Abstract
Metazoans have evolved ways to engage only the most appropriate cells for long-term tissue development and homeostasis. In many cases, competitive interactions have been shown to guide such cell selection events. In Drosophila, a process termed cell competition eliminates slow proliferating cells from growing epithelia. Recent studies show that cell competition is conserved in mammals with crucial functions like the elimination of suboptimal stem cells from the early embryo and the replacement of old T-cell progenitors in the thymus to prevent tumor formation. Moreover, new data in Drosophila has revealed that fitness indicator proteins, required for cell competition, are also involved in the culling of retinal neurons suggesting that ‘fitness fingerprints’ may play a general role in cell selection.
Current Opinion in Cell Biology 2014, 31:16–22
This review comes from a themed issue on Cell cycle, differentiation and disease
Edited by Stefano Piccolo and Eduard Batlle
For a complete overview see the Issue and the Editorial
Available online 12th July 2014
http://dx.doi.org/10.1016/j.ceb.2014.06.011
0955-0674/© 2014 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Darwinian cell competition in the body
Darwin's theory of natural selection has revolutionized our understanding of how organisms evolve. Often, the essence of his theory is formulated with ‘the fittest survive’, a term first coined by Herbert Spencer, to summarize the ideas of Darwin that better adapted organisms will live to have more offspring. In 1881, zoologist Wilhelm Roux argued that Darwinian competition and selection had not been considered for the development of tissues and organs. In his view, cells within our bodies were also likely to compete for space and limited resources. Such ‘fights’ among slightly varying ‘parts of our bodies’ would result in the ‘selective breeding’ of the most durable and the elimination of less durable parts (cells).
Along similar lines, Santiago Ramon y Cajal proposed a few years later that developing neurons may be engaged in a competitive struggle for space and nutrition, an idea which gained support in the framework of the neurotrophic theory and the discovery of nerve growth factor by Rita-Levi Montalcini and its isolation by Stanley Cohen in 1960 [1]. During nervous system development, large proportions of neurons die in almost every region of the nervous system. The normal death of these neurons occurs during a limited time window coinciding with target innervation [2]. Up to now, a large body of evidence has shown that neurons compete for limiting amounts of target-derived or paracrine factors, which support the survival of only a fraction of the initially generated neurons, thus potentially eliminating unfit or less suitable neurons from a larger population [3]. This provides a mechanism how the right number and probably also the right quality of neurons are chosen to innervate given target tissues. Many aspects of the neurotrophic theory have been molecularly proven, such as identification of further target and paracrine-derived survival factors and their corresponding receptors on developing neurons [4], but how exactly optimal neurons are identified is less clear.
In Drosophila, a process known as cell competition [5] eliminates cells with heterozygous mutations in ribosomal protein genes (Minute cells) through a mechanism that has been proposed to involve competition for extracellular factors and apoptosis [6]. Various genetic studies in Drosophila have established, that apart from Minute mutations (Figure 1a), also reduced growth factor signaling, lowered anabolic capacity or altered apico-basal polarity represent triggers for competitive interactions, which have been recently reviewed elsewhere [7, 8, 9].
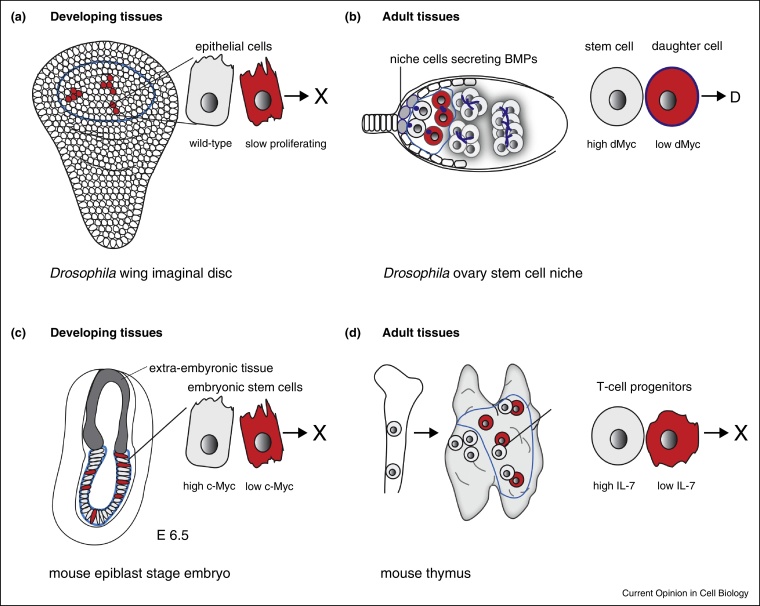
Figure 1.
Cell competition in Drosophila and mouse tissues.Cell competition occurs in Drosophila among epithelial cells of developing wing imaginal discs (a). In adult flies, stem cells in the ovary germline niche compete with their daughters and among each other for niche-derived factors (b). Cell competition in mice has been found to occur at the epiblast stage among pluripotent embryonic stem cells around embryonic day 6.5 (E6.5) (c). In adult mice, competitive interactions take place among resident and fresh bone marrow-derived T-cell progenitors in the thymus. Blue lines mark areas of competition. The cross symbolizes apoptotic elimination, whereas D stands for niche exit and differentiation.
In some situations, it has been shown that mutant cells can become ‘supercompetitors’ and behave as winners by outcompeting wild-type cells, which now turn into losers. For example, clones with elevated levels of Drosophila myc (dmyc), the homolog of the human c-Myc protooncogene, can convert into such supercompetitors. Supercompetitor cells expand in developing fly epithelia by inducing apoptosis in surrounding wild-type cells based on short range cell–cell interactions [10, 11]. The ‘enrichment’ in supercompetitor (winner) clones is morphologically silent [10] because it is balanced by the concomitant loss of wild-type cells.
Although cell competition normally occurs in proliferating tissues, a recent study by Tamori and Deng has revealed that competitive interactions can also play a role in the postmitotic Drosophila follicular epithelium [12••, 13]. The authors showed that follicular cells with heterozygous mutations in ribosomal protein genes (Minutes) or reduced levels of mahjong (mahj), a regulator of apico-basal polarity [14], are selectively lost by apoptosis from follicular epithelia, whereas no cell death was triggered in tissues made entirely of Minute or mahj−/− cells. In contrast, other factors known to trigger competition in mitotic epithelia (dMyc, activated growth factor signaling or apico-basal tumor suppressor genes) do not play a role in this type of competition. As a further difference, the eliminated cells due to competition are not replaced by cell proliferation. Instead, remaining winner cells increase in size by accelerating their endocycles in a process named compensatory cellular hypertrophy [12••].
To summarize, the outcome of both classical cell competition and supercompetition is a Darwinian-like selection, leading to long-term survival of certain cells over others.
The growing functions of cell competition
Until recently, work on cell competition was mainly carried out in Drosophila and relied heavily on the analysis of two experimentally induced populations (e.g. wild-type vs. mutant cells) in mosaic epithelia. These limitations raised important questions: is cell competition conserved in mammals and does it play a relevant physiologic role in non-manipulated tissues?
An initial study on chimeric mice described that Minute cells were also eliminated from mouse embryonic tissue, but mechanistic insight was limited [15]. In 2010, Tamori et al. showed that mahj−/− cells are outcompeted from mammalian epithelial layers formed by cultured Madin-Darby canine kidney cells [14], which suggested a conserved role for cell competition in Drosophila and mammals. And in that same year, Bondar and Medzhitov revealed competitive interactions among p53 mutant and wild-type hematopoietic stem cells in mice [16]. In the next section, we will focus on the latest advances in the field.
Selection of optimal stem cells to construct tissues
In Drosophila, a type of physiologic competition has been described in the ovary stem cell niche, where high dMyc-expressing stem cells compete with low dMyc-expressing daughter cells for niche-derived factors [17] (Figure 1b). This natural competition was proposed to create sharp differentiation boundaries and eliminate suboptimal stem cells from the niche by triggering differentiation rather than cell death. The analysis of mosaic stem cell niches furthermore revealed that dMyc-overexpressing stem cells replaced adjacent wild-type stem cells within several days without changing tissue architecture, whereas other growth promoting mutations (e.g. PTEN, a negative regulator of insulin signaling) strongly activated stem cell proliferation without inducing stem cell competition.
In a recent study, Vermeulen et al., have followed stem cell dynamics in mosaic mouse intestinal crypts harboring stem cells with intestinal-tumor associated mutations [18•]. The authors show that stem cells expressing an oncogenic Kras variant or lacking both copies of the negative Wnt regulator Apc gain a competitive advantage and preferentially replace wild-type stem cells without changing the overall patterns of proliferation or differentiation of the intestinal epithelium. In the case of apc−/− stem cells, competition is likely to be mediated by Myc, which is responsible for most Wnt target gene activation following Apc loss [19], although not formally addressed in this study. Interestingly, stem cells with mutations in p53 only started to outcompete wild-type cells in colitis-affected intestines, where the fitness of surrounding cells is reduced due to chronic inflammation [18•]. These findings support the current perception that cell competition may be implicated in early, morphologically silent events of cancer development [20].
Apart from intestinal crypts, which seem promising to analyze competition among stem cells [18•], elegant genetic tools and in vitro systems have been developed in the past year to study cell competition in mammals [21••, 22••]. Two new studies in mice have revealed that cell competition is important to select optimal embryonic stem cells during development [21••, 22••] (Figure 1c). Tristan Rodriguez and his team found that several types of viable, but fitness-compromised stem cells are eliminated from mouse embryonic stem cell (ESC) cultures due to cell competition. By co-culturing wild-type and different ‘unfit’ mouse ESCs for up to four days in differentiation-promoting media, they could show that cells with strongly reduced bone morphogenetic (BMP) signaling, compromised autophagy or with tetraploid genomes were selectively eliminated from mixed cultures, whereas they grew normally in monocultures [21••]. Moreover, a co-culture of two populations with compromised fitness did not show signs of competition, indicating that this system may be employed in the future to assess if certain fitness deficits are stronger than others (e.g. autophagy vs. slow proliferation). Cells with defective BMP signaling are also outcompeted from developing fly epithelia [6]. In Drosophila, loser cells can be protected from competition by overactivation of the BMP pathway (i.e. Dpp signaling). This suggests that loser cells may at least partly die because they compete less efficiently for growth/survival signals both in Drosophila and mammals [22••, 6].
In a second study, Miguel Torres and his group focused their attention on early mouse embryonic development, namely the epiblast stage (Figure 1c) [22••]. The epiblast is already implanted embryonic tissue, still composed of pluripotent stem cells, which will differentiate subsequently to form all three germ layers during gastrulation. At around embryonic day 6.5 (E6.5) apoptosis peaks in the epiblast indicating that a large fraction of cells are being eliminated. Miguel Torres and colleagues successfully developed a system to create random genetic mosaics (iMOS-System) in the mouse epiblast, which can be followed afterwards by marker proteins [22••]. When inducing a subset of cells with higher c-Myc levels, they observed supercompetition, meaning that embryonic tissues analyzed a few days post mosaic induction, consisted mainly of c-Myc overexpressing cells [22••]. This relative enrichment of supercompetitor cells did not occur if cell death was prevented by the expression of an apoptosis inhibitor in surrounding wild-type cells. These findings demonstrate that, as in Drosophila, the relative expansion of winner cells is dependent on the purging of cells with lower relative levels of Myc.
Both groups describe that ‘loser stem cells’ in their systems express lower levels of c-Myc protein compared to the winner population [21••, 22••] and that the relative difference in Myc protein correlates with the extent of competition observed in the mouse embryo [22••]. However, it was the analysis of endogenous c-Myc expression in the epiblast, which provided the key to understand the physiologic role of cell competition: up to E6.75, epiblast stem cells showed intrinsic variations in c-Myc protein expression, whereas by day E7.6, these heterogeneities had vanished and all persisting stem cells expressed high levels of c-Myc protein. By monitoring which cells were dying in the epiblast, Clavería et al. could demonstrate that Caspase-3 activation preferentially occurred in stem cells with lower c-Myc levels [22••]. Altogether, these findings provide strong evidence that natural Myc-driven cell competition results in selection of embryonic stem cells with high anabolic capacity. This optimization of epiblast stem cells may be crucial, since they represent the building blocks for all future tissues and competition would ensure that only ‘prime material’ will be considered.
The analysis of cell competition in mice revealed high similarity to what is known from Drosophila. The above-mentioned studies did only observe different results with respect to potential diffusible factors involved in cell competition. Strikingly, Sancho et al. found that competition-dependent cell death was even triggered in situations where direct cell–cell contact was prevented between ESCs, by culturing wild-type cells in a separate compartment above BMP-compromised cells [21••]. In contrast, Miguel Torres and colleagues saw that conditioned media from ESCs undergoing supercompetition due to mosaic dMyc overexpression, was not sufficient to trigger apoptosis in healthy wild-type ESCs [22••]. A secreted killing signal has been previously postulated based on competition assays with insect cells [23, 24], but its production seemed to require initial cell–cell interaction between competing cells. Finally, Clavería et al. showed that in the mouse epiblast and ESC cultures, loser cells are engulfed by neighbors. In the future, it will be interesting to know whether the engulfment step in mammals plays a causal role to induce death, as proposed by the laboratory of Nicholas Baker [25] or if it is just required to clear apoptotic debris, as we believe it is the case in Drosophila [26].
Cell competition as an intrinsic tumor suppressor mechanism
Cell competition may be important during development, but what about adult tissues with a high turnover rate?
In a recent work, Martins et al. addressed the questions whether replacement of ‘old’ thymus-resident T cell progenitors by new bone marrow-derived stem cells may show typical features of cell competition [27••, 28]. They indeed found evidence that thymus-resident and incoming progenitors compete for the hematopoietic growth factor IL-7 (Figure 1d). Fresh progenitors immigrating from the bone marrow seemed to compete more efficiently for IL7, which led to induction of the pro-survival protein Bcl-2. The authors propose a model wherein IL-7 availability is limited for thymus-resident progenitors as long as there is a steady supply of new progenitors to the thymus [27••]. Therefore, Bcl-2 levels tend to drop in thymus-resident progenitors during competition, leading to their death. Intriguingly, when the arrival of new progenitors (and therefore competition) was abolished, resident progenitors over-proliferated, ultimately resulting in tumor formation. These results suggest that naturally occurring cell competition is required to renew the pool of T-cell progenitors periodically with fresh cells from the bone marrow. If this turnover is prevented, older progenitors turn into cancerous cells. In this case, cell competition acts as a tumor suppressor mechanism to prevent cancer in the thymus through negative selection of potentially hazardous progenitors. It is not known yet why progenitors in the thymus get predisposed to cancerous transformation. Possibilities include the exposure to a cancer-promoting signal from the thymus environment or accumulation of defects while self-renewing and giving rise to new T-cells. Alternatively, thymus progenitors may already arrive to the thymus with a pre-defined expiry date (e.g. due to shortened telomeres [29]), after which they get out of control.
Taken together, these new findings highlight the importance of competitive interactions in cell quality control in mammals.
Recognition and elimination of suboptimal cells by fitness indicator proteins
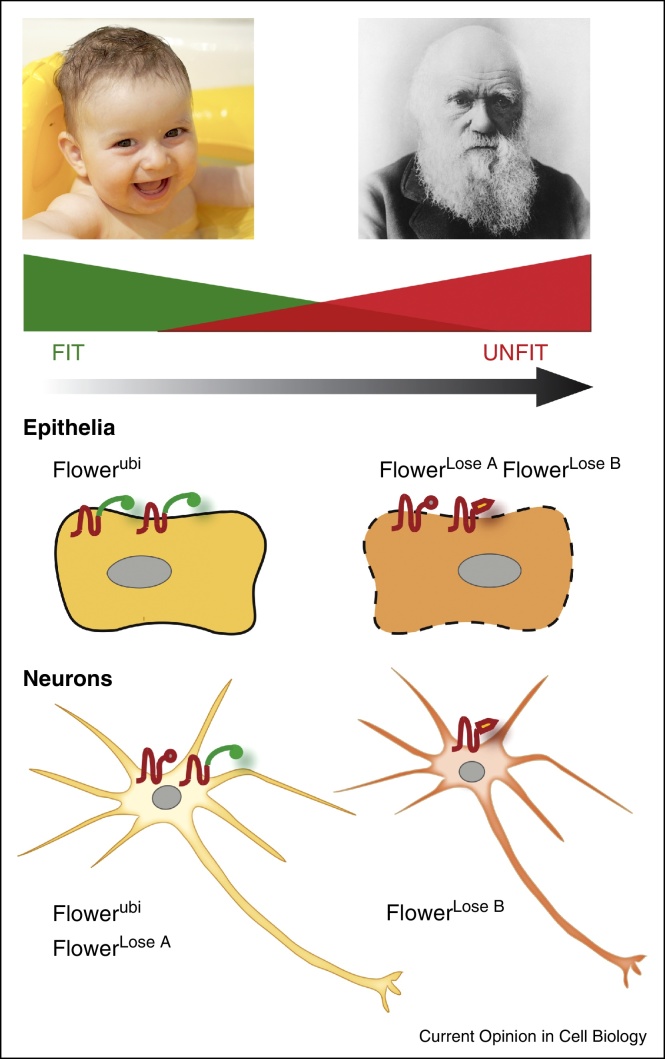
Several experiments on cell competition in flies indicate that trophic theories may be too simplistic to explain cell competition. In Drosophila, the amount of survival factor cells compete for is often not limiting, but cell selection still occurs because cells can compare their fitness directly thanks to fitness indicator proteins. In Drosophila, cells display information about their fitness state via different isoforms of the conserved transmembrane protein Flower. Suboptimal epithelial cells, for example, are detected and eliminated because they express a set of Flower Lose isoforms, which is not present on the more vigorous surrounding cells [30] (Figure 2). By means of this surface code, which changes gradually as a cell turns unfit, cells are able to monitor the ‘health’ of their neighbors (Figure 2).
Figure 2.
Tissue-specific fitness fingerprints of Drosophila cells. Cells in Drosophila are capable of discriminating aspects of cellular fitness based on extracellularly exposed Flower proteins in a tissue-specific manner [30, 31••]. These fitness fingerprints change as cells become gradually unfit. Through yet unknown mechanisms, cells are able to ‘read’ the fitness status of neighboring cells, similar to humans, which rely on specific features to determine the age of a person (wrinkles, graying hair, eye bags, etc.).
A recent study by Merino et al. describes that such Flower ‘fitness fingerprints’ also regulate the culling of unwanted neurons in the fly retina [31••]. The authors observed that neurons signal intact fitness by a neuron-specific Flower fitness fingerprint, which is distinct from the one used in epithelia (Figure 2). Neurons in incomplete photoreceptor units, in turn, express a specific Flower Lose isoform, which induces their elimination. In this case, the purged neurons are not replaced by fitter ones, revealing that Flower proteins can mediate cell selection in processes that are distinct from cell competition [31••]. Strikingly, when all neurons in the retina were forced to present the apoptosis-triggering Flower Lose isoform, the excess neurons persisted and the neuronal network was not refined [31••].
The fact that Flower fitness fingerprints can provide information about the ‘quality of neurons’ is exciting and opens the door to explore Flower functions in neurobiology. It may for example be interesting to assess if certain Flower fingerprints correlate with states of neuronal health, which are known to range from highly resilient to very vulnerable depending on conditions such as electrical activity, expression of anti-apoptotic genes or the availability of neurotrophic factors [32]. Fitness marks on neurons may also guide neuronal selection during human or mouse adult neurogenesis in the hippocampus, where competitive interactions are known to occur [33, 34], or during early neural development, where apoptosis is thought to occur in proliferating neural precursors [35].
To discriminate between cell eliminations triggered by direct cell–cell comparison of fitness status (e.g. Flower marks) and cell deaths resulting from unsuccessful competition for external survival factors (e.g. developing neurons requiring NGF), we propose to use the terms direct and indirect cell competition, respectively, as employed in ecology to describe competition among animals (direct) and for common resources (indirect competition) [36].
Conclusions and outlook
Research in the last twenty years has substantially advanced our understanding of quality control mechanisms within a cell such as targeting of misfolded proteins to the proteasome, removal of faulty mRNAs by nonsense-mediated mRNA decay and error corrections by DNA repair mechanisms.
Cell competition now provides a mechanism, how cell quality can be monitored at the tissue level from development to adult tissue homeostasis, possibly even in postmitotic tissues. Recent studies in mice have shown that cell competition is conserved in mammals and plays an important physiologic role in eliminating viable, but slightly fitness-compromised cells. Meanwhile, numerous studies in flies and mice have established that the cell competition response detects and targets a wide range of cellular defects reducing viable cell fitness, indicating that cell quality is monitored with great sensitivity. Not only competition, but also supercompetition can occur in mice. The propensity to tumor development seems to be the down side of cell competition, which selects cells based on relative cell fitness. Nevertheless, It appears that the advantages (efficient cell quality control) and versatility (fitness fingerprints) of the pathway normally outweighs this inherent risk to support cancer development.
The consequences of lack of competition are only at the beginning of being understood but are likely to affect a wide range of processes such as tissue homeostasis, regeneration, aging and cancer, whereby a first study describing cell competition-like processes during liver regeneration in mice has already been published [37]. The possibility that fitness fingerprints involved in competition may have been adopted for other cell selection processes offers an exciting new route of research. Further investigations in this direction can show if Flower marks play similar roles in sculpting and maintaining optimal neural networks in higher organisms with expected impact on normal neurological function and disease.
What is cellular fitness?
Imbalances in cell fitness can arise due to transcriptional noise [38], unequal exposure to survival factors or stressors or upon random acquisition of mutations. Cell surveillance mechanisms based on cellular fitness are therefore thought to improve tissue quality and prevent premature organ dysfunction.
The term ‘high fitness’ is widely used in ecology and evolutionary biology to describe that an organism is better adapted and will live to have more offspring, which will inherit the advantageous trait, based on Darwin's theory of natural selection. Relative ecological fitness, in turn, usually describes an individual's potential to survive and reproduce in the face of natural selection, compared to the average fitness exhibited by the other members of the population. Biologist usually do not need to know in which conditions an organisms is fitter than another, because often the inherent advantage or disadvantage of a trait is only revealed in retrospect in an evolutionary or ecological context.
Because of the vague definition of fitness, philosophers have pointed out with good reason that the concepts of fitness and natural selection lack a description of what they would refer to as ‘reference environment’ [39], in which a trait would indeed increase or decrease fitness. Similar aspects are true for the concept of cell fitness. Mutations that negatively affect cell fitness are also identified in retrospect. The study of cell competition in flies and mammals has revealed that cellular fitness cannot be determined as an absolute value. Relative fitness differences are decisive if a cell type survives in a given ‘reference environment’ or not, for example, suboptimal cells are only outcompeted when surrounded by fitter neighbors, but survive when neighboring cells also show reduced fitness. Similarly, epithelial cells with four copies of Drosophila myc do only behave as supercompetitors when in contact with wild-type cells, whereas they do not expand if embedded among equal cells (4x myc) with identical fitness. These findings show that relative and not absolute ‘fitness’ values decide over a cell's continuance in the tissue and that high fitness in the context of a multicellular organism is only beneficial to a certain degree, since overly fit cells may contribute to cancer development.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We thank Prof. Carlo C Maley and Dr. Athena Aktipis for bringing to our attention distinctions made between direct and indirect competition in the field of ecology.
Work in our laboratories is funded by the European Research Council, Swiss National Science Foundation, Josef Steiner Cancer Research Foundation, Japanese-Swiss S&T program and the Swiss Cancer League.
Contributor Information
Eduardo Moreno, Email: eduardo.moreno@izb.unibe.ch, emoreno@izb.unibe.ch.
Christa Rhiner, Email: christa.rhiner@izb.unibe.ch.
References
- 1.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 2.Oppenheim R.W. Cell death in the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 3.Raff M.C. Social controls on cell survival and cell death. Nature. 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- 4.Simi A., Ibáñez C.F. Assembly and activation of neurotrophic factor receptor complexes. Dev Neurobiol. 2010;70:323–331. doi: 10.1002/dneu.20773. [DOI] [PubMed] [Google Scholar]
- 5.Morata G., Ripoll P. Minutes: mutants of Drosophila autonomously affecting cell division rate. Dev Biol. 1975;42:211–221. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- 6.Moreno E., Basler K., Morata G. Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature. 2002;416:755–759. doi: 10.1038/416755a. [DOI] [PubMed] [Google Scholar]
- 7.Vincent J.P., Fletcher A.G., Baena-Lopez L.A. Mechanisms and mechanics of cell competition in epithelia. Nat Rev Mol Cell Biol. 2013;14:581–591. doi: 10.1038/nrm3639. [DOI] [PubMed] [Google Scholar]
- 8.Baillon L., Basler K. Reflections on cell competition: growth, proliferation, morphogens and apoptosis. Semin Cell Dev Biol. 2014 doi: 10.1016/j.semcdb.2014.04.034. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 9.Patel P.H., Edgar B.A. Tissue design: how Drosophila tumors remodel their neighborhood. Semin Cell Dev Biol. 2014 doi: 10.1016/j.semcdb.2014.03.012. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 10.Moreno E., Basler K. dMyc transforms cells into super-competitors. Cell. 2004;117:117–129. doi: 10.1016/s0092-8674(04)00262-4. [DOI] [PubMed] [Google Scholar]
- 11.de la Cova C., Abril M., Bellosta P., Gallant P., Johnston L.A. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117:107–116. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- 12••.Tamori Y., Deng W.M. Tissue repair through cell competition and compensatory cellular hypertrophy in postmitotic epithelia. Dev Cell. 2013;25:350–363. doi: 10.1016/j.devcel.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a hormone-inducible system to create suboptimal cells in the follicular epithelium, this study shows that competition can also mediate cell elimination in postmitotic tissues.
- 13.Tamori Y., Deng W.M. Compensatory cellular hypertrophy: the other strategy for tissue homeostasis. Trends Cell Biol. 2014;24:230–237. doi: 10.1016/j.tcb.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamori Y., Bialucha C.U., Tian A.G., Kajita M., Huang Y.C., Norman M., Harrison N., Poulton J., Ivanovitch K., Disch L., Liu T., Deng W.M., Fujita Y. Involvement of Lgl and Mahjong/VprBP in cell competition. PLoS Biol. 2010;8:e1000422. doi: 10.1371/journal.pbio.1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliver E.R., Saunders T.L., Tarlé S.A., Glaser T. Ribosomal protein L24 defect in belly spot and tail (Bst), a mouse Minute. Development. 2004;131:3907–3920. doi: 10.1242/dev.01268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bondar T., Medzhitov R. p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell. 2010;6:309–322. doi: 10.1016/j.stem.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhiner C., Díaz B., Portela M., Poyatos J.F., Fernández-Ruiz I., López-Gay J.M., Gerlitz O., Moreno E. Persistent competition among stem cells and their daughters in the Drosophila ovary germline niche. Development. 2009;136:995–1006. doi: 10.1242/dev.033340. [DOI] [PubMed] [Google Scholar]
- 18•.Vermeulen L., Morrissey E., van der Heijden M., Nicholson A.M., Sottoriva A., Buczacki S., Kemp R., Tavaré S., Winton D.J. Defining stem cell dynamics in models of intestinal tumor initiation. Science. 2013;342:995–998. doi: 10.1126/science.1243148. [DOI] [PubMed] [Google Scholar]; This paper shows that stem cells with an activating mutation in Kras or loss of apc outcompete wild-type stem cells in the colonic crypts of the mouse intestine without changing overall tissue architecture. Moreover, they show that mutations in p53 may confer a competitive advantage depending on the ‘fitness status’ of surrounding cells.
- 19.Sansom O.J., Meniel V.S., Muncan V., Phesse T.J., Wilkins J.A., Reed K.R., Vass J.K., Athineos D., Clevers H., Clarke A.R. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446:676–679. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- 20.Moreno E. Is cell competition relevant to cancer? Nat Rev Cancer. 2008;8:141–147. doi: 10.1038/nrc2252. [DOI] [PubMed] [Google Scholar]
- 21••.Sancho M., Di-Gregorio A., George N., Pozzi S., Sánchez J.M., Pernaute B., Rodríguez T.A. Competitive interactions eliminate unfit embryonic stem cells at the onset of differentiation. Dev Cell. 2013;26:19–30. doi: 10.1016/j.devcel.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Co-cultures of different types of mouse embryonic stem cells reveal that c-Myc driven cell competition eliminates unfit embryonic stem cells including cells with compromised BMP-signaling, autophagy or tetraploid genomes during early steps of differentiation.
- 22••.Clavería C., Giovinazzo G., Sierra R., Torres M. Myc-driven endogenous cell competition in the early mammalian embryo. Nature. 2013;500:39–44. doi: 10.1038/nature12389. [DOI] [PubMed] [Google Scholar]; This study reveals for the first time naturally occurring cell competition in mice during early embryonic development, which leads to enrichment of pluripotent stem cells with high c-Myc levels and therefore anabolic capacity before the onset of differentiation.
- 23.Senoo-Matsuda N., Johnston L.A. Soluble factors mediate competitive and cooperative interactions between cells expressing different levels of Drosophila Myc. Proc Natl Acad Sci U S A. 2007;104:18543–18548. doi: 10.1073/pnas.0709021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Portela M., Casas-Tinto S., Rhiner C., López-Gay J.M., Dominguez O., Soldini D., Moreno E. Drosophila SPARC is a self-protective signal expressed by loser cells during cell competition. Dev Cell. 2010;19:562–573. doi: 10.1016/j.devcel.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Li W., Baker N.E. Engulfment is required for cell competition. Cell. 2007;129:1215–1225. doi: 10.1016/j.cell.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 26.Lolo F.N., Casas-Tintó S., Moreno E. Cell competition time line: winners kill losers, which are extruded and engulfed by hemocytes. Cell Rep. 2012;2:526–539. doi: 10.1016/j.celrep.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 27••.Martins V.C., Busch K., Juraeva D., Blum C., Ludwig C., Rasche V., Lasitschka F., Mastitsky S.E., Brors B., Hielscher T., Fehling H.J., Rodewald H.R. Cell competition is a tumour suppressor mechanism in the thymus. Nature. 2014 doi: 10.1038/nature13317. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]; The authors show that cell competition regulates the turnover and quality of T-cell progenitors in the mouse thymus. If the competitive interaction among bone-marrow derived and thymus resident T-cell progenitors is suppressed, the old thymus resident progenitors overproliferate and finally turn into cancerous cells.
- 28.Moreno E. Darwinian Tumor suppression in the thymus. Nature. 2014 (Epub ahead of print) [Google Scholar]
- 29.Bernardes de Jesus B., Blasco M.A. Telomerase at the intersection of cancer and aging. Trends Genet. 2013;29:513–520. doi: 10.1016/j.tig.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhiner C., López-Gay J.M., Soldini D., Casas-Tinto S., Martín F.A., Lombardia L., Moreno E. Flower forms an extracellular code that reveals the fitness of a Cell to its neighbors in Drosophila. Dev Cell. 2010;18:1–14. doi: 10.1016/j.devcel.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 31••.Merino M.M., Rhiner C., Portela M., Moreno E. “Fitness fingerprints” mediate physiological culling of unwanted neurons in Drosophila. Curr Biol. 2013;23:1300–1309. doi: 10.1016/j.cub.2013.05.053. [DOI] [PubMed] [Google Scholar]; This paper highlights the importance of fitness indicator proteins for cell selection processes in Drosophila. It shows the existence of neuron-specific fitness fingerprints, which can mediate cell selection during developmentally regulated apoptosis.
- 32.Bell K.F., Hardingham G.E. The influence of synaptic activity on neuronal health. Curr Opin Neurobiol. 2011;21:299–305. doi: 10.1016/j.conb.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao C., Deng W., Gage F.H. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 34.Bergami M., Berninger B. A fight for survival: the challenges faced by a newborn neuron integrating in the adult hippocampus. Dev Neurobiol. 2012;72:1016–1031. doi: 10.1002/dneu.22025. [DOI] [PubMed] [Google Scholar]
- 35.de la Rosa E.J., de Pablo F. Cell death in early neural development: beyond the neurotrophic theory. Trends Neurosci. 2000;23:454–458. doi: 10.1016/s0166-2236(00)01628-3. [DOI] [PubMed] [Google Scholar]
- 36.Keddy P.A. 2nd ed. Springer; 2001. Competition. ISBN-10:1402002297. [Google Scholar]
- 37.Oertel M., Menthena A., Dabeva M.D., Shafritz D.A. Cell competition leads to a high level of normal liver reconstitution by transplanted fetal liver stem/progenitor cells. Gastroenterology. 2006;130:507–520. doi: 10.1053/j.gastro.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 38.Bahar R., Hartmann C.H., Rodriguez K.A., Denny A.D., Busuttil R.A., Dollé M.E., Calder R.B., Chisholm G.B., Pollock B.H., Klein C.A., Vijg J. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature. 2006;441:1011–1014. doi: 10.1038/nature04844. [DOI] [PubMed] [Google Scholar]
- 39.Abrams M. What determines biological fitness? The problem of the reference environment. Synthese. 2009;166:21–40. [Google Scholar]