Abstract
Purpose
To develop a bio-assay that would be able to directly test gastrointestinal and/or dissolution samples to determine lipase activity and inhibition by Orlistat.
Methods
Enzyme assays were performed with porcine pancreatic lipase and para-Nitrophenyl Palmitate (pNPP) in pH 8.0 reaction buffer at 37°C. Substrate hydrolysis was monitored by absorbance changes at 410 nm. The dissolution of two Orlistat formulations was tested with a USP II apparatus. Samples were HPLC analyzed to determine release profile in addition to being diluted and directly assayed for inhibitory effect.
Results
The lipase-pNPP system demonstrates linearity and Michalis-Menten kinetics with a Km=2.7 ± 0.2 μM and Kcat = 0.019 s−1. Orlistat showed highly potent and time dependent inhibition with 5 ng/ml effecting 50% activity after 5 minutes in the Lipase-pNPP system. Dissolution studies showed a correlation of the drug release profile to the inhibitory effect of dissolution samples in the assay.
Conclusions
The lipase-pNPP method can be used as an in vitro assay to monitor orlistat inhibition from drug release or dissolution samples.
Keywords: Orlistat, Bioassay, Lipase, Inhibition, Dissolution
Introduction
Lipase plays a key role in dietary fat absorption by hydrolyzing triglycerides to monoglycerides and free fatty acids. As such, lipase inhibition may be a possible pathway to reduce total caloric intake. Orlistat is a potent lipase inhibitor that is sold as a weight loss aid in prescription and over-the-counter formulations. It has been demonstrated that orlistat is an irreversible inhibitor that covalently binds to serine 152 residue of lipase [1], which causes a pronounced in vitro as well as in vivo inhibition of gastric and pancreatic lipases [2,3]. Previous studies on this time dependent inhibition have used two-phase emulsion monitoring activity with a pH stat, which limits sample throughput [4,5].
Several studies have also revealed the significant effect of modifying the formulation of the orlistat on lipase inhibitory activity [6]. It was found in one study that nano-sized particles of orlistat can be used for enhanced in vitro dissolution rate and lipase inhibition [7]. However, to optimize the formulation for pharmacodynamic effect would require different time-consuming and costly clinical studies. Hence, there is a need for a simplified in vitro method to guide the selection of a suitable composition of formulation to optimize the release profile for obtaining better pharmacodynamic effect of orlistat products.
To address this need, a dynamic in vitro single-phase system was developed to monitor lipase inhibition, enabling rapid screening of multiple test samples. This system was able to provide a simulation of the in vivo lipase activity and is capable of directly testing dissolution or gastrointestinal (GI) fluid samples to assess lipase inhibition by orlistat. To validate the developed assay, two different formulations of orlistat were monitored for their lipase inhibitory activity from drug release samples.
Materials and Methods
Materials
Porcine Pancreatic Lipase (Type II) (EC 3.1.1.3), sodium deoxycholate, sodium phosphate monobasic, isopropanol and p-nitrophenyl palmitate (pNPP) were purchased from Sigma Aldrich (USA). Tetrahydrolipistatin (Orlistat, THL) was provided by Roche. UV-transparent 96-well plates were used for colorimetric quantification on a Tecan Infinite M200 spectrophotometer.
Lipase preparation
Since the majority of triglyceride hydrolysis is done by pancreatic lipase, porcine pancreatic lipase was used as a model enzyme. Crude lipase was dissolved in reaction buffer (10 mg/ml) and centrifuged at 7000 g for 10 minutes to remove insolubles [8].
In vitro assay for determination of lipase inhibition
Lipase assays were performed in a 96-well, clear, flat bottomed plate with 200 μl reaction volume. pNPP was used as a substrate with a reaction buffer of 50 mM sodium phosphate, 5 mM sodium deoxycholate, and 10% isopropanol at pH 8.0 [9-11]. Lipase assays used a 200 μl reaction volume and substrate conversion was monitored with a Tecan Infinite M200 spectrophotometer at 410 nm. All assays were run at 37°C and reported results are the average of six replicates that were blank subtracted.
Estimation of enzyme kinetics
To characterize the system, the kinetics of this single phase system was analyzed. Enzyme linearity of was shown by finding the initial rate for increasing enzyme concentrations. For determination of the kinetic constants, enzyme concentration was fixed at 0.01 mg/ml (the lipase concentration was determined per an established calibration curve, data not shown) and the substrate concentration was varied. Minimization of the sum of squares gave a fit to the Michalis-Menten equation.
Estimation of time dependent inhibition
Pre-incubation and co-treatment studies were performed to verify single phase irreversible binding. For pre-incubation, the lipase and orlistat were added together and incubated at 37°C for varying times before substrate was added and the initial rate (<2 minutes) was recorded.
For co-treatment, the substrate and orlistat were added together and the rate was monitored continuously. To find the activity as a function of time, the derivative of a 2nd order polynomial fit was taken to find the instantaneous slope, which was normalized to the positive control.
Dissolution studies
Two solid dosage forms of orlistat were compared using this pNPP assay, Formulation 1 and Formulation 2. A Hanson USP II dissolution apparatus was used to determine release profiles of the formulations. The dissolution media used was 3% Sodium Lauryl Sulphate (SLS), pH 6.0 at 37°C. 2ml samples were manually withdrawn and filtered with a 0.45 μm Poly tetra fluoro ethylene (PTFE) filter, discarding the first ml of filtrate. Samples were analyzed on an Agilent 1100 HPLC with a Waters nova-pak C18 3.9×150 mm, 4 μ particle size HPLC column to determine the active concentration. Dissolution samples were diluted and incubated with lipase and tested for activity with pNPP.
Results
Enzyme kinetics
The enzyme demonstrated a high degree of linearity for the range of concentrations considered (0-2.5 mg/ml) as shown in figure 1. Lipase-pNPP single phase catalysis exhibits Michalis-Menten kinetics with a Km=2.7 ± 0.2 μM and Kcat=0.019 s−1 as shown in figure 2.
Figure 1.
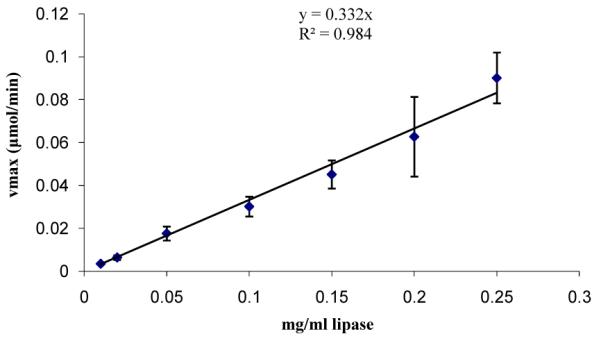
Lipase activity demonstrating linearity with pNPP (0.5 mM) at 37°C in reaction buffer (50 mM sodium phosphate, 5 mM sodium deoxycholate, 10% isopropanol at pH 8.0).
Figure 2.
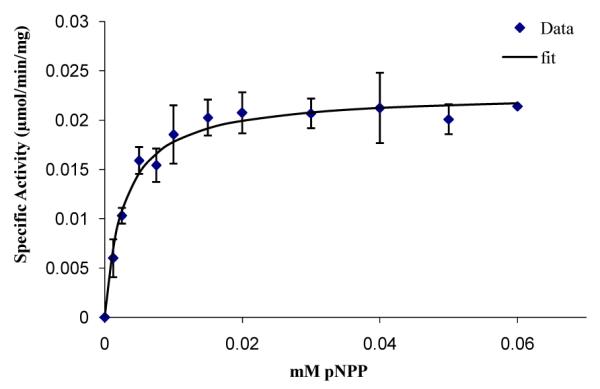
Lipase activity demonstrating Michalis-Menten kinetics (Km=2.7 ± 0.2 μM and Kcat=0.019 s-1) with pNPP at 37°C in reaction buffer (50 mM sodium phosphate, 5 mM sodium deoxycholate, 10% isopropanol at pH 8.0).
Lipase inhibition
Lipase inhibition assays showed that orlistat is a potent, time dependent inhibitor in single phase solutions. In the pre-incubation study, an exponential decrease in the observed initial rate of the enzyme was observed as shown in figure 3.
Figure 3.
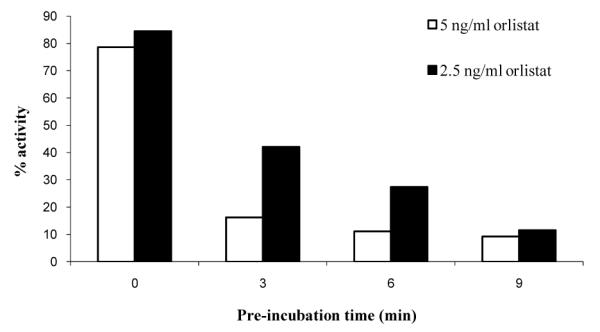
Pre-Incubation Inhibition Kinetics. 0.05 mg/ml lipase, 0.2 mM pNPP, 10% isopropanol, 50 mM Na phosphate pH 8, 5 mM Na deoxycholate.
Co-treatment inhibition kinetics showed a linear decrease in the enzyme activity with time (Figure 4). For the incubation results, all 2nd order fits had a very good fit (R2>0.99), which provided linear decreases in the activity with time. Lipase activity was completely negated with 20 ng/ml orlistat in a sample with 0.1 mg/ml lipase.
Figure 4.
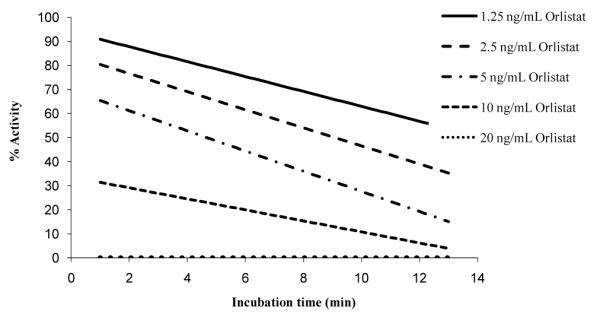
Co-treatment inhibition kinetics. 0.05 mg/ml lipase, 0.2 mM pNPP, 10% isopropanol, 50 mM Na phosphate pH 8, 5 mM Na deoxycholate with varying concentrations of orlistat.
Dissolution studies
Typical and repeatable dissolution profiles were observed using a USP II apparatus. Two different formulations were able to be distinguished in the dissolution test under identical conditions, with Formulation 1 showing a faster initial release rate.
Dissolution samples produced an inhibitory effect that correlates to the release profile. The effects were most notable with the formulation 2, where small differences in the initial rate of release correspond to significantly higher inhibition (Figure 5).
Figure 5.
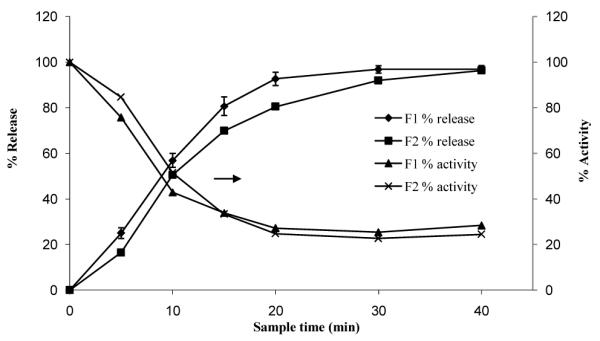
Correlation between percent release and corresponding lipase activity for two formulations of orlistat.
Discussion
The study demonstrated the utility of an in vitro single phase assay that would be able to directly test GI and/or dissolution samples to determine lipase activity and inhibition by orlistat.
The results confirmed and expanded the findings of previous two-phase studies that orlistat is an irreversible, time-dependent inhibitor of lipase [1,3,5,12-14], despite using a single-phase reaction.
In two phase emulsion studies, coalescence of emulsion particles may gave rise to reduced surface area to the point where reaction rates may become limited in part by substrate. In addition, the resulting heterogeneity caused by “creaming” of the emulsion may compromise the ability to procure representative subsamples for conducting the lipase assay. Therefore, the developed single phase assay is arguably better method to evaluate lipase activity [15].
Enzyme linearity over a range of physiologically relevant concentrations and adherence to Michalis-Menten kinetics provide a simple and robust system for testing dissolution or GI samples. Additionally, the resolution that this assay provides can distinguish between minor differences in enzyme inhibition. The ability to directly evaluate dissolution samples is validated by the correlation with the HPLC release profile. In combination with bio-dissolution system, the method could be developed as a bio-method to determine the suitability of formulation (such IR or SR) for orlistat-type drugs.
Limitations of this method may arise when examining interfacial effects of excipients. A potential example of this is sucrose ester, which alters the surface tension of micelles in an emulsion [16]. As such, its effects would not be able to be seen in a single phase assay.
Optimizing excipient formulations may allow for lower dosages of the active, but will require improved in vitro-in vivo correlations (IVIVC) to predict pharmacodynamic behavior before human trials. Future studies using this assay with a biorelevant dissolution method and human lipase or GI fluid will verify the sensitivity of the assay in addition to providing a more accurate IVIVC.
Conclusions
We have developed a very sensitive, single-phase assay for orlistat that mimics the time-dependent inhibition seen in emulsions. Additionally, we have shown a successful proof of concept for using dissolution samples in an in vitro assay as a method of estimating in vivo efficacy.
Acknowledgements
Daniel Lewis was supported by the GSK internship program. The authors would like to thank Ms. P. Tiwari for the assistance in preparation of this manuscript.
Abbreviations
- pNPP
para-Nitrophenyl Palmitate
- GI
Gastrointestinal
- pNPA
para-Nitrophenyl Acetate
- DPG
1,2-Dioleoyl-3-Pyrenedecanoyl-rac-Glycerol
- THL
Tetrahydrolipistatin
- IVIVC
In vitro-in vivo Correlation
References
- 1.Hadvary P, Sidler W, Meister W, Vetter W, Wolfer H. The lipase inhibitor tetrahydrolipstatin binds covalently to the putative active site serine of pancreatic lipase. J Biol Chem. 1991;266:2021–2027. [PubMed] [Google Scholar]
- 2.Sternby B, Hartmann D, Borgstrom B, Nilsson A. Degree of in vivo inhibition of human gastric and pancreatic lipases by Orlistat (Tetrahydrolipstatin, THL) in the stomach and small intestine. Clin Nutr. 2002;21:395–402. doi: 10.1054/clnu.2002.0565. [DOI] [PubMed] [Google Scholar]
- 3.Hadvary P, Lengsfeld H, Wolfer H. Inhibition of pancreatic lipase in vitro by the covalent inhibitor tetrahydrolipstatin. Biochem J. 1988;256:357–361. doi: 10.1042/bj2560357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosseneu M, Taveirne MJ, Caster H, Van Biervliet JP. Hydrolysis of very-low-density lipoproteins labeled with a fluorescent triacylglycerol: 1,3-dioleoyl-2-(4-pyrenylbutanoyl)glycerol. Eur J Biochem. 1985;152:195–198. doi: 10.1111/j.1432-1033.1985.tb09182.x. [DOI] [PubMed] [Google Scholar]
- 5.Negre A, Salvayre R, Dousset N, Rogelle P, Dang QQ, et al. Hydrolysis of fluorescent pyrenetriacylglycerols by lipases from human stomach and gastric juice. Biochim Biophys Acta. 1988;963:340–348. doi: 10.1016/0005-2760(88)90300-1. [DOI] [PubMed] [Google Scholar]
- 6.Carrière F, Renou C, Ransac S, Lopez V, De Caro J, et al. Inhibition of gastrointestinal lipolysis by Orlistat during digestion of test meals in healthy volunteers. Am J Physiol Gastrointest Liver Physiol. 2001;281:G16–G28. doi: 10.1152/ajpgi.2001.281.1.G16. [DOI] [PubMed] [Google Scholar]
- 7.Dolenc A, Govedarica B, Dreu R, Kocbek P, Srcic S, et al. Nanosized particles of orlistat with enhanced in vitro dissolution rate and lipase inhibition. Int J Pharm. 2010;396:149–155. doi: 10.1016/j.ijpharm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Lehner R, Verger R. Purification and characterization of a porcine liver microsomal triacylglycerol hydrolase. Biochemistry. 1997;36:1861–1868. doi: 10.1021/bi962186d. [DOI] [PubMed] [Google Scholar]
- 9.Gupta N, Rathi P, Gupta R. Simplified para-nitrophenyl palmitate assay for lipases and esterases. Anal Biochem. 2002;311:98–99. doi: 10.1016/s0003-2697(02)00379-2. [DOI] [PubMed] [Google Scholar]
- 10.Liu D, Yu Y, Wong WKR, Zhao X, Zhang J. An enzyme-like polyclonal antibody capable of catalyzing ester hydrolysis. Enzyme and Microbial Technology. 1997;20:24–31. [Google Scholar]
- 11.Winkler UK, Stuckmann M. Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J Bacteriol. 1979;138:663–670. doi: 10.1128/jb.138.3.663-670.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duque M, Graupner M, Stutz H, Wicher I, Zechner R, et al. New fluorogenic triacylglycerol analogs as substrates for the determination and chiral discrimination of lipase activities. J Lipid Res. 1996;37:868–876. [PubMed] [Google Scholar]
- 13.Gargouri Y, Chahinian H, Moreau H, Ransac S, Verger R. Inactivation of pancreatic and gastric lipases by THL and C12:0-TNB: a kinetic study with emulsified tributyrin. Biochim Biophys Acta. 1991;1085:322–328. doi: 10.1016/0005-2760(91)90136-6. [DOI] [PubMed] [Google Scholar]
- 14.Dousset N, Negre A, Salvayre R, Rogalle P, Dang QQ, et al. Use of a fluorescent radiolabeled triacylglycerol as a substrate for lipoprotein lipase and hepatic triglyceride lipase. Lipids. 1988;23:605–608. doi: 10.1007/BF02535605. [DOI] [PubMed] [Google Scholar]
- 15.Lowe ME. Assays for pancreatic triglyceride lipase and colipase. Methods Mol Biol. 1999;109:59–70. doi: 10.1385/1-59259-581-2:59. [DOI] [PubMed] [Google Scholar]
- 16.Sarda L, Desnuelle P. [Actions of pancreatic lipase on esters in emulsions] Biochim Biophys Acta. 1958;30:513–521. doi: 10.1016/0006-3002(58)90097-0. [DOI] [PubMed] [Google Scholar]


