Dear editor
Our team, general surgeons and gynecologists look constantly for ways to prevent postsurgical adhesions, and hence, we appreciate the platform you have established through multiple publications.1–3 This is especially because postsurgical adhesions may result in several complications such as the small bowel obstruction, secondary infertility, dyspareunia, chronic abdominal/pelvic pain and many others.
Prevention of postsurgical adhesions is still an unsolved problem in spite of the suggested modifications of current surgical methods and application of various barriers, sprays, and use of other antiadhesive medications. We have already pointed out that a design of ideal nanoparticles should become a target of personalized adhesion prevention strategy in the future4,5 and therefore, we read with great interest the article by Shin et al that was recently published in your journal.6 This article explores the potential of postoperative adhesion prevention by nanofibers of poly(lactic-co-glycolic acid) (PLGA) loaded with epigallocatechin-3-O-gallate (EGCG), which is the most bioactive polyphenolic compound extracted from green tea.
The authors demonstrated that the effects of PLGA barrier loaded with EGCG in the prevention of adhesion are associated with the expression of its active compound. EGCG possesses an antioxidant activity as well as a prolonged activated partial thromboplastin time (aPTT) as reported by in vitro experiments.6 The authors also demonstrated the significantly inhibited inflammation, neovascularization and fibrosis in animals treated with PLGA barrier loaded with EGCG compared with PLGA treatment only. In addition, the use of EGCG was associated with significantly reduced severity and extent of posttraumatic adhesions when compared with that of Interceed TC7.6
As we reported before, an increased severity of adhesion was associated with accelerated activation of spontaneous chemiluminescence (SCL) of peritoneal macrophages (Figure 1). Two different adhesion barriers, TC7 with the size 0.3×3.5 cm (Ethicon Company, Johnson & Johnson) and Fibrin Glue (Beriplast, Behring, Germany), applied on suture lines made during experimental surgery in an incised rat uterine horn were compared with similar lesions sutured using catgut 4/0 or prolene 4/0 (Ethicon Company, Johnson & Johnson) without application of barriers.7,8 Peritoneal fluid was collected from the abdominal cavity of euthanized animals, and SCL patterns of freshly isolated macrophages were measured every day in three rats from each group. Changes in SCL were recorded and analyzed in comparison with the dynamic macroscopic and histological features of postsurgical acute inflammation, wound healing, and adhesion formation, which were observed under the transmission electron microscopy. Experiments were done with the approval of the Institutional Local Ethical committee (Academician V.I. Kulakov Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia).7
Figure 1.
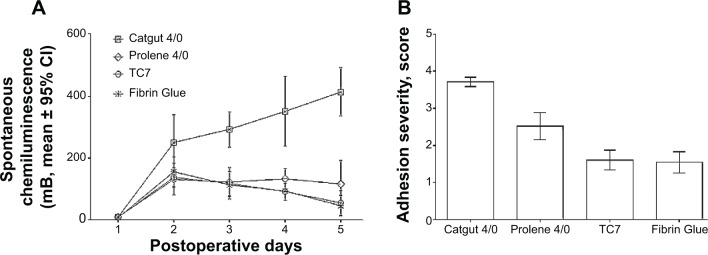
Postsurgical spontaneous chemiluminescence activity of freshly isolated peritoneal macrophages (A) and adhesion severity (B) after application of two different adhesion barriers, TC7 with the size 0.3×3.5 cm (Ethicon Company, Johnson & Johnson) and Fibrin Glue (Beriplast, Behring, Germany), on suture lines made during experimental surgery in an incised rat uterine horn were compared with similar lesions sutured using catgut 4/0 or prolene 4/0 (Ethicon Company, Johnson & Johnson) without application of barriers.
Abbreviation: CI, confidence interval.
In addition, we also found that there were reduced inflammation, neovascularization, and mild sclerotic changes after the application of both barriers7 as shown by Shin et al.6 Further studies evaluating the ability of EGCG to scavenge reactive oxygen species in peritoneal macrophages and cause prolonged activation of partial thromboplastin time in peritoneal fluid will enhance our current understanding of the related adhesion formation signaling pathways.
Acknowledgments
This work was supported by the Russian Science Foundation (grant no 14-31-00024).
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest or financial ties to disclose. The authors alone are responsible for the content and writing of the paper.
References
- 1.Yang B, Gong C, Zhao X, et al. Preventing postoperative abdominal adhesions in a rat model with PEG-PCL-PEG hydrogel. Int J Nanomedicine. 2012;7:547–557. doi: 10.2147/IJN.S26141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao X, Deng X, Wei X, et al. Novel thermosensitive hydrogel for preventing formation of abdominal adhesions. Int J Nanomedicine. 2013;8:2453–2463. doi: 10.2147/IJN.S46357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Q, Li L, Wang N, et al. Biodegradable and thermosensitive micelles inhibit ischemia-induced postoperative peritoneal adhesion. Int J Nanomedicine. 2014;9:727–734. doi: 10.2147/IJN.S55497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mynbaev OA, Eliseeva MY, Malvasi A, Tinelli A. Challenging nanoparticles: a target of personalized adhesion prevention strategy. Int J Nanomedicine. 2014;9:3375–3376. doi: 10.2147/IJN.S67938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mynbaev OA, Eliseeva MY, Tinelli A, et al. A personalized adhesion prevention strategy: Arslan E, Talih T, Oz B, Halaclar B, Caglayan K, Sipahi M. Comparison of lovastatin and hyaluronic acid/carboxymethyl cellulose on experimental created peritoneal adhesion model in rats. Int J Surg. 2014;12(2):120–124. doi: 10.1016/j.ijsu.2013.11.010. [DOI] [PubMed] [Google Scholar]; Int J Surg. 2014;12(9):901–905. doi: 10.1016/j.ijsu.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Shin YC, Yang WJ, Lee JH, et al. PLGA nanofiber membranes loaded with epigallocatechin-3-O-gallate are beneficial to prevention of postsurgical adhesions. Int J Nanomedicine. 2014;9:4067–4078. doi: 10.2147/IJN.S68197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mynbaev OA. Etiology, pathogenesis and prevention principles of postoperative adhesions: ScD thesis. Moscow: 1997. p. 45. Russian. [Google Scholar]
- 8.Myinbayev OA, Rubliova KI. Use of chemiluminescent method for evaluation of postoperative adhesion formation after reproductive pelvic surgery: an experimental study. Am J Reprod Immunol. 1996;36(4):238–241. doi: 10.1111/j.1600-0897.1996.tb00170.x. [DOI] [PubMed] [Google Scholar]


