assay (EMSA). RPE and Bay11-7082 inhibited LPS-induced NF-κB DNA-binding activity in RIE and BMM. Bars represent the mean ± standard error of the mean from three different experiments (*P < 0·05, compared to unstimulated cells; †P < 0·05, compared to LPS stimulation). RPE, rice prolamin extract; RIE, rat intestinal epithelial cell; BMM, bone marrow-derived macrophage.
RPE ameliorates DSS-induced experimental colitis
We tested the therapeutic efficacy of RPE in a murine model of chemical-induced acute intestinal inflammation. Mice were fed RPE chow (0·05–0·2%) or control chow (AIN-76) for 3 days before being exposed to 3% DSS in drinking water ad libitum or tapwater control. Mice were then killed after an additional 6 days and inflammation was evaluated using clinical and histological parameters. RPE-fed, DSS-exposed mice showed less significant weight loss than control diet-fed, DSS-exposed mice (P < 0·05) (Fig. 5). RPE-fed, DSS-exposed mice had significantly longer colons than control diet-fed, DSS-exposed mice (P < 0·05) (Fig. 6). Histological score of colon of RPE-fed, DSS-exposed mice indicated a significant decrease in colon inflammation compared to control diet-fed, DSS-exposed mice (P < 0·05) (Fig. 7). Serum levels of MCP-1, IL-6 and TNF-α were quantified by ELISA. RPE-fed, DSS-exposed mice showed the lower level of MCP-1, IL-6 and TNF-α than those of control diet-fed, DSS-exposed mice (P < 0·05) (Fig. 8).
Fig. 5.
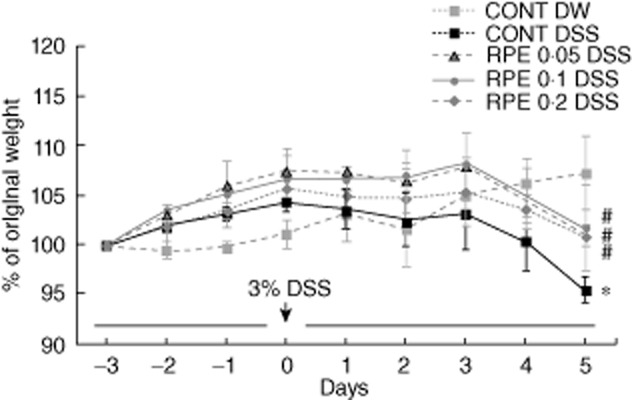
Rice prolamin extract (RPE) ameliorates DSS-induced experimental colitis as measured by weight loss. Mice were prefed RPE-chow (0·05, 0·1, 0·2%) or control chow (CONT) (AIN-76) for 3 days (loading period). After this time, mice were given 3% dextran sulphate sodium (DSS) in drinking water for an additional 6 days. Weight loss in response to DSS is presented as a percentage of the starting weight (*P < 0·05 versus control; †P < 0·05 versus DSS alone). RPE-fed, DSS-exposed mice showed less significant weight loss than control diet-fed, DSS-exposed mice by day 6 (P < 0·05). Values are mean ± standard error of the mean; n = 6 in each group.
Fig. 6.
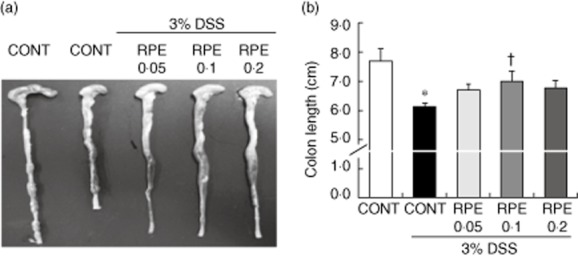
Rice prolamin extract (RPE) ameliorates dextran sulphate sodium (DSS)-induced experimental colitis as measured by colon length. (a) Mice (six per group) were killed at designated time-points, the entire colon was dissected and then directly imaged in a light imaging box. (b) RPE-fed, DSS-exposed mice had significantly longer colons than control diet-fed, DSS-exposed mice (*P < 0·05 versus control; †P < 0·05 versus DSS alone). CONT = control chow.
Fig. 7.
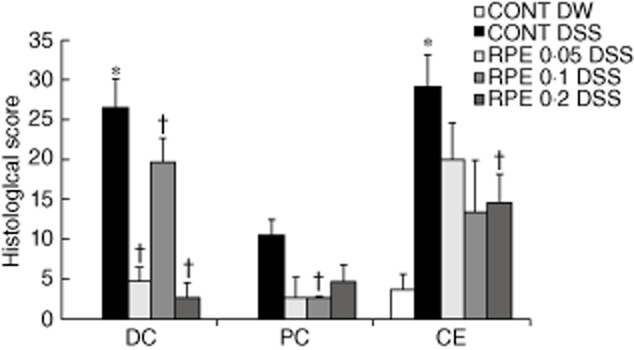
Rice prolamin extract (RPE) ameliorates dextran sulphate sodium (DSS)-induced experimental colitis as measured by histological score. The proximal, distal colon and caecum of DSS-exposed mice were evaluated and coded sections were scored using a validated scoring system with a scale of 0–40. Values are mean ± standard error of the mean; n = 6 in each group. Histological score of colon of RPE-fed, DSS-exposed mice indicated a significant decrease in colon inflammation compared to control diet-fed, DSS-exposed mice (*P < 0·05 versus control; †P < 0·05 versus DSS alone).
Fig. 8.
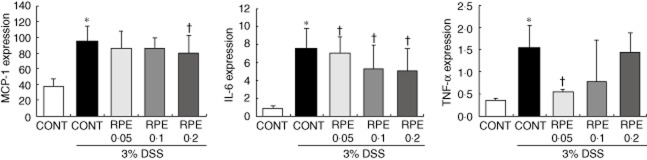
Rice prolamin extract (RPE) reduces serum levels of proinflammatory mediators in dextran sulphate sodium (DSS)-induced experimental colitis. Serum levels of monocyte chemotactic protein (MCP)-1, interleukin (IL)-6 and tumour becrosis factor (TNF)-α were quantified by enzyme-linked immunosorbent assay (ELISA). RPE-fed, DSS-exposed mice showed the lower level of MCP-1, IL-6 and TNF-α than those of control diet-fed, DSS-exposed mice (*P < 0·05 versus control; †P < 0·05 versus DSS alone). Values are mean ± standard error of the mean; n = 6 in each group; CONT = control chow.
Impact of RPE on apoptosis and cell proliferation following DSS-induced intestinal injury
A balance of apoptosis and cell proliferation determines normal tissue homeostasis. A key feature of intestinal homeostasis is the host's ability to maintain the integrity of the epithelium and promote repair mechanisms following various injury insults. Therefore, we reasoned that RPE could decrease intestinal epithelial cell apoptosis in response to DSS-induced injury. Because molecular alterations probably precede clinical signs of colitis and histopathological evidence of inflammation, which occurred at approximately days 4–5 in our model, signs of apoptosis and cell proliferation were evaluated at day 3 of DSS. In general, apoptotic cells were found at the surface of the epithelium. Interestingly, immunohistochemical analysis showed a decrease of activated caspase-3-positive cells in RPE-fed, DSS-exposed mice, compared to control diet-fed, DSS-exposed mice (Fig. 9a). We next tested the impact of RPE on intestinal epithelial cell proliferation in response to DSS-induced injury. In RPE-fed, DSS-exposed mice, PCNA-positive cells were located mainly in the lower third of the crypts, whereas in control diet-fed, DSS-exposed mice, PCNA-positive cells were observed in the lower to middle third of the crypts. Expression of the proliferative marker PCNA within intact colonic crypts decreased in RPE-fed, DSS-exposed mice, compared to control diet-fed, DSS-exposed mice (Fig. 9b). These findings indicate that RPE treatment attenuates colon inflammation through the modulation of intestinal apoptosis and cell proliferation.
Fig. 9.
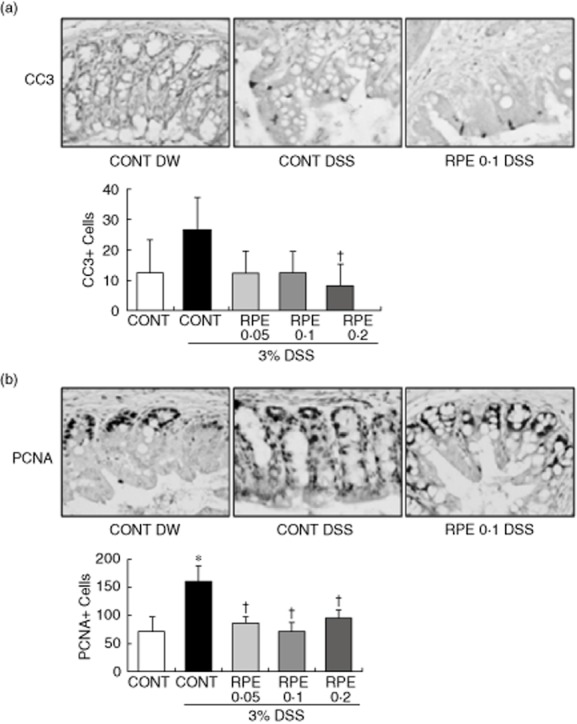
Impact of rice prolamin extract (RPE) on apoptosis and cell proliferation following dextran sulphate sodium (DSS)-induced intestinal injury. Immunohistochemical analyses of proliferating cell nuclear antigen (PCNA) and cleaved caspase-3 staining were performed in the colons harvested from mice. (a) Cleaved caspase-3 immunoreactivity was found predominantly in the nuclei of cells. Cleaved caspase 3-positive cells in RPE-fed, dextran sulphate sodium (DSS)-exposed mice were decreased, compared to control diet-fed, DSS-exposed mice (†P < 0·05 versus DSS alone). (b) PCNA immunoreactivity was found predominantly in the nuclei of cells. RPE-fed, DSS-exposed mice showed significant down-regulation of PCNA staining, compared to control diet-fed, DSS-exposed mice (*P < 0·05 versus control; †P < 0·05 versus DSS alone). CONT = control chow.
RPE inhibits NF-κB activation in DSS-induced experimental colitis
We explored whether anti-inflammatory effect of RPE in response to DSS-induced intestinal injury is associated with inhibition of NF-κB activity. Western blotting showed that DSS-induced IκBα phosphorylation/degradation and phosphorylation of NF-κB/p65 was blocked by RPE treatment (Fig. 10). These findings indicate that RPE treatment attenuates acute colon inflammation by inhibiting NF-κB activation.
Fig. 10.
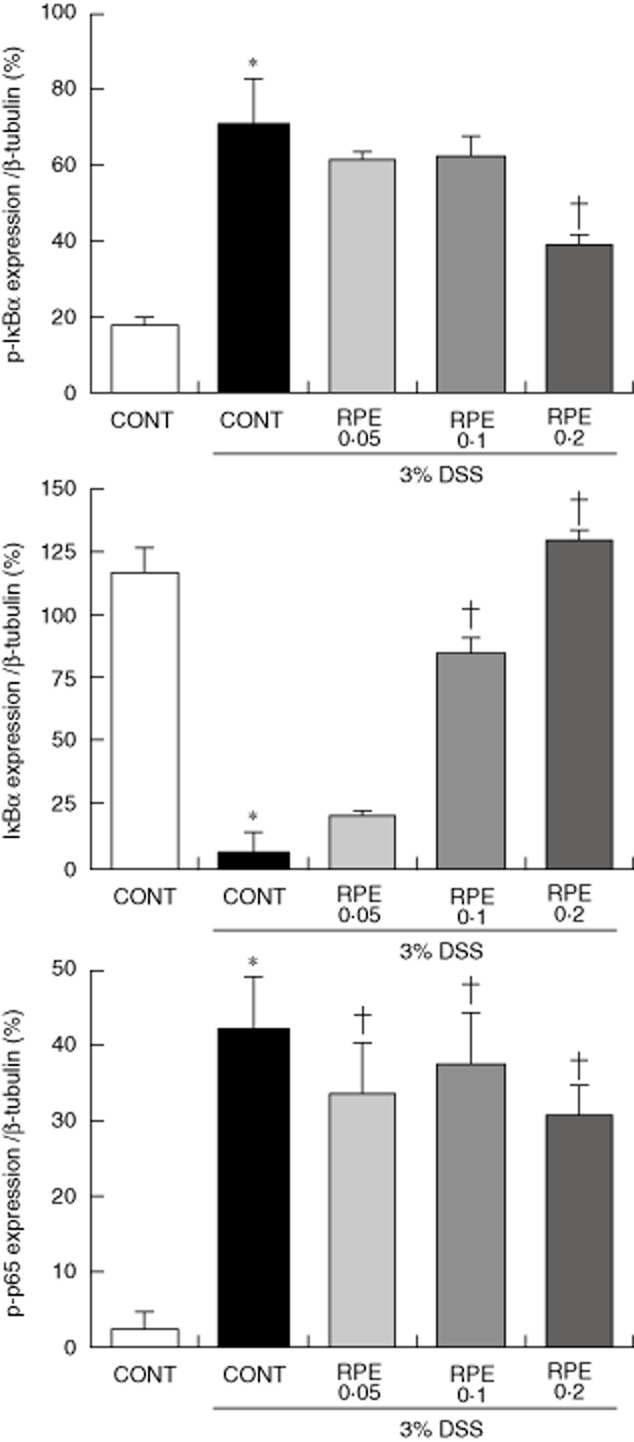
Rice prolamin extract (RPE) inhibits nuclear factor (NF)-κB/p65 activation in dextran sulphate sodium (DSS)-induced experimental colitis. Mice (n = 6 per group) were killed after 6 (for total colon protein) days of 3% DSS exposure, respectively, and protein (20 μg) was subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). Western blotting for IκBα, phospho-IκBα, phospho-p65 and β-tubulin was performed. Band intensities were quantified by densitometry. Results are representative of three independent experiments. DSS-induced IκBα phosphorylation/degradation and phosphorylation of NF-κB/p65 were blocked by RPE (*P < 0·05 versus control; †P < 0·05 versus DSS alone). CONT, control chow.
Discussion
The majority of proteins in rice grain are found in the rice bran fraction. Rice bran proteins are mainly storage proteins, and storage proteins influence nutritional quality for humans and their functional properties are important to the utilization of cereal grain 3,4. Four protein fractions in rice bran have been classified by solubility, aggregation and molecular weight protein, as follows: water-soluble albumin, salt-soluble globulin, alkali-soluble glutelin and alcohol-soluble prolamin. Solubility and aggregation properties would aid in choosing the extraction solvent and the molecular weight data would help in the identification of the extracted protein 3,4. The extract condition of each rice protein fraction must be optimized for high protein recovery efficiency because the development of denaturation of rice protein for extraction processing may influence the nutritional and pharmaceutical application of rice protein. Until now, several different methods have been developed to extract each rice protein fraction. Generally, rice proteins are extracted from defatted rice flour 32. In our study, rice prolamin was extracted effectively from defatted rice flour by using 70% ethanol as solvent, and did not show any aggregation and thermographic denaturation during extraction processing.
Previous studies have clarified that rice and rice fraction mediate anti-inflammatory effects by down-regulating NF-κB, which in turn reduces expression of inflammatory enzymes and proinflammatory cytokines in human peripheral blood mononuclear cells, aortic endothelial cells and mouse macrophage cells 36–38. Recently, rice prolamin has been shown to be effective as an active rice ingredient capable of activating anti-leukaemia immunity in human leukaemia U937 cells 31. Therefore, rice prolamin may have anti-inflammatory activity. However, until now the direct effects of RPE on NF-κB signalling in intestinal inflammation have not been investigated. Therefore, to identify the bioactive ingredients responsible for the biological response modification of rice and rice fraction, this study isolated prolamin fraction from rice and examined its effect on anti-inflammatory activity.
First, we investigated the possible anti-inflammatory activity of RPE by analysing the effect on the expression of proinflammatory mediators and NF-κB signalling in both LPS-stimulated non-immune (RIE) and immune cells (BMM). RPE profoundly inhibited the production of proinflammatory mediators, including MCP-1, IL-6 and TNF-α, in LPS-stimulated RIE and BMM. Also, RPE inhibited IκBα phosphorylation/degradation, NF-κB/p65 nuclear translocation and DNA-binding activity of NF-κB in LPS-stimulated RIE and BMM. The pharmacological NF-κB inhibitor inhibited DNA-binding activity of NF-κB in LPS-stimulated RIE and BMM like RPE. Therefore, RPE has an anti-inflammatory activity in LPS-stimulated RIE and BMM by virtue of its ability to reduce the production of proinflammatory mediators via the blockage of NF-κB signalling.
Previously, rice and rice fractions including fermented brown rice, enzyme-treated rice fibre and phytosteryl ferulates ameliorated intestinal inflammation in a DSS-induced experimental colitis model 27–29. Next, to assess the therapeutic efficacy of RPE on intestinal inflammation, we used a DSS-induced experimental colitis model. In our study, RPE-fed, DSS-exposed mice displayed significantly less weight loss, longer colons and lower histological score than control diet-fed, DSS-exposed mice. Eventually, RPE ameliorated intestinal inflammation in DSS-induced experimental colitis model. We explored whether the anti-inflammatory activity of RPE in DSS-induced experimental colitis is associated with blocking of NF-κB signalling. DSS-induced IκBα phosphorylation/degradation and phosphorylation of NF-κB/p65 in colonic tissues were blocked by RPE.
Apoptosis and cell proliferation must be properly balanced to maintain normal homeostasis. However, colitis development and progression result from the imbalance between apoptosis and cell proliferation 39,40. Thus, we sought to investigate the impact of RPE on intestinal apoptosis and cell proliferation following DSS-induced colitis. A significant decrease of caspase-3-activated positive cells was evident in RPE-fed, DSS-exposed mice compared to DSS-exposed mice, indicating an inhibitory effect of RPE apoptosis. Also, expression of the proliferative marker, PCNA, within intact colonic crypts decreased significantly in RPE-fed, DSS-exposed mice compared to control diet-fed, DSS-exposed mice. These findings suggest that RPE treatment may improve intestinal epithelial response to DSS-induced injury.
In conclusion, RPE treatment blocked the production of LPS-induced proinflammatory mediators and NF-κB activation in RIE and BMM. RPE treatment ameliorated intestinal inflammation, inhibited NF-κB signalling and affected intestinal apoptosis and cell proliferation in DSS-induced experimental colitis. Therefore, RPE treatment ameliorates intestinal inflammation by inhibiting NF-κB activation and modulating intestinal apoptosis and cell proliferation.
Acknowledgments
This study was supported by a grant (110116-3) from High Value-added Food Technology Development Program, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
Disclosure
All authors declare that they have no conflicting interests.
Author contributions
C. Y. C., Y. L. P. and N. K. carried out the study and designed the experiments. D. S. M. and J. S. K. contributed reagents/materials/analysis tools. C. Y. C. and Y. E. J. analysed the data. B. W. A. and H. S. K. supervised work and corrected the manuscript. Y. E. J. conceived and designed experiments and wrote the manuscript. All authors read and approved the final manuscript.
References
- 1.Shewry PR, Halford NG. Cereal seed storage proteins: structures, properties and role in grain utilization. J Exp Bot. 2002;53:947–958. doi: 10.1093/jexbot/53.370.947. [DOI] [PubMed] [Google Scholar]
- 2.Khush GS. Origin, dispersal, cultivation and variation of rice. Plant Mol Biol. 1997;35:25–34. [PubMed] [Google Scholar]
- 3.Mills EN, Jenkins JA, Alcocer MJ, Shewry PR. Structural, biological, and evolutionary relationships of plant food allergens sensitizing via the gastrointestinal tract. Crit Rev Food Sci Nutr. 2004;44:379–407. doi: 10.1080/10408690490489224. [DOI] [PubMed] [Google Scholar]
- 4.Fabian C, Ju YH. A review on rice bran protein: its properties and extraction methods. Crit Rev Food Sci Nutr. 2011;51:816–827. doi: 10.1080/10408398.2010.482678. [DOI] [PubMed] [Google Scholar]
- 5.Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Adv Data. 2004;343:1–19. [PubMed] [Google Scholar]
- 6.Joo YE. Natural product-derived drugs for the treatment of inflammatory bowel diseases. Intest Res. 2014;12:103–109. doi: 10.5217/ir.2014.12.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Islam MS, Nagasaka R, Ohara K, et al. Biological abilities of rice bran-derived antioxidant phytochemicals for medical therapy. Curr Top Med Chem. 2011;11:1847–1853. doi: 10.2174/156802611796235099. [DOI] [PubMed] [Google Scholar]
- 8.Ishizone S, Maruta F, Suzuki K, et al. In vivo bactericidal activities of Japanese rice-fluid against H. pylori in a Mongolian gerbil model. Int J Med Sci. 2007;4:203–208. doi: 10.7150/ijms.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murakami M, Ota H, Sugiyama A, et al. Suppressive effect of rice extract on Helicobacter pylori infection in a Mongolian gerbil model. J Gastroenterol. 2005;40:459–466. doi: 10.1007/s00535-005-1570-7. [DOI] [PubMed] [Google Scholar]
- 10.Matsuhashi T, Otaka M, Odashima M, et al. Protective effect of a novel rice extract against ethanol-induced gastric mucosal injury in rat. Dig Dis Sci. 2007;52:434–441. doi: 10.1007/s10620-006-9571-9. [DOI] [PubMed] [Google Scholar]
- 11.Jayaraj AP, Tovey FI, Clark CG, Rees KR, White JS, Lewin MR. The ulcerogenic and protective action of rice and rice fractions in experimental peptic ulceration. Clin Sci (Lond) 1987;72:463–466. doi: 10.1042/cs0720463. [DOI] [PubMed] [Google Scholar]
- 12.Tomita H, Kuno T, Yamada Y, et al. Preventive effect of fermented brown rice and rice bran on N-methyl-N′-nitro-N-nitrosoguanidine-induced gastric carcinogenesis in rats. Oncol Rep. 2008;19:11–15. [PubMed] [Google Scholar]
- 13.Kawabata K, Tanaka T, Murakami T, et al. Dietary prevention of azoxymethane-induced colon carcinogenesis with rice-germ in F344 rats. Carcinogenesis. 1999;20:2109–2115. doi: 10.1093/carcin/20.11.2109. [DOI] [PubMed] [Google Scholar]
- 14.Kuno T, Hirose Y, Yamada Y, et al. Chemoprevention of mouse urinary bladder carcinogenesis by fermented brown rice and rice bran. Oncol Rep. 2006;15:533–538. [PubMed] [Google Scholar]
- 15.Kuno T, Hirose Y, Hata K, et al. Preventive effect of fermented brown rice and rice bran on N-nitrosomethylbenzylamine induced esophageal tumorigenesis in rats. Int J Oncol. 2004;25:1809–1815. [PubMed] [Google Scholar]
- 16.Katayama M, Sugie S, Yoshimi N, et al. Preventive effect of fermented brown rice and rice bran on diethylnitrosoamine and phenobarbital-induced hepatocarcinogenesis in male F344 rats. Oncol Rep. 2003;10:875–880. [PubMed] [Google Scholar]
- 17.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 18.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Podolsky DK, Xavier RJ. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 20.Dharmani P, Chadee K. Biologic therapies against inflammatory bowel disease: a dysregulated immune system and the cross talk with gastrointestinal mucosa hold the key. Curr Mol Pharmacol. 2008;1:195–212. doi: 10.2174/1874467210801030195. [DOI] [PubMed] [Google Scholar]
- 21.Karrasch T, Jobin C. NF-κ B and the intestine: friend or foe? Inflamm Bowel Dis. 2007;14:114–124. doi: 10.1002/ibd.20243. [DOI] [PubMed] [Google Scholar]
- 22.Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-κB. Cell Death Differ. 2006;13:759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- 23.Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence T, Bebien M, Liu GY, Nizet V, Karin M. IKKα limits macrophage NF-κB activation and contributes to the resolution of inflammation. Nature. 2005;434:1138–1143. doi: 10.1038/nature03491. [DOI] [PubMed] [Google Scholar]
- 25.Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 26.Lorenz RG, McCracken VJ, Elson CO. Animal models of intestinal inflammation: ineffective communication between coalition members. Springer Semin Immunopathol. 2005;27:233–247. doi: 10.1007/s00281-005-0208-4. [DOI] [PubMed] [Google Scholar]
- 27.Kataoka K, Ogasa S, Kuwahara T, et al. Inhibitory effects of fermented brown rice on induction of acute colitis by dextran sulfate sodium in rats. Dig Dis Sci. 2008;53:1601–1608. doi: 10.1007/s10620-007-0063-3. [DOI] [PubMed] [Google Scholar]
- 28.Islam MS, Murata T, Fujisawa M, et al. Anti-inflammatory effects of phytosteryl ferulates in colitis induced by dextran sulphate sodium in mice. Br J Pharmacol. 2008;154:812–824. doi: 10.1038/bjp.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komiyama Y, Andoh A, Fujiwara D, et al. New prebiotics from rice bran ameliorate inflammation in murine colitis models through the modulation of intestinal homeostasis and the mucosal immune system. Scand J Gastroenterol. 2011;46:40–52. doi: 10.3109/00365521.2010.513062. [DOI] [PubMed] [Google Scholar]
- 30.Onda Y, Kawagoe Y. Oxidative protein folding: selective pressure for prolamin evolution in rice. Plant Signal Behav. 2011;6:1966–1972. doi: 10.4161/psb.6.12.17967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen YJ, Chen YY, Wu CT, Yu CC, Liao HF. Prolamin, a rice protein, augments anti-leukaemia immune response. J Cereal Sci. 2010;51:189–197. [Google Scholar]
- 32.Ju ZY, Hettiarachchy NS, Rath N. Extraction, denaturation and hydrophobic properties of rice flour proteins. J Food Sci. 2001;66:229–232. [Google Scholar]
- 33.Chambers TJ, Owens JM, Hattersley G, Jat PS, Noble MD. Generation of osteoclast-inductive and osteoclastogenic cell lines from the H-2KbtsA58 transgenic mouse. Proc Natl Acad Sci USA. 1993;90:5578–5582. doi: 10.1073/pnas.90.12.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 35.Williams KL, Fuller CR, Dieleman LA, et al. Enhanced survival and mucosal repair after dextran sodium sulfate-induced colitis in transgenic mice that overexpress growth hormone. Gastroenterology. 2001;120:925–937. doi: 10.1053/gast.2001.22470. [DOI] [PubMed] [Google Scholar]
- 36.Kumar KS, Vijayan V, Bhaskar S, Krishnan K, Shalini V, Helen A. Anti-inflammatory potential of an ethyl acetate fraction isolated from Justicia gendarussa roots through inhibition of iNOS and COX-2 expression via NF-κB pathway. Cell Immunol. 2012;272:283–289. doi: 10.1016/j.cellimm.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 37.Lin CP, Lin YL, Huang PH, Tsai HS, Chen YH. Inhibition of endothelial adhesion molecule expression by Monascus purpureus-fermented rice metabolites, monacolin K, ankaflavin, and monascin. J Sci Food Agric. 2011;91:1751–1758. doi: 10.1002/jsfa.4371. [DOI] [PubMed] [Google Scholar]
- 38.Park HJ, Han ES, Park DK, Lee C, Lee KW. An extract of Phellinus linteus grown on germinated brown rice inhibits inflammation markers in RAW264·7 macrophages by suppressing inflammatory cytokines, chemokines, and mediators and up-regulating antioxidant activity. J Med Food. 2010;13:1468–1477. doi: 10.1089/jmf.2010.1131. [DOI] [PubMed] [Google Scholar]
- 39.Kiechle FL, Zhang X. Apoptosis: biochemical aspects and clinical implications. Clin Chim Acta. 2002;326:27–45. doi: 10.1016/s0009-8981(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 40.Kenneth NS, Duckett CS. IAP proteins: regulators of cell migration and development. Curr Opin Cell Biol. 2012;24:871–875. doi: 10.1016/j.ceb.2012.11.004. [DOI] [PubMed] [Google Scholar]


