Abstract
Objectives
1) To determine whether pRIFLE (Pediatric Risk, Injury, Failure, Loss, End Stage Renal Disease) criteria serves to characterize the pattern of Acute Kidney Injury (AKI) in critically ill pediatric patients; and 2) to identify if pRIFLE score will predict morbidity and mortality in our patient´s cohort.
Design
Prospective Cohort.
Setting
Multidisciplinary, tertiary care, 10- bed PICU.
Patients
266 patients admitted to PICU from November 2009 to November 2010.
Interventions
None.
Measurements and Main Results
The incidence of AKI in the PICU was 27.4%, of which 83.5% presented within 72hrs of admission to the PICU. Patients with AKI were younger, weighed less, were more likely to be on in fluid overload ≥10%, and were more likely to be on inotropic support, diuretics or amino glycosides. No difference in gender, use of other nephrotoxins, or mechanical ventilation was observed. Fluid overload ≥10% was an independent predictor of morbidity and mortality.
In multivariate analysis, AKI-Injury and Failure categories, as defined by pRIFLE, predicted mortality, hospital length of stay, and PICU length of stay.
Conclusions
In this cohort of critically ill pediatric patients, AKI identified by pRIFLE and fluid overload ≥ 10% predicted increased morbidity and mortality. Implementation of pRIFLE scoring and close monitoring of fluid overload upon admission may help develop early interventions to prevent and treat AKI in critically ill children.
Keywords: Acute kidney injury; pRIFLE, children; fluid overload; outcome
Introduction
Acute renal failure is a frequent clinical complication in critical care that is constituted by an acute drop in renal function, with manifestations ranging from minimal elevation of serum creatinine concentration, to anuric renal failure. Recognizing the need for a more uniform definition, the Acute Dialysis Quality Initiative Group, consisting of specialists in adult and pediatric nephrology and critical care, established a system allowing for the classification of acute renal failure severity.1,2, Through this workgroup, the term Acute Kidney Injury (AKI) was introduced, and a new classification system, termed the RIFLE criteria, was proposed for use in critically ill adult patients.2 RIFLE (acronym for Risk of renal dysfunction, Injury to the kidney, Failure of kidney function, Loss of kidney function, and End-stage Renal Disease) stratifies patients based on changes in serum creatinine (Scr) levels from baseline and/or a decrease in urine output (Uop)3. The RIFLE criteria have been adopted by most intensive care units and nephrology societies as a way to define AKI, and they have been shown to independently predict hospital length of stay, healthcare costs, morbidity, and mortality in adult and pediatric patients4,5,6,7.
Prospective pediatric studies of AKI are limited.4,8 Recently, Ackan-Arikan et al developed a modified version of the RIFLE criteria for pediatric patients, called pRIFLE, shown in Table 1. Their proposed pRIFLE criteria are based on a decrease in estimated creatinine clearance (eCCl) and in urine output (Uop) based on weight. In their cohort, 82% of 150 mechanically ventilated pediatric patients developed AKI as diagnosed by pRIFLE, and they did so predominantly within the first week of admission to the Pediatric Intensive Care Unit (PICU). The mortality of these patients was 14.6%, compared to 11.1% for those with normal renal function.4. Plotz et al described a smaller retrospective cohort that identified AKI diagnosed by pRIFLE to be associated with higher mortality9. No other risk factors such as nephrotoxins or fluid overload, were evaluated in this study.
Table1.
pRIFLE Classification
| Category | Estimated Creatinine Clearance* | Urine Output |
|---|---|---|
| Risk (R) | Decrease by 25% | < 0.5 mL/kg/hr for 8 h |
| Injury (I) | Decrease by 50% | < 0.5 mL/kg/hr for 16 h |
| Failure (F) | Decrease by 75% or < 35 mL/min/1.73 m2 | < 0.3 mL/kg/hr for 24 hr or anuric for 12 h |
| Loss (L) | Loss of renal function > 4 weeks | |
| End-Stage (E) | End Stage Renal Disease | |
Calculated with Schwartz equation: Length (cm) × K (constant) / serum creatinine
Fluid overload is emerging as an important marker of morbidity and mortality in critically ill patients, especially in patients requiring continuous renal replacement therapy.10,11 Fluid overload (FO%), defined as: [(Total fluid in (L) – Total fluid out (L)) / admission weight (kg)] × 100], has been found to be an important predictive factor in critically ill children. Higher FO% has been associated with higher mortality in patients receiving continuous renal replacement therapy, with higher oxygenation index, and longer length of stay10,11,12,13. However, there are very few prospective studies that assess risk factors for AKI, fluid overload, and the utility of the pRIFLE criteria for identifying AKI in a general pediatric intensive care population. Most studies are retrospective, and in patients with very high morbidity and mortality (mechanical ventilation, inotropes, post-transplant, post-cardiovascular surgery)8.
The goals of this prospective study were to: 1) determine whether the pRIFLE criteria serve to characterize patterns of AKI in critically ill pediatric patients in a Level 1 PICU; 2) determine the prevalence, demographic, and clinical characteristics of AKI in our cohort; 3) establish the association between fluid overload, AKI, and mortality in our population; and 4) identify whether pRIFLE scoring can predict morbidity (hospital and PICU length of stay) and mortality in our patients.
Materials and Methods
A prospective analysis of patients admitted to a multidisciplinary, academic, tertiary care PICU in San Juan, Puerto Rico, between November 2009 and November 2010, was performed. This 10 bed PICU cares for medical, surgical, and trauma patients, as well as patients from all pediatric subspecialties. It does not have immediate post solid organ transplant or bone marrow transplant patients, or immediate post-op cardiovascular surgery patients. Inclusion criteria for the study were: admission to the PICU for 24 hours or more, and age between 1 month and 21 years. All patients with preexisting end stage renal disease or renal transplantation were excluded. In patients with multiple admissions to the PICU during the same hospital admission, only the first PICU admission was taken into account. The Institutional Review Board (IRB) of the University of Puerto Rico Medical Sciences Campus (UPRMSC), School of Medicine (SoM) approved the study protocol.
Demographic, laboratory, and clinical data, including urine output and serum creatinine, were collected daily for the first 14 days of admission to the PICU. Estimated creatinine clearance (eCCl) was calculated using the Schwartz formula (Table 1). For baseline eCCl, the patient was assumed to have normal renal function and assigned a baseline eCCl of 120 mL/min/1.73 m2. Patients were assigned to the appropriate pRIFLE classification (Table 1) if criteria for urine output, serum creatinine, or both, were fulfilled. If both eCCl and Uop criteria were met, the more severe of the pRIFLE strata achieved was utilized for analysis. Hospital length of stay, PICU length of stay, and mortality, were also recorded. The primary outcome measure was mortality, and the secondary outcomes were PICU length of stay and hospital length of stay, which were classified as measures of morbidity.
To calculate fluid overload, the previously published equation by Goldstein et al11 was used: [(Total fluid in (L) – Total fluid out (L)) / admission weight (kg)] × 100. A score of 10% or more was considered to be indicative of fluid overload.
Statistical Analysis
The statistical software STATA version 11.0 (STATA Corp., College Station, TX, USA) was used to performed the statistical analyses. Univariate analysis was done using measures of central tendency and dispersion (mean, standard deviation, median, and range) for continuous variables. Categorical variables were described using frequencies and proportions. Bivariate analysis was done using the unpaired t test (or Mann-Whitney U test as applicable) for continuous variables; categorical variables were evaluated using Pearson chi-square (or Fisher’s exact test). To evaluate the risk of AKI with the study outcomes (hospital and PICU length of stay, and mortality), contingency tables and odd ratios (OR) with their 95% confidence intervals (CI) were calculated. Finally, two main analyses were performed to evaluate the risk of AKI with each study outcome. In the first analysis, the definition of AKI included patients categorized as Risk, Injury and Failure. The second analysis defined AKI as patients with Injury and Failure, and the patients categorized as AKI Risk were included in the No-AKI group. Each analysis was performed using unconditional logistic regression, and three models were constructed: 1) an unadjusted model, 2) an age-adjusted model, and 3) a multivariate model adjusting for age, fluid overload, and PRISM score. In addition, the role of fluid overload as a predictor of outcome (hospital and PICU length of stay, and mortality) was also evaluated. The statistical significance was set at p<0.05.
Results
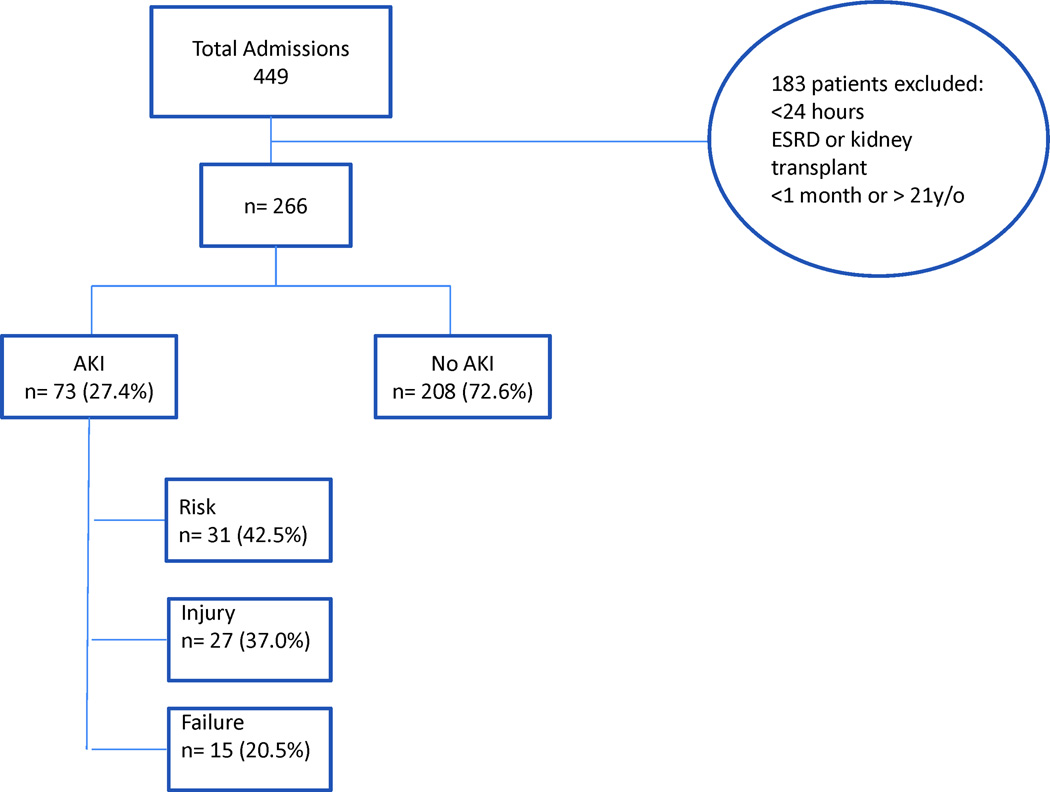
A total of 449 patient admissions were recorded in the PICU during the 12 month study period; 183 patients were excluded, resulting in a total of 266 admissions evaluated (Figure 1). The mean age of patients was 7.0 years (range from 1 month to 19.9 years of age). Demographic data is shown in Table 2. Acute Kidney Injury was diagnosed in 73 (27.4%) patients in the PICU using the pRIFLE score. Sixty five patients (89%) of those diagnosed with AKI (n=73) in the PICU fulfilled pRIFLE criteria by eCCl; eight (11%); of those patients with AKI (n=73) fulfilled pRIFLE criteria by Uop during the study period. The admission diagnoses of all patients (AKI and no-AKI) were classified according to etiology. Most patients were classified under the category of respiratory diagnoses, followed by neurologic diagnoses, as illustrated in Table 3.
Figure 1.
Diagram of admissions during study period and incidence of Acute Kidney Injury (AKI)
Table 2.
Demographic Data and Clinical Characteristics of Study Population
| Variable | Study population (N= 266) |
No AKI (n= 193) |
AKI (n= 73) |
p-value |
|---|---|---|---|---|
| Age, yrs | ||||
| Mean (SD) | 7.0 (6.0) | 8.4 (5.8) | 3.5 (5.1) | <0.001 |
| Median (range) | 6.0 (0–19) | 8.0 (0–19) | 0.7 (0–18) | |
| Weight, Kg | ||||
| Mean (SD) | 27.8 (22.0) | 32.1 (20.9) | 16.3 (20.9) | <0.001 |
| Median (range) | 20.0 (1.8–93) | 28.0 (1.9–90) | 7.3 (3.0–93) | |
| Sex: Male | 156 (58.7) | 108 (56.0) | 48 (65.8) | 0.148 |
| Insurance | 0.086 | |||
| Private | 59 (22.2) | 48 (24.9) | 11 (15.1) | |
| Government | 207 (77.8) | 145 (75.1) | 62 (84.9) | |
| Inotropes | 48 (18.1) | 27 (14.0) | 21 (28.8) | 0.005 |
| Diuretics | 85 (32.0) | 43 (22.3) | 42 (57.5) | <0.001 |
| Amino glycosides | 96 (36.1) | 62 (32.1) | 34 (46.6) | 0.029 |
| Vancomycin | 86 (32.3) | 59 (30.6) | 27 (37.0) | 0.318 |
| Contrast study | 67 (25.2) | 53 (27.5) | 14 (19.2) | 0.165 |
| NSAIDs | 5 (1.9) | 4 (2.1) | 1 (1.4) | >0.999 |
| Assisted Ventilation | 194 (72.9) | 139 (72.0) | 55 (75.3) | 0.586 |
| Fluid overload (≥10%) | 97 (36.5) | 54 (28.0) | 43 (58.9) | <0.001 |
| PRISM II score (n=249) | ||||
| Mean (SD) | 6.0 (5.3) | 5.7 (5.1) | 6.6 (5.7) | 0.213 |
| Median (range) | 4.0 (0–27) | 4 (0–26) | 6 (0–27) | |
| Death | 21 (7.9) | 12 (6.2) | 9 (12.3) | 0.099 |
| LOS hospital, days | ||||
| Median (25th, 75th) | 15 (8, 30) | 14 (7, 28) | 20 (10, 39.5) | 0.041† |
| ≥20 | 105 (41.5) | 69 (37.3) | 36 (52.9) | 0.025 |
| LOS PICU, days | ||||
| ≥ 15 | 42 (15.8) | 20 (10.4) | 22 (30.1) | <0.001 |
SD: Standard Deviation; NSAIDs: Non-Steroidal Anti-inflammatory Drugs;
Mann-Whitney test
Table 3.
Diagnostic categories of study population (n=266)
| Diagnostic category n(%) | |||
|---|---|---|---|
| Respiratory | 100 (37.6) | 66 (34.2) | 34 (46.6) |
| Neurologic | 18 (6.7) | 10 (5.2) | 8 (11.0) |
| Cardiovascular/Shock | 67 (25.2) | 59 (30.6) | 8 (10.9) |
| Hematologic/Coagulation | 29 (10.9) | 17 (8.8) | 12 (16.4) |
| Metabolic | 25 (9.4) | 22 (11.4) | 3 (4.1) |
| Other | 27 (10.2) | 19 (9.8) | 8 (11.0) |
Table 2 displays the comparison of characteristics between patients classified as having AKI and those classified as having no-AKI according to the pRIFLE score. As can be seen in Figure 1, 73 patients (27.4%) developed AKI as diagnosed by the pRIFLE score; 31 patients (42.5%) met the Risk criteria, 27 patients (37.0%) met the Injury criteria, and 15 patients (20.5%) met the Failure criteria. Among all of the patients diagnosed with AKI, 83.5% (61/73) developed AKI within the first 72 hours of admission to the PICU, regardless of each patient`s specific pRIFLE score. Patients with AKI were younger and weighed less than patients without AKI (p<0.001). Among the clinical characteristics studied, there was a statistically significant increase in inotrope and diuretic use among AKI patients. The only nephrotoxins significantly associated with an increased incidence of AKI were the amino glycosides. No statistical difference was noted among the other measured parameters (Table 2). Fluid overload ≥10% was more prevalent in patients who developed AKI versus patients who did not develop AKI (58.9% v. 28.0% p <0.001).
Outcomes of AKI
Patients with AKI had longer hospital lengths of stay than patients with no-AKI (14 (IQR 7,28) days vs 20 (IQR 10, 39.5) days, p = 0.041). The median hospital length of stay of the whole study population was 15 (IQR 8,30) days.
A total of 21 patients died (7.8% of the whole study population). Of the patients with AKI (n=73), 9 patients (12.3%) died, compared to 12 patients (6.2%) who died among the patients with no-AKI (n=193), (p= 0.099). In order to assess the impact of severe AKI, mortality and length of stay (LOS) where re-evaluated in a sub-analysis consisting of two groups: no-AKI and AKI Risk (labeled “no-AKI-R”), compared with AKIe Injury and Failure (labeled “AKI-IF”).. Mortality was significantly higher in the AKI-IF group when compared to the no-AKI-R group (16.7% v 6.25% p=0.03).
Fluid overload ≥10% (FO10%) was associated with increased mortality (crude OR 3.1 95th CI 1.2–7.8), and longer PICU and hospital LOS, even when adjusted for age and PRISM II score (OR 12.7 95% CI 4.6–34.2; OR 3.1 95% CI 1.67–5.67 respectively).
Three binary logistic regression models were done to assess whether the degree of AKI (Risk or “R”, Injury or “I”, and Failure or “F”) could predict morbidity and mortality in PICU patients (Table 3). In the first model, mortality in patients with AKI diagnosed by pRIFLE was analyzed, and AKI was not significantly associated with increased mortality. In the second model, univariate analysis suggested that patients with AKI diagnosed by pRIFLE were at increased risk for longer hospital length of stay [OR (95% CI) 1.89 (1.00–3.32)]. However, when the multivariate analysis was done, with adjustments for age, sex, FO10%, and PRISM II score, the significance was lost. In the third model, PICU length of stay was analyzed in patients with AKI diagnosed by pRIFLE. Univariate analysis suggested that patients with AKI were at increased risk of longer PICU length of stay [OR (95% CI) 3.73 (1.89–7.38)], and this association persisted when adjusted for age. When the model was adjusted for age, FO10% and PRISM II score in multivariate analysis, the significance was lost. Multivariate analyses for no-AKI and AKI-R compared to AKI-I & F were also performed to evaluate the impact AKI severity on: survival, hospital LOS, and PICU LOS (Table 4). Patients with AKI-I & F had higher mortality and longer hospital LOS than patients with no-AKI and AKI-R, in both the crude and the age adjusted models. AKI-I & F were significantly associated with increased PICU LOS even after adjustments for age, FO10%, and PRISM II score (OR 4.16 95% CI 1.72–10.06). These models show that AKI-I & F, as identified by pRIFLE, are independent predictors of mortality, increased hospital length of stay, and PICU length of stay
Table 4.
Multivariate binary logistic regression models for Acute Kidney Injury (No-AKI vs. AKI [R, I or F]) morbidity and mortality prediction in PICU (Pediatric Intensive Care Unit)
| AKI† OR (95% CI) |
|||
|---|---|---|---|
| Outcome variables | Unadjusted | Age-adjusted | Age, fluid overload, PRISM II score |
| Mortality | 2.12 (0.85–5.27) | 2.61 (0.96–7.09) | 1.94 (0.62–6.12) |
| LOS Hospital (≥20 days) | 1.89 (1.07–3.32) | 1.64 (0.89–3.00) | 1.21 (0.62–2.34) |
| LOS PICU (≥15 days) | 3.73 (1.89–7.38) | 2.93 (1.40–6.12) | 2.11 (0.91–4.86) |
AKI: acute kidney injury (R,I or F);
Reference category: No-AKI ; OR: odds ratio; CI: confidence interval; fluid overload (≥10%), PRISM II score (≥10)
Discussion
This prospective study shows that the pRIFLE score is a useful instrument to identify pediatric AKI in critically ill children. It was demonstrated that pRIFLE is an accurate marker of increased morbidity and mortality. In our study, 24.3% of the patients presented AKI, identified by pRIFLE score. Rates of pediatric AKI in literature vary greatly and are difficult to compare. In our study, 27.4% of the patients presented AKI, as identified by pRIFLE, whereas the rate of AKI reported in Ackan-Arikan et al, for example, was 82%. Our study population is different, however, in that it includes all PICU admissions, regardless of mechanical ventilator or inotropic support. Other retrospective studies have calculated a lower prevalence of AKI than the prevalence in our study. Schneider et al described a prevalence of AKI of 10% in a retrospective cohort study. However, the Schneider et al study utilized the adult RIFLE criteria in order to identify AKI, which does not utilize eCCl calculations, and likely underestimates the prevalence of pediatric AKI.6. Our study provides the literature with a heterogenous cohort of critically ill patients without continuous renal replacement therapy (CRRT) in which to study the development and outcomes of AKI.
AKI, as defined by the pRIFLE score, occurred early in the PICU course of most patients, often within the first 72 hours of admission. This supports previous pediatric studies where children develop organ failure, including AKI, early in their PICU courses.4, 6,14
Fluid overload, which had been previously found to be predictive of mortality in children receiving CRRT, was found to be independently associated with longer intensive care and hospital stays.12, 13 Fluid overload as the first sign of kidney dysfunction is an emerging concept in the literature. In this study, fluid overload ≥10% had a stronger association with increased hospital LOS and PICU LOS than did AKI15.
In our cohort, patients with AKI were younger and weighed less. This finding is consistent with previous published observations6,8, although others have found no association between AKI and patient’s age or weight.16
The majority of patients with AKI in our study were identified using the creatinine clearance criteria (eCCl) for pRIFLE and not the urine output criteria (Uop). Nonetheless, Uop criteria did identify 11% of the cases. Additional studies may be needed to determine if urine output is really necessary for an early AKI diagnosis. It was also demonstrated that a decrease in urine output was not an early marker of AKI, which seems contrary to our current understanding of kidney function in the setting of AKI.
Previous studies have reported amino glycoside-associated AKI in almost a quarter of the patients receiving amino glycosides.17, 18 Furthermore, the presence of additional renal insults, including concomitant vancomycin or furosemide use in the critical care population, may increase risk of nephrotoxicity with amino glycosides.19–21 Our study demonstrated an increase in AKI for patients receiving amino glycosides, although it found no association between AKI and vancomycin or contrast agents. This finding warrants the continuous monitoring of amino glycoside levels and kidney function in this patient population.
Patients with AKI had more inotrope and diuretic use than those without AKI. This is most likely due to the patients` severity of illness. Patients with known signs of AKI, such as initial low urine output and/or signs of excessive total body fluid, are placed on diuretics early in their PICU course. Similarly, patients in an unstable hemodynamic state are at increased risk for both inotrope use and ischemic AKI in the setting of multi-organ damage. Interestingly, however, PRISM II scores did not vary among groups. Additional studies should be conducted in order to further define the relationship between AKI and these clinical characteristics.
A potential limitation of our study is that it assumes a baseline creatinine clearance (eCCl) of 120 mL/min/1.73 m2 for all patients, as the baseline creatinine levels were unknown for most patients. This could lead to misdiagnosis of patients with chronic kidney disease and a low eCCl at baseline as having AKI. In addition, restricting our data set to each patient`s initial admission to the PICU could have limited the number of data sets in the study. Patients who were admitted for less than 24 hours were excluded from the study, since a majority of post-operative elective cases fall within this time frame and were not of interest to our analysys. However, we recognize that extremely ill patients may die within hours of admission, and they would have been excluded from our study.
In this study, AKI-I & F defined by the pRIFLE classification predicted increased morbidity and mortality in our pediatric population. The pRIFLE score has proven to be useful in a wide range of disease states and age groups, and should be considered an effective indicator of pediatric AKI in PICU patients. Implementation of pRIFLE scoring in every PICU admission may provide clinicians with an additional instrument to develop preventive and early therapeutic interventions in critically ill children at risk of AKI. Similarly, fluid overload ≥10% can be a useful clinical parameter in PICU management, as it also predicted mortality and length of stay despite adjustments for age and PRISM II scores.
Conclusion
Through this prospective study, we have shown that the pRIFLE criteria serve to characterize AKI in critically ill pediatric patients. Patients with AKI as diagnosed by pRIFLE had increased hospital length of stay, increased PICU length of stay, and increased mortality. These results confirm that the pRIFLE score is a valuable clinical tool that should be utilized in PICU practice to identify patients at risk for, or patients with, AKI.
The pRIFLE classification system is an accurate marker of increased morbidity and mortality in children in a wide range of disease states and age groups, and it should prove useful in clinical practice Future studies may contemplate analyzing the effect of early interventions to prevent and treat AKI in critically ill patients who show no improvement in renal function during their first 24–48 hours of admission. These may ultimately serve to improve management of these patients and provide improvements in the delivery of quality care. Finally, fluid overload should be studied further as a potential target for additional early interventions in critically ill children, and as a clinical tool for monitoring goals of care.
Table 5.
Multivariate binary logistic regression models for Acute Kidney Injury (No-AKI/R vs. I/F) morbidity and mortality prediction in PICU (Pediatric Intensive Care Unit)
| AKI‡ OR (95% CI) |
|||
|---|---|---|---|
| Outcome variables | Unadjusted | Age-adjusted | Age, fluid (≥10), PRISM II score |
| Mortality | 3.00 (1.13–7.96) | 3.36 (1.21–9.27) | 2.43 (0.74–8.02) |
| LOS Hospital (≥20 days) | 2.31 (1.15–4.64) | 2.06 (1.01–4.20) | 1.50 (0.70–3.25) |
| LOS PICU (≥15 days) | 6.25 (2.97–13.15) | 5.26 (2.45–11.30) | 4.16 (1.72–10.06) |
AKI: acute kidney injury;
Reference category: No-AKI/R ; OR: odds ratio; CI: confidence interval; fluid overload (≥10%), PRISM II score (≥10)
Acknowledgments
This publication was possible by grants from the National Center for Research Resources (U54 RR 026139-01A1) and the National Institute on Minority Health and Health Disparities (8U54 MD 007587-03) from the National Institutes of Health. Its contents are solely the responsibility of authors and do not necessarily represent the official view of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have not disclosed any potential conflict of interest.
Contributor Information
Yadira A. Soler, University of Puerto Rico Medical Sciences Campus, Department of Pediatrics - Critical Care, ysoler@hotmail.com, 787-960-0967.
Mariely Nieves-Plaza, University of Puerto Rico Medical Sciences Campus, mariely.nieves1@upr.edu, 787-759-0306 ext 0306.
Mónica Prieto, Weill-Cornell Medical College, monica.m.prieto@gmail.com.
Ricardo García-De Jesús, University of Puerto Rico Medical Sciences Campus, Department of Pediatrics - Critical Care, Ricardo.garcia@upr.edu, 787-777-3535 ext 7186, 7184.
Marta Suárez-Rivera, University of Puerto Rico Medical Sciences Campus, Department of Pediatrics - Nephrology, marta.suarez@upr.edu, 787-510-9138.
References
- 1.Kellum JA, Levin N, Bourman C, Lameire N. Developing a consensus classification system for acute renal failure. Curr Opin Crit Care. 2002;8:509–514. doi: 10.1097/00075198-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Bagga A, Bakkaloglu A, Deverajan P, Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Wamock DG, Joannidis M, Levin A Acute Kidney Injury Network. Improving outcomes from Acute Kidney Injury: Report of an Initiative. Pediatr Nephrol. 2007;22:1655–1658. doi: 10.1007/s00467-007-0565-4. [DOI] [PubMed] [Google Scholar]
- 3.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akcan-Arikan A, Zappitelli M, Loftis LL, Wasburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with Acute Kidney Injury. Kidney Int. 2007;71:1028–1035. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 5.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between Acute Renal Failure and mortality following cardiac surgery. JAMA. 1998;104:343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 6.Schneider J, Khemani R, Grushkin C, Bart R. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med. 2010;38:933–939. doi: 10.1097/CCM.0b013e3181cd12e1. [DOI] [PubMed] [Google Scholar]
- 7.Bunchman TE, McBryde KD, Mottes TE, Gardner JJ, Maxvold NJ, Brophy PD. Pediatric acute renal failure: outcome by modality and disease. Pediatr Nephrol. 2001;16:1067–1071. doi: 10.1007/s004670100029. [DOI] [PubMed] [Google Scholar]
- 8.Basu R, Devarajan P, Wong H, Wheeler D. An update and review of acute kidney injury in pediatrics. Pediatr Crit Care Med. 2011 May;12(3):339–347. doi: 10.1097/PCC.0b013e3181fe2e0b. [DOI] [PubMed] [Google Scholar]
- 9.Plotz F, Bouma A, Van Wijk J, Kneyber M, Bokenkamp A. Pediatric Acute Kidney Injury in the ICU: an independent evaluation of pRIFLE criteria. Int Care Med. 2008;34:1713–1717. doi: 10.1007/s00134-008-1176-7. [DOI] [PubMed] [Google Scholar]
- 10.Symons J, Chua A, Somers M, Baum M, Bunchman T, Benfield M, Brophy P, Blowey B, Fortenberry J, Chand D, Flores F, Hackbarth R, Alexander S, Mahan J, McBryde K, Goldstein S. Demographic Characteristics of Pediatric Continuous Renal Replacement Therapy: A report of the Prospective Continuous Renal Replacement Therapy Registry. Clin J Am Soc Nephrol. 2007;2:732–738. doi: 10.2215/CJN.03200906. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein SL, Currier H, Graf CD, Cosio CC, Brewer ED, Sachdeva R. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107:1309–1312. doi: 10.1542/peds.107.6.1309. [DOI] [PubMed] [Google Scholar]
- 12.Foland JA, Fortenberry JD, Warshaw BL, Pettignano R, Merritt RK, Heard ML, Rogers K, Reid C, Tanner AJ, Easley KA. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32:1771–1776. doi: 10.1097/01.ccm.0000132897.52737.49. [DOI] [PubMed] [Google Scholar]
- 13.Ackan-Arikan A, Zappitelli M, Goldstein S, Naipaul A, Jefferson L, Loftis L. Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med. 2012;13:253–258. doi: 10.1097/PCC.0b013e31822882a3. [DOI] [PubMed] [Google Scholar]
- 14.Proulx F, Gauthier M, Nadeau D, et al. Timing and predictors of death in pediatric patients with multiple organ system failure. Crit Care Med. 1994;22:1025–1031. doi: 10.1097/00003246-199406000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Basu R, Chawla L, Wheeler D, Goldstein S. Renal angina: an emerging paradigm to identify children at risk for acute kidney injury. Pediatr Nephrol. 2012;27:1067–1078. doi: 10.1007/s00467-011-2024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zappitelli M, Bernier P, Saczkowski R, Tchervenkov C, Gottesman R, Dancea A, Hyder A, Alkandari O. A small post-operative rise in serum creatinine predicts Acute Kidney injury in children undergoing cardiac surgery. Kidney Int. 2009;76:885–892. doi: 10.1038/ki.2009.270. [DOI] [PubMed] [Google Scholar]
- 17.Selby N, Shaw S, Woodier N, Fluck R, Kolhe N. Gentamicin-associated Acute Kidney Injury. Q J Med. 2009;102:873–880. doi: 10.1093/qjmed/hcp143. [DOI] [PubMed] [Google Scholar]
- 18.Rybak MJ, Abate BJ, Kang SL, Ruffing MJ, Lerner SA, Drusano GL. Prospective evaluation of the effect of an amino glycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob Agents Chemother. 1999;43:1549–1555. doi: 10.1128/aac.43.7.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prins JM, Weverling GJ, de Blok K, van Ketel RJ, Speelman P. Validation and nephrotoxicity of a simplified once-daily amino glycoside dosing schedule and guidelines for monitoring therapy. Antimicrob Agents Chemother. 1996;40:2494–2499. doi: 10.1128/aac.40.11.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]