Abstract
BACKGROUND
Thromboelastography (TEG) is used to diagnose perturbations in clot formation and lysis that are characteristic of acute traumatic coagulopathy (ATC). With novel functional fibrinogen (FF) TEG, fibrin- and platelet-based contributions to clot formation can be elucidated to tailor resuscitation and thromboprophylaxis. We sought to describe the longitudinal contributions of fibrinogen and platelets to clot strength after injury, hypothesizing that low levels of functional fibrinogen and a low contribution of fibrinogen to clot strength on admission would be associated with coagulopathy, increased transfusion requirements, and worse outcomes.
METHODS
603 longitudinal plasma samples were prospectively collected from 251 critically-injured patients at a single Level 1 Trauma Center from 0–120h. TEG maximal amplitude (MA), FF MA, FF levels (FLEV), von Clauss fibrinogen, and standard coagulation measures were performed in parallel. Percentage contributions of FF (%MAFF) and platelets (%MAplatelets) were calculated as each MA divided by overall kaolin TEG MA.
RESULTS
Coagulopathic patients (INR>=1.3) had significantly lower admission %MAFF than non-coagulopathic patients (24.7% vs. 31.2%, p<0.05). Patients requiring plasma transfusion had a significantly lower admission %MAFF (26.6% vs. 30.6%, p<0.05). Higher admission %MAFF was predictive of reduced mortality (HR 0.815, p<0.001). %MAplatelets was higher than %MAFF at all time points, decreased over time, and stabilized at 72 hours (69.4% at 0h, 56.2% at 72h). In contrast, %MAFF increased over time and stabilized at 72 hours (30.6% at 0h, 43.8% at 72).
CONCLUSION
FF TEG affords differentiation of fibrin- versus platelet-based clot dynamics. Coagulopathy and plasma transfusion were associated with a lower %MAFF. Despite this importance of fibrinogen, platelets had a greater contribution to clot strength at all time points after injury. This suggests that attention to these relative contributions should guide resuscitation and thromboprophylaxis, and that antiplatelet therapy may be of under-recognized importance to thromboprophylaxis after trauma.
LEVEL OF EVIDENCE
Level IV; prognostic
Keywords: Functional fibrinogen, clot strength, thromboprophylaxis
BACKGROUND
The accurate identification and treatment of acute traumatic coagulopathy (ATC) is of utmost importance in the care of trauma patients, as these patients have significant associated morbidity and mortality (1–4). Indeed, the 25–33% of severely injured patients who suffer from ATC often present with uncontrolled hemorrhage and have a high incumbent mortality unless rapid hemostatic resuscitation is instituted. Because of this, the timely identification and treatment of ATC is crucial. Viscoelastic testing via thromboelastography (TEG) is poised to replace traditional plasma-based tests (INR, PTT) in the diagnosis of perturbations in clot formation and lysis characteristic of ATC, as well as the guidance of resuscitation and thromboprophylaxis (5–10). TEG measures clot formation and lysis in real-time in a sample of whole blood by producing a characteristic tracing from which several parameters are identified.
Clot formation is the end result of a sequential protease activation cascade resulting in platelet activation and fibrin deposition and crosslinking. The TEG parameter maximal amplitude (MA) is purported to be a measure of total clot strength, but until recently the relative contributions of platelets and fibrin to this clot strength have been unknown. Historically, the function of fibrinogen was represented by the TEG parameters kinetic time and alpha angle, as they are measures of the rapidity of fibrin buildup and crosslinking, but the correlations between these measures and fibrinogen levels measured by the traditional von Clauss assay (11) have been suboptimal (12, 13). Recently however, the relative fibrin- and platelet-based contributions to clot formation can be elucidated with by the addition of the functional fibrinogen (FF) TEG. Harr et al. recently validated FF TEG in a small cohort of trauma patients (12). This assay monitors activated clot formation in the presence of a glycoprotein IIb/IIIa receptor blocker. In this modified TEG assay, all viscoelastic parameters measured are the result of fibrin deposition alone because the glycoprotein IIb/IIIa receptor blocker antagonizes the platelet contribution to clot strength (MA), and therefore any remaining clot strength (MA) is due to the fibrin contribution (14, 15). From this FF TEG, two important parameters are obtained. The first, functional fibrinogen maximal amplitude (FF MA) is the maximal clot strength due to the polymerization of fibrin alone. The second, functional fibrinogen level (FLEV) is calculated by analytical software through a transformation of the FF MA to approximate the concentration of ‘functional’ fibrinogen contained in the sample (16). This crucial differentiation between platelet and fibrin contributions to clot strength can assist in tailoring both early resuscitation and later thromboprophylaxis. We sought to explore the longitudinal relative contributions of fibrinogen and platelets to clot strength after injury, hypothesizing that a low level of functional fibrinogen (FLEV) and a low contribution of fibrinogen to clot strength on admission would be associated with coagulopathy, transfusion requirements, and worse outcomes.
METHODS
Longitudinal plasma samples were prospectively collected from 251 critically-injured trauma patients at a single Level 1 Trauma Center on arrival and at 2, 3, 4, 6, 12, 24, 48, 72, 96, and 120 hours after admission to a Level I urban trauma intensive care unit (ICU).
Our methodology for collection of whole blood for viscoelastic testing has been described previously (17). Briefly, admission samples were collected via initial placement of a 16G or larger peripheral intravenous line; subsequent samples were collected via indwelling arterial catheters. Standard laboratory vacuum-sealed tubes containing 3.2% (0.109 mol/L) sodium citrate were used for all draws. After a waiver of consent was applied for initial blood draws, informed consent was obtained from all patients, as approved by the University of California Committee on Human Research. A total of 603 samples were analyzed on 251 patients. Demographics, resuscitation data, clinical laboratory results, and outcomes were collected in parallel. Point-of-care thromboelastography (TEG) was performed to assess viscoelastic properties of clot formation with the TEG 5000 (Haemonetics; Niles, Il) immediately after sample collection. One mL of citrated whole blood was added to a manufacturer-standardized vial containing the clotting activator kaolin and mixed. Following this, 340 uL was transferred from the kaolin vial to the TEG cup, warmed to 37°C, and recalcified with 20 uL of 0.2 mol/L CaCl2. For the FF TEG, 500 uL of citrated blood was added to the FF vial (kaolin + glycoprotein IIb/IIIa antagonist) and mixed; 340 uL was then transferred to the TEG cup, and warmed and recalcified as above. In parallel, plasma fibrinogen concentration was assayed by the von Clauss method (11) and plasma-based standard coagulation measures were performed. Platelet contribution to clot strength was calculated as MATEG−MAFF=MAplatelets. Percentage contributions of FF (%MAFF) and platelets (%MAplatelets) were calculated as each respective MA divided by the overall kaolin TEG MA. Coagulopathy was defined by admission INR>=1.3. Thrombocytopenia was defined by platelets <= 200. Multi-organ failure was defined using the Denver Postinjury Multiple Organ Failure Score (18–20).
Data are presented as mean (SD), median (interquartile range), or percentage; univariate comparisons were made using Student’s t test for normally distributed data, Wilcoxon rank sum or Kruskal Wallis testing for skewed data, and Fisher’s exact test for proportions. Intergroup comparisons between multiple groups were only judged significant when corrected for multiple comparisons using a standard Bonferroni correction. Linear regression was used to assess correlations between prospectively collected TEG values and laboratory values. Cox proportional hazards regression was used to identify predictors of mortality. An [alpha] = 0.05 was considered significant. All analysis was performed by the authors using Stata version 12 (StataCorp, College Station, TX).
RESULTS
The 251 patients were a standard trauma population: median age 35 years (24–50 years), 80.7% male, and blunt mechanism of injury in 52.6%. A median ISS score of 9 and median admission base deficit of −3.1 (−8.7−1.5) reflected an injured population (Table 1). The median INR on admission was 1.1; coagulopathy (INR>=1.3) was present in 16.7% of patients. Median admission platelet count and fibrinogen were within respective normal ranges (platelet 274×109/L, IQR 229–335; fibrinogen 221 mg/dL, IQR 163–294; Table 1). Platelets contributed a median of 69.5% and fibrinogen a median of 30.5% to clot strength on admission (Table 1). 31.6% of the cohort was transfused with packed red blood cells (pRBC), 18.9% with plasma (FFP), and 14.2% with platelets within 24 hours. Multi-organ failure developed during admission in 4.4%, and in-hospital mortality was 10.2% (Table 1).
TABLE 1.
Patient Demographics/Outcomes
| N=251 | |
|---|---|
| Age (years) | 35 (24–50) |
| Male | 80.7% |
| BMI (kg/m2) | 27.2 +/− 6.4 |
| Blunt mechanism | 52.6% |
| Injury severity score | 9 (1–19) |
| Admit GCS | 15 (9–15) |
| Pre-hospital crystalloid volume (mL) | 50 (0–250) |
| Admit temperature (°) | 36.4 +/− 0.9 |
| Admit pH | 7.3 +/− 0.1 |
| Admit base deficit | −3.2 +/− 6.1 |
| Admit INR >=1.3 | 16.7% |
| Admit INR | 1.1 (1.0–1.2) |
| Admit PTT (sec) | 28.2 (26.4–31.5) |
| Admit platelets <=200×109/L | 13.4% |
| Admit platelets (x 109/L) | 274 (229–335) |
| Admit fibrinogen (mg/dL) | 221 (163–294) |
| Admit FLEV (mg/dL) | 367.4 +/−88.5 |
| Fibrinogen percent contribution to clot at admit | 30.5% (27.6–34.2) |
| Platelet percent contribution to clot at admit | 69.5% (65.8–72.4) |
| Transfused pRBC in 24 hours | 31.6% |
| Transfused FFP in 24 hours | 18.9% |
| Transfused platelets in 24 hours | 14.2% |
| Total hospital days | 4 (2–10) |
| Total ICU days (to 28 days) | 0 (0–4) |
| Ventilator free days (to 28 days) | 28 (24–28) |
| Acute lung injury | 7.8% |
| Multi-organ failure | 4.4% |
| Mortality at discharge | 10.2% |
Patient demographics for the 251 patients. Data are mean +/− SD, median (inter-quartile range), or percentage. Data for skewed variables reported as median with inter-quartile ranges. Ventilator free days are counted for the first 28 days of hospitalization. Patients who expired received 0 ventilator free days.
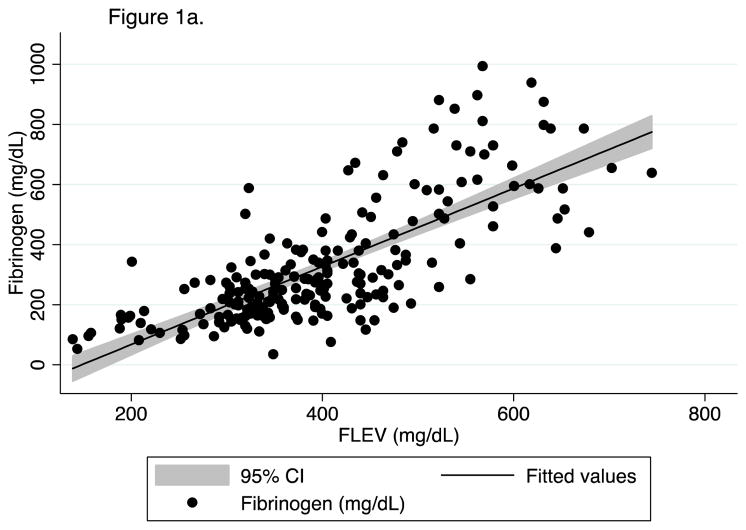
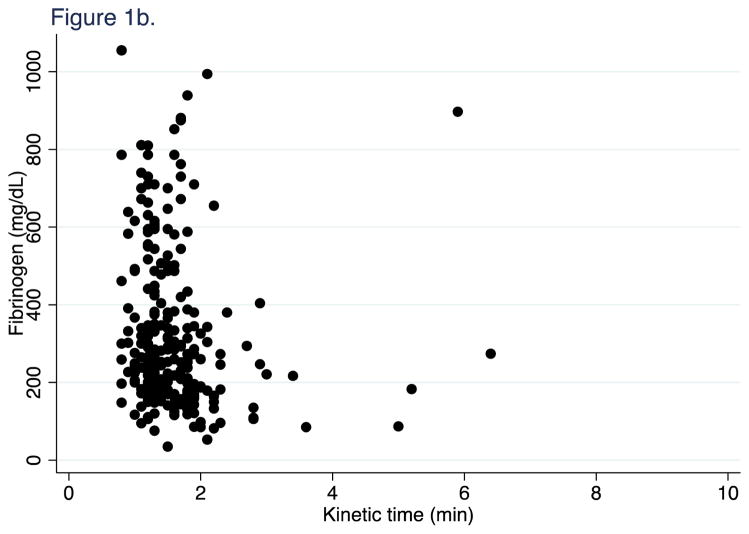
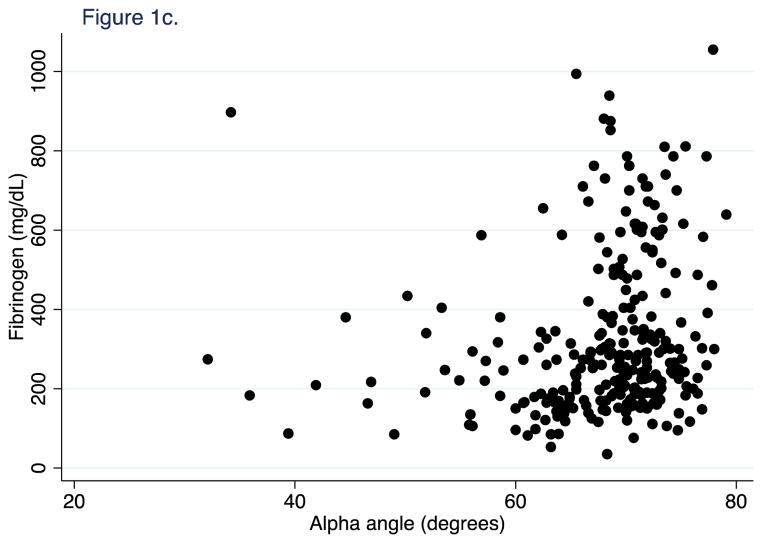
First, we confirmed in our patient population the recently published finding from Harr et al. (12) that FLEV correlates with standard von Clauss fibrinogen better than the historic TEG measures of fibrinogen function (kinetic time and alpha angle). 603 FF TEGs were performed on longitudinal samples from 251 patients. For all time points, FLEV correlated strongly with von Clauss fibrinogen levels (R2=0.57, p<0.001; Figure 1a), but weakly with TEG kinetic time and alpha angle (R2=0.01, p=0.095; and R2=0.03, p=0.004, Figure 1b & 1c). We then confirmed that clot strength (CK MA) and FLEV correlated on each day out to 5 days (0h R2=0.55, p<0.001; 24h R2=0.56, p<0.001; 48h R2=0.44, p<0.001; 72h R2=0.61, p<0.001; 96h R2=0.64, p<0.001; 120h R2=0.49, p<0.001).
Figure 1.
Figure 1a. Correlation of FLEV on fibrinogen at all time points.
FLEV (functional fibrinogen level) mg/dL. Fibrinogen (mg/dL).
Figure 1b. Correlation of kinetic time on fibrinogen at all time points
Kinetic time (min). Fibrinogen (mg/dL).
Figure 1c. Correlation of alpha angle on fibrinogen at all time points.
Alpha angle (degrees). Fibrinogen (mg/dL).
Next, for univariate comparisons between patients with different levels of functional fibrinogen (FLEV) on admission, we isolated patients with high and low functional fibrinogen levels from mid-range functional fibrinogen levels by dividing the cohort into percentiles by FLEV. Patients in the 0–25th FLEV percentile had median values of 281 mg/dL (IQR 235–307); those in the 25th–75th FLEV percentile had median values of 370 mg/dL (IQR 343–398); and those in the 75th–100th percentile had median values of 461 mg/dL (IQR 440–501; Table 2). The patients in the lowest FLEV percentile were younger than those in the highest FLEV percentile (‘low’ median age 26.5 years, IQR 23–41; ‘high’ median age 40 years, IQR 33–51.5; p<0.017 corrected for multiple comparisons), but had no statistically significant differences in injury severity or admission base deficit (all p>0.05; Table 2).
TABLE 2.
Patient Demographics/Outcomes by admit FLEV percentiles
| 0–25th Percentile (N=54) | 25–75th Percentile (N=95) | 75–100th Percentile (N=48) | p-value | |
|---|---|---|---|---|
| Age (years) | 26.5 (23–41) | 33.5 (24–49) | 40 (33–52) | 0.003 |
| Male | 91.0% | 75.5% | 78.7% | 0.071 |
| BMI (kg/m2) | 26.1 +/−4.4 | 27.3 +/− 6.8 | 31.4 +/− 8.3 | 0.017 |
| Blunt mechanism | 48.1% | 48.4% | 41.7% | 0.738 |
| Injury severity score | 4 (1–21) | 4.5 (1–11.5) | 9 (1–14) | 0.468 |
| Admit GCS | 15 (9–15) | 15 (10–15) | 15 (13.5–15) | 0.239 |
| Pre-hospital crystalloids volume (mL) | 100 (0–250) | 50 (0–100) | 62.5 (0–120) | 0.059 |
| Admit temperature (°) | 36.5 +/− 0.7 | 36.5 +/− 0.7 | 36.6 +/− 0.7 | 0.628 |
| Admit pH | 7.26 +/− 0.2 | 7.34 +/− 0.1 | 7.32 +/− 0.1 | 0.006 |
| Admit base deficit | −3.7 +/− 6.4 | −1.7 +/− 4.7 | −3.2 +/− 6.2 | 0.518 |
| Admit INR >=1.3 | 25.0% | 7.4% | 7.9% | 0.015 |
| Admit INR | 1.1 (1–1.25) | 1.1 (1–1.1) | 1.1 (1–1.1) | 0.077 |
| Admit PTT (sec) | 18.8 (26.8–33.0) | 27.7 (25.8–29.7) | 28.1 (26.3–32.8) | 0.027 |
| Admit platelets <=200×109/L | 20.8% | 7.4% | 2.6% | 0.036 |
| Admit platelets (x 109/L) | 238 (202–282) | 275.5 (230–317) | 289.5 (254–374) | 0.001 |
| Admit fibrinogen (mg/dL) | 159 (139–201) | 228 (170–300) | 276 (225–382) | <0.001 |
| Admit FLEV (mg/dL) | 281 (235–307) | 370 (343–398) | 461 (440–501) | <0.001 |
| Fibrinogen percent contribution to clot at 0h | 25.6 (22.2–27.3) | 30.6 (29.2–32.5) | 36.8 (35.0–38.8) | <0.001 |
| Platelet percent contribution to clot at 0h | 74.4 (72.7–77.8) | 69.4 (67.5–70.8) | 63.2 (61.3–65.0) | <0.001 |
| Transfused pRBC in 24 hours | 29% | 14.3% | 33.3% | 0.030 |
| Transfused FFP in 24 hours | 22.4% | 8.3% | 5.5% | 0.033 |
| Transfused platelets in 24 hours | 16.3% | 7.1% | 8.3% | 0.262 |
| Total hospital days | 3 (1–7) | 3 (1–5.5) | 3 (2–8) | 0.889 |
| Total ICU days (to 28 days) | 0.5 (0–3) | 0 (0–2) | 0 (0–2) | 0.201 |
| Ventilator free days (to 28 days) | 28 (23–28) | 28 (26–28) | 28 (28–28) | 0.092 |
| Acute lung injury | 13% | 4.7% | 6.7% | 0.529 |
| Multi-organ failure | 5.6% | 1.1% | 4.2% | 0.197 |
| Mortality at discharge | 12% | 9.4% | 5.4% | 0.658 |
Patient demographics for the 197 patients with FLEV at 0 hour. Data are mean +/− SD, median (inter-quartile range), or n (%) as indicated. For not-normally distributed variables reported as median with inter-quartile ranges. Ventilator free days are counted for the first 28 days of hospitalization. Patients who expired received 0 ventilator free days.
However, those in the lowest admission FLEV percentile had the highest rate of coagulopathy (25%) and thrombocytopenia (20.8%), and not surprisingly the lowest von Clauss fibrinogen levels (159 mg/dL, IQR 139–201, p<0.017 corrected for multiple comparisons; all p<0.05; Table 2). Patients in both the lowest and highest admission FLEV percentiles had higher packed red blood cell transfusion requirements (29% in ‘low’; 14.3% in ‘mid’; 33.3% in ‘high’; p=0.030). However, the patients in the lowest FLEV percentile had the highest plasma transfusion requirements (22.4% in ‘low’; 8.3% in ‘mid’; 5.5% in ‘high’; p=0.033). Those patients in the lowest FLEV percentile trended toward higher mortality at discharge (12% in ‘low’; 9.4% in ‘mid’; 5.4% in ‘high’; non-significant, p=0.658; Table 2). Higher admission FLEV predicted reduced mortality in an unadjusted model (hazard ratio 0.991, p=0.005), whereas von Clauss fibrinogen did not (hazard ratio 1.00, p=0.601).
Next, we compared patients with low, intermediate, and high clot strength by CK MA percentiles (mean CK MA 57.7mm vs. 65.4mm vs. 72.6mm, p=0.0010, Supplement 1). The 51 patients with the lowest clot strength (mean CK MA 57.7) were younger (mean age 36.1 vs. 37.3 vs. 44.9, p<0.017 corrected for multiple comparisons), but had no significant difference in mean injury severity (13.2 vs. 10.1 vs. 9.9, p=0.1493, Supplement 1) than those patients with intermediate or high clot strength. As expected, those with the lowest clot strength had the highest percentage with coagulopathy (26.1% vs. 11.3% vs. 8.9%, p=0.0470), but no statistically significant differences in transfusion needs (24h transfusion of pRBC/FFP 28.2% vs. 21.4% vs. 27.9%, p=0.5990, Supplement 1) compared to those with intermediate or high clot strength. We then stratified by high and low FLEV levels, split at the mean for patients in each percentile of clot strength (Table 3). Notably, the 24 patients with low clot strength and low FLEV trended toward higher rates of coagulopathy than those with low clot strength and high FLEV (34.8% vs. 10.0%, p=0.0760). Additionally, they had significantly worse outcomes, requiring more transfusion of RBC/FFP in 24h (40.9% vs. 4.8%, p=0.0090), longer ICU stays (median 1 day vs. 0 day, p=0.0089), fewer ventilator-free days (median 27 days vs. 28 days, p=0.0172), and trended toward a higher mortality (26.1% vs. 4.8%, p=0.0970, Table 3). However, the patients with normal and high clot strength similarly stratified by FLEV levels had no significant differences in coagulopathy, transfusion, or mortality (Supplement 2 & 3).
TABLE 3.
Demographics/Outcomes of patients with low clot strength, stratified by low/high FLEV
| Low FLEV (N=24) | High FLEV (N=24) | p-value | |
|---|---|---|---|
| Age (years) | 35.0 +/− 20.2 | 35.4 +/− 16.0 | 0.800 |
| Male | 91.7% | 83.3% | 0.666 |
| BMI (kg/m2) | 25.1 +/− 3.3 | 26.4 +/− 5.8 | 0.651 |
| Blunt mechanism | 58.3% | 33.3% | 0.147 |
| Injury severity score | 17.0 +/− 21.1 | 6.7 +/− 8.0 | 0.108 |
| Admit GCS | 10 (7–26) | 15 (14–15) | 0.008 |
| Pre-hospital crystalloid volume (mL) | 100 (0–250) | 50 (0–250) | 0.746 |
| Admit temperature (°) | 36.1 +/− 0.84 | 36.6 +/− 0.4 | 0.083 |
| Admit pH | 7.21 +/− 0.13 | 7.33 +/− 0.11 | 0.010 |
| Admit base deficit | −5.30 +/− 7.41 | −3.04 +/− 5.70 | 0.765 |
| Admit INR >=1.3 | 34.8% | 10.0% | 0.076 |
| Admit platelets <=200×109/L | 17.4% | 25.0% | 0.405 |
| Admit fibrinogen (mg/dL) | 151.5 (119.5–177) | 211 (152–300) | 0.056 |
| Admit FLEV (mg/dL) | 262.8 (196.15–287.4) | 328.5 (319.3–350.4) | <0.001 |
| Admit CK MA (mm) | 57.1 +/− 4.5 | 58.3 +/− 2.6 | 0.571 |
| Fibrinogen percent contribution to clot at 0h | 24.1 (18.5–26.6) | 31.4 (29.7–34.0) | <0.001 |
| Platelet percent contribution to clot at 0h | 75.9 (73.4–81.5) | 68.6 (66.0–70.0) | <0.001 |
| Transfused in 24 hours (RBC/FFP) | 40.9% | 4.8% | 0.009 |
| Transfused platelets in 24 hours | 18.1% | 4.8% | 0.345 |
| Total hospital days | 3 (1–7) | 2 (1–4) | 0.572 |
| Total ICU days (to 28 days) | 1 (0–3) | 0 (0–0) | 0.009 |
| Ventilator free days (to 28 days) | 27 (0–28) | 28 (28–28) | 0.017 |
| Acute lung injury | 22.2% | 0.0% | 0.156 |
| Thromboembolic complication | 4.3% | 0.0% | 0.523 |
| Multi-organ failure | 8.3% | 0.0% | 0.489 |
| Mortality at discharge | 26.1% | 4.8% | 0.097 |
Patient demographics for the 48 patients with low clot strength and admission FLEV (functional fibrinogen level) data. Data are mean +/− SD, median (inter-quartile range), or n (%) as indicated. Data for skewed variables reported as median with inter-quartile ranges. Ventilator free days are counted for the first 28 days of hospitalization. Patients who expired received 0 ventilator free days.
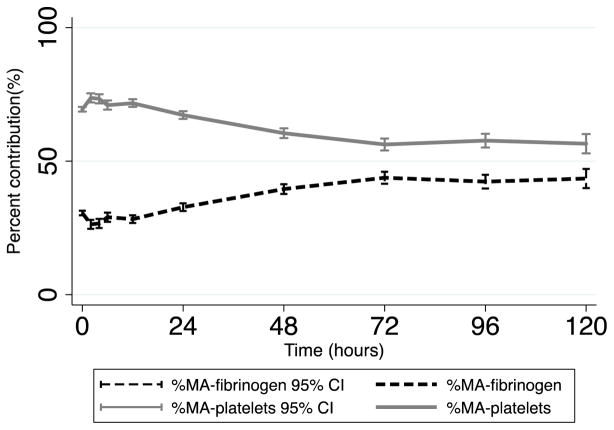
Following this, we calculated the percent contribution of fibrin to clot strength (%MAFF) and the percent contribution of platelets (%MAplatelets) to clot strength at all time points after injury as described in the methods, and performed univariate comparisons by outcomes. Coagulopathic patients (INR>=1.3) had significantly lower admission %MAFF than non-coagulopathic patients (Table 4; 24.7% vs. 31.2%, p=0.009). In addition, patients requiring plasma transfusion had a significantly lower admission %MAFF (Table 4; 26.6% vs. 30.6%, p=0.030). Patients who expired by 24 hours had significantly lower admission %MAFF than those who survived (Table 4; 16.8% vs. 30.6% p=0.010). In fact, higher admission %MAFF was predictive of reduced mortality in an unadjusted model (hazard ratio 0.871, p<0.001). Using linear regression, we found that a 10% increase in admission %MAFF was associated with an INR decrease of 0.1, 24 hour red blood cell transfusion decrease of 2.3 units, and 24 hour plasma transfusion decrease by 1.7 units (all p<0.05). Most notable, %MAplatelets was higher than %MAFF at all time points, decreased over time, and stabilized at 72 hours (69.4% at 0h, 56.2% at 72h; Figure 2). In contrast, %MAFF increased over time and stabilized at 72 hours (30.6% at 0h, 43.8% at 72h; Figure 2).
TABLE 4.
Mean percent contribution of fibrinogen to clot strength by outcomes
| Percent contribution of fibrinogen | p-value | |
|---|---|---|
| Alive at 24 hours | 30.6 +/− 5.5 | 0.010 |
| Dead at 24 hours | 16.8 +/− 10.3 | |
| Tranfused pRBC in 24 hours | 29.3 +/− 7.9 | 0.519 |
| Not transfused pRBC in 24 hours | 30.4 +/− 5.3 | |
| Tranfused FFP in 24 hours | 26.6 +/− 8.1 | 0.030 |
| Not transfused FFP in 24 hours | 30.6 +/− 5.5 | |
| Transfused platelets in 24 hours | 27.4 +/− 8.6 | 0.259 |
| Not transfused platelets in 24 hours | 30.5 +/− 5.6 | |
| Plts<=200×109/L | 27.7 +/− 6.4 | 0.069 |
| Plts>200×109/L | 30.7 +/− 6.0 | |
| INR<1.3 | 31.2 +/− 5.04 | 0.009 |
| INR>=1.3 | 24.7 +/− 9.4 |
Mean percent contribution of fibrinogen to clot strength at admission for the 197 patients with admission functional fibrinogen testing. Data are mean +/− SD.
Figure 2. Mean percent contribution to clot strength over time.
%-MA fibrinogen (mean percent contribution of fibrinogen to clot strength). %-MA platelets (mean percent contribution of platelets to clot strength).
DISCUSSION
Fundamental hemostatic capacity can be attributed to a combination of the rapidity of formation, absolute strength, and breakdown of clot. These mutual determinants of clot formation (or failure thereof) account for the dynamic spectrum that spans from thrombosis to hemorrhage. Fibrin and platelets are the primary contributors to the absolute clot strength, which is represented by the TEG parameter MA, yet until now the relative contribution of fibrin deposition to clot strength over time after injury has been unknown. Additionally, knowledge regarding the relative contribution of platelets to hemostasis has been missing. These crucial knowledge gaps may contribute to the polar differences in international resuscitation practices and thromboprophylaxis. Addressing these unknown relative contributions of fibrinogen and platelets to clot strength after injury are of critical importance for evidence-based guidance of early resuscitation and later thromboprophylaxis (21), given opposing fibrinogen-based European and platelet-based United States resuscitation practices (22–26).
With the addition of the TEG functional fibrinogen test, novel differentiation of fibrin- versus platelet-based clot dynamics can assist in tailoring both early resuscitation and later thromboprophylaxis. Our data confirms recently published work by Harr et al. (12) suggesting that functional fibrinogen levels correlate with standard fibrinogen levels better than the historic TEG measures of fibrin buildup and crosslinking (kinetic time and alpha angle). In addition, we found that patients with low admission functional fibrinogen levels are more commonly coagulopathic and require more transfusions, suggesting a role for FF testing in the evaluation of ATC and the future prediction of transfusion needs. Most importantly, patients with higher admission functional fibrinogen levels had reduced mortality whereas patients with higher von Clauss fibrinogen did not; In fact, for a 1mg/dL increase in functional fibrinogen levels, there was a 1% decrease in the risk of mortality at discharge (hazard ratio 0.991, p=0.005). This new functional measurement may better predict mortality in real-time compared to standard laboratory fibrinogen levels.
Notably, in stratification of patients into low, intermediate, and high clot strength, the finding that only in patients with the lowest clot strength does a low functional fibrinogen level associate with coagulopathy, transfusion needs, and worse outcomes (without a difference in injury severity) suggests that the level of functional fibrinogen is of critical importance in patients with an overall deficiency in the strength of the clot. Injured patients with poor overall clot strength and a low functional fibrinogen level may be the appropriate target population for transfusion with fibrinogen containing products.
We next demonstrated that in injured patients, both coagulopathy and need for plasma transfusion were associated with a lower percent contribution of fibrinogen to clot strength. In addition, a higher admission percent contribution to clot strength predicted reduced mortality. For a 1% increase in the contribution of fibrinogen to clot strength, there was a 12.9% decrease in the risk of mortality at discharge (hazard ratio 0.871, p<0.001). These findings suggest a major importance of fibrinogen to the hemostatic function of the clot. However, despite the importance we found of fibrinogen function in relation to coagulopathy, transfusion needs and mortality, we demonstrated that platelets have a larger contribution to clot strength at all time points after injury. While specific perturbations of fibrinogen and platelets and their role in the functional dynamics of ATC are not completely characterized, our findings suggest that attention to the relative contribution of fibrinogen and platelet function should guide both early resuscitation and later thromboprophylaxis.
As with other single-center prospective studies of traumatic coagulation, several limitations exist. Fibrinogen accounts for approximately 20% of clot strength in normal individuals (12, 21), however our study demonstrates that after injury, fibrinogen accounts for 30.5% of clot strength on admission which increased to 43.5% over the ensuing 5 days. Like all physiologic responses after injury, the injury response is very different from a normal population and in order to characterize biomarkers such as functional fibrinogen, we must characterize them in a trauma population in relation to outcomes. Fibrinogen is an acute phase reactant and may be on the whole higher in patients after injury. Alternatively, the increase in the fibrinogen contribution to clot strength after injury may be compensatory for the platelet dysfunction seen after injury (27, 28). Additionally, while we can calculate the relative contributions of fibrinogen and platelets to clot strength, it remains unknown what the importance of each is in ATC. More research is needed to better understand what the critical functional deficit is here. Patients with an overall deficit in clot strength and low functional fibrinogen levels may be the appropriate target population for fibrinogen containing resuscitation, however it is unclear whether fibrinogen transfusion will actually correct this deficit. Additionally, given that we demonstrated a critical role of functional fibrinogen levels on outcome, the finding that platelets contribute more to clot after injury than fibrinogen leaves the critical issue of the functional role of platelets unanswered. The functional role of platelets needs to be addressed. Additionally, if anti-platelet therapy is of under-recognized importance for thromboprophylaxis, the clinical use of anti-platelet agents after trauma needs significant study.
In conclusion, given our findings that low functional fibrinogen levels and a low percent contribution of fibrinogen to clot strength on admission were associated with coagulopathy, plasma transfusion and mortality, early attention to correction of functional fibrinogen deficits may be a useful resuscitation goal. Deeper understanding of the importance of fibrinogen levels during resuscitation may prompt more rapid correction of deficits by earlier, more liberal use of fibrinogen concentrates. Appealingly, as plasma units also contain fibrinogen, a component of the observed benefit of plasma-based resuscitation strategies may also be related to earlier correction of fibrinogen deficits. Both of these issues deserve ongoing study, for which FF TEG will be a critical tool. Concordantly, given that platelets played a greater role in clot strength at all time points after injury, these data confirm recent findings that antiplatelet therapy may be of under-recognized importance to adequate thromboprophylaxis after trauma (21). In fact, the routine use of heparin-based thromboprophylaxis after trauma, by failing to inhibit platelets, may under-treat the predominant contributor to clot strength at all time points after injury. This issue is of utmost importance to the appropriate treatment of the continued epidemic of hypercoagulability after trauma. Future investigations into hypercoagulability after injury using functional fibrinogen TEG to examine these relative contributions are underway. Indeed we understand that the coagulation milieu after injury is dynamic and complex and it is in this multivariate context that the optimal role of functional fibrinogen testing will be understood and further studied.
Supplementary Material
Acknowledgments
Funding: Supported by NIH GM-085689 (MJC), NIH T32 GM-08258-20 (MEK), NIH HL090833 and HL110969 (CSC), and NIH T32 GM-008258-25 (LZK).
Footnotes
AUTHOR CONTRIBUTIONS:
LZK, MEK, and MJC contributed to study design, data collection, data analysis, data interpretation, writing, and clinical revision.
BJR, CSC, RFV contributed to study design, data collection, and clinical revision.
Conflicts of interest: None
Meetings: Oral presentation at the 2013 annual meeting of the American Association for the Surgery of Trauma in San Francisco, CA 9/2013
Contributor Information
Lucy Z Kornblith, Email: lucy.kornblith@ucsfmedctr.org.
Matthew E Kutcher, Email: matthew.kutcher@ucsfmedctr.org.
Brittney J Redick, Email: redickb@sfghsurg.ucsf.edu.
Carolyn S Calfee, Email: carolyn.calfee@ucsf.edu.
Ryan F Vilardi, Email: vilardir@sfghsurg.ucsf.edu.
Mitchell Jay Cohen, Email: mcohen@sfghsurg.ucsf.edu.
References
- 1.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. The Journal of trauma. 2003;55(1):39–44. doi: 10.1097/01.TA.0000075338.21177.EF. Epub 2003/07/12. [DOI] [PubMed] [Google Scholar]
- 2.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. The Journal of trauma. 2003;54(6):1127–30. doi: 10.1097/01.TA.0000069184.82147.06. Epub 2003/06/19. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MJ, Call M, Nelson M, Calfee CS, Esmon CT, Brohi K, Pittet JF. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Annals of surgery. 2012;255(2):379–85. doi: 10.1097/SLA.0b013e318235d9e6. Epub 2011/12/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen MJ, West M. Acute traumatic coagulopathy: from endogenous acute coagulopathy to systemic acquired coagulopathy and back. The Journal of trauma. 2011;70(5 Suppl):S47–9. doi: 10.1097/TA.0b013e31821a5c24. Epub 2011/08/24. [DOI] [PubMed] [Google Scholar]
- 5.Kheirabadi BS, Crissey JM, Deguzman R, Holcomb JB. In vivo bleeding time and in vitro thrombelastography measurements are better indicators of dilutional hypothermic coagulopathy than prothrombin time. The Journal of trauma. 2007;62(6):1352–9. doi: 10.1097/TA.0b013e318047b805. discussion 9–61. Epub 2007/06/15. [DOI] [PubMed] [Google Scholar]
- 6.Martini WZ, Cortez DS, Dubick MA, Park MS, Holcomb JB. Thrombelastography is better than PT, aPTT, and activated clotting time in detecting clinically relevant clotting abnormalities after hypothermia, hemorrhagic shock and resuscitation in pigs. The Journal of trauma. 2008;65(3):535–43. doi: 10.1097/TA.0b013e31818379a6. Epub 2008/09/12. [DOI] [PubMed] [Google Scholar]
- 7.Park MS, Martini WZ, Dubick MA, Salinas J, Butenas S, Kheirabadi BS, Pusateri AE, Vos JA, Guymon CH, Wolf SE, et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. The Journal of trauma. 2009;67(2):266–75. doi: 10.1097/TA.0b013e3181ae6f1c. discussion 75–6. Epub 2009/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plotkin AJ, Wade CE, Jenkins DH, Smith KA, Noe JC, Park MS, Perkins JG, Holcomb JB. A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. The Journal of trauma. 2008;64(2 Suppl):S64–8. doi: 10.1097/TA.0b013e318160772d. Epub 2008/04/11. [DOI] [PubMed] [Google Scholar]
- 9.Holcomb JB, Minei KM, Scerbo ML, Radwan ZA, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Khan S, et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Annals of surgery. 2012;256(3):476–86. doi: 10.1097/SLA.0b013e3182658180. Epub 2012/08/08. [DOI] [PubMed] [Google Scholar]
- 10.Carroll RC, Craft RM, Langdon RJ, Clanton CR, Snider CC, Wellons DD, Dakin PA, Lawson CM, Enderson BL, Kurek SJ. Early evaluation of acute traumatic coagulopathy by thrombelastography. Translational research : the journal of laboratory and clinical medicine. 2009;154(1):34–9. doi: 10.1016/j.trsl.2009.04.001. Epub 2009/06/16. [DOI] [PubMed] [Google Scholar]
- 11.Clauss A. Rapid physiological coagulation method in determination of fibrinogen. Acta haematologica. 1957;17(4):237–46. doi: 10.1159/000205234. Epub 1957/04/01. Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens. [DOI] [PubMed] [Google Scholar]
- 12.Harr JN, Moore EE, Ghasabyan A, Chin TL, Sauaia A, Banerjee A, Silliman CC. Functional fibrinogen assay indicates that fibrinogen is critical in correcting abnormal clot strength following trauma. Shock. 2013;39(1):45–9. doi: 10.1097/SHK.0b013e3182787122. Epub 2012/12/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeger V, Willi S, Liu T, Yeh DD, De Moya M, Zimmermann H, Exadaktylos AK. The Rapid TEG alpha-Angle may be a sensitive predictor of transfusion in moderately injured blunt trauma patients. The Scientific World Journal. 2012;2012:821794. doi: 10.1100/2012/821794. Epub 2012/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson JJ, Waly HM, Wilson JM. Fundamentals of coagulation and glycoprotein IIb/IIIa receptor inhibition. American heart journal. 1998;135(4):S35–42. doi: 10.1016/s0002-8703(98)70296-0. Epub 1998/04/16. [DOI] [PubMed] [Google Scholar]
- 15.Mousa SA, Forsythe MS. Comparison of the effect of different platelet GPIIb/IIa antagonists on the dynamics of platelet/fibrin-mediated clot strength induced using thromboelastography. Thrombosis research. 2001;104(1):49–56. doi: 10.1016/s0049-3848(01)00336-x. Epub 2001/10/05. [DOI] [PubMed] [Google Scholar]
- 16.Fluger I, Maderova K, Simek M, Hajek R, Zapletalova J, Lonsky V. Comparison of functional fibrinogen assessment using thromboelastography with the standard von Clauss method. Biomedical papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia. 2012;156(3):260–1. doi: 10.5507/bp.2011.035. Epub 2012/06/05. [DOI] [PubMed] [Google Scholar]
- 17.Kutcher ME, Cripps MW, McCreery RC, Crane IM, Greenberg MD, Cachola LM, Redick BJ, Nelson MF, Cohen MJ. Criteria for empiric treatment of hyperfibrinolysis after trauma. The journal of trauma and acute care surgery. 2012;73(1):87–93. doi: 10.1097/TA.0b013e3182598c70. Epub 2012/06/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC. Postinjury multiple organ failure: a bimodal phenomenon. The Journal of trauma. 1996;40(4):501–10. doi: 10.1097/00005373-199604000-00001. discussion 10–2. Epub 1996/04/01. [DOI] [PubMed] [Google Scholar]
- 19.Sauaia A, Moore FA, Moore EE, Lezotte DC. Early risk factors for postinjury multiple organ failure. World journal of surgery. 1996;20(4):392–400. doi: 10.1007/s002689900062. Epub 1996/05/01. [DOI] [PubMed] [Google Scholar]
- 20.Sauaia A, Moore EE, Johnson JL, Ciesla DJ, Biffl WL, Banerjee A. Validation of postinjury multiple organ failure scores. Shock. 2009;31(5):438–47. doi: 10.1097/SHK.0b013e31818ba4c6. Epub 2008/10/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harr JN, Moore EE, Chin TL, Ghasabyan A, Gonzalez E, Wohlauer MV, Banerjee A, Silliman CC, Sauaia A. Platelets are dominant contributors to hypercoagulability after injury. The journal of trauma and acute care surgery. 2013;74(3):756–62. doi: 10.1097/TA.0b013e3182826d7e. discussion 62–5. Epub 2013/02/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schochl H, Nienaber U, Maegele M, Hochleitner G, Primavesi F, Steitz B, Arndt C, Hanke A, Voelckel W, Solomon C. Transfusion in trauma: thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy. Crit Care. 2011;15(2):R83. doi: 10.1186/cc10078. Epub 2011/03/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schochl H, Cotton B, Inaba K, Nienaber U, Fischer H, Voelckel W, Solomon C. FIBTEM provides early prediction of massive transfusion in trauma. Crit Care. 2011;15(6):R265. doi: 10.1186/cc10539. Epub 2011/11/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schochl H, Forster L, Woidke R, Solomon C, Voelckel W. Use of rotation thromboelastometry (ROTEM) to achieve successful treatment of polytrauma with fibrinogen concentrate and prothrombin complex concentrate. Anaesthesia. 2010;65(2):199–203. doi: 10.1111/j.1365-2044.2009.06188.x. Epub 2009/12/10. [DOI] [PubMed] [Google Scholar]
- 25.Schochl H, Nienaber U, Hofer G, Voelckel W, Jambor C, Scharbert G, Kozek-Langenecker S, Solomon C. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care. 2010;14(2):R55. doi: 10.1186/cc8948. Epub 2010/04/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schochl H, Posch A, Hanke A, Voelckel W, Solomon C. High-dose fibrinogen concentrate for haemostatic therapy of a major trauma patient with recent clopidogrel and aspirin intake. Scandinavian journal of clinical and laboratory investigation. 2010;70(6):453–7. doi: 10.3109/00365513.2010.500396. Epub 2010/07/14. [DOI] [PubMed] [Google Scholar]
- 27.Kutcher ME, Redick BJ, McCreery RC, Crane IM, Greenberg MD, Cachola LM, Nelson MF, Cohen MJ. Characterization of platelet dysfunction after trauma. The journal of trauma and acute care surgery. 2012;73(1):13–9. doi: 10.1097/TA.0b013e318256deab. Epub 2012/06/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wohlauer MV, Moore EE, Thomas S, Sauaia A, Evans E, Harr J, Silliman CC, Ploplis V, Castellino FJ, Walsh M. Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. Journal of the American College of Surgeons. 2012;214(5):739–46. doi: 10.1016/j.jamcollsurg.2012.01.050. Epub 2012/04/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.