Abstract
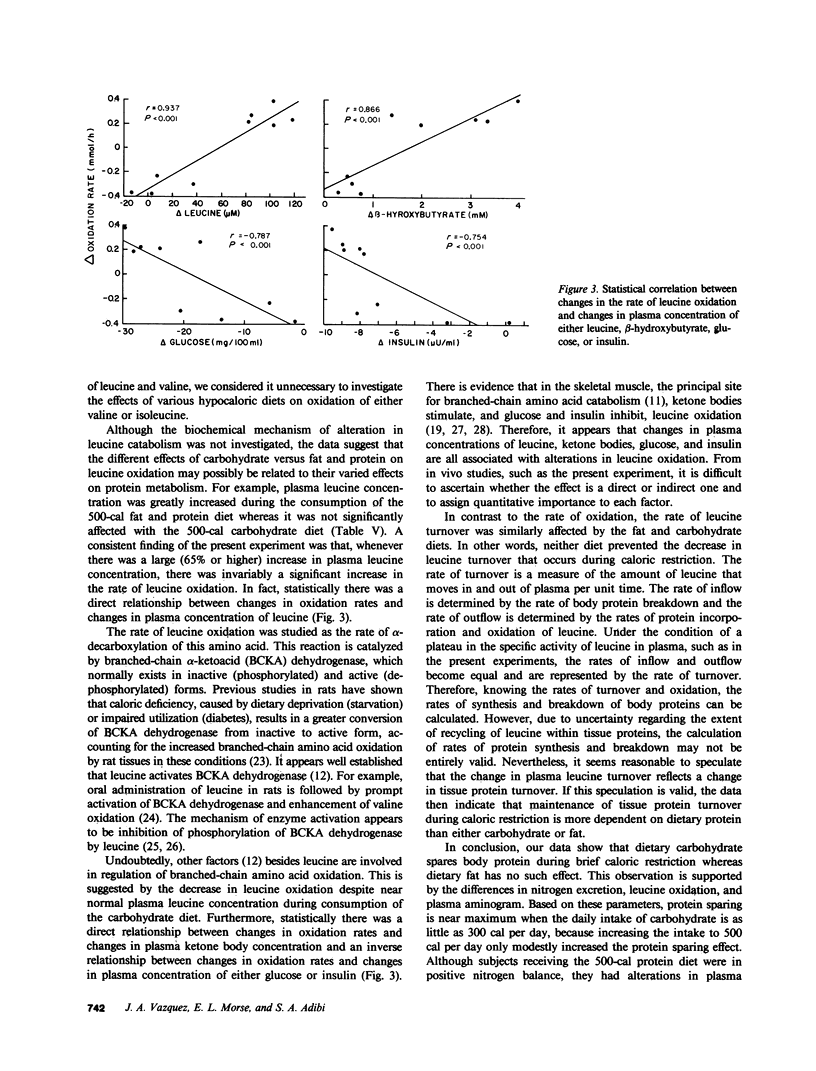
To assess the effect of each dietary caloric source on the catabolism of branched-chain amino acids, we investigated the rate of leucine oxidation before and after obese volunteers consumed one of the following diets for one week: (a) starvation, (b) 300 or 500 cal of fat/d, (c) 300 or 500 cal of carbohydrate/d, (d) 300 or 500 cal of protein/d, (e) a mixture of carbohydrate (300 cal/d) and fat (200 cal/d), or (f) a mixture of carbohydrate (300 cal/d) and protein (200 cal/d). Starvation significantly increased the rate of leucine oxidation (1.4 +/- 0.11 vs. 1.8 +/- 0.16 mmol/h, P less than 0.01). The same occurred with the fat and protein diets. In sharp contrast, the 500-cal carbohydrate diet significantly decreased the rate of leucine oxidation (1.3 +/- 0.13 vs. 0.6 +/- 0.09 mmol/h, P less than 0.01). The same occurred when a portion of the carbohydrate diet was isocalorically replaced with either fat or protein. The cumulative nitrogen excretion during the fat diet and starvation was not significantly different. As compared with the fat diets, the carbohydrate diets on the average reduced the urinary nitrogen excretion by 12 g/wk. Nitrogen balance was positive during the consumption of the 500-cal protein diet, but negative during the consumption of carbohydrate-protein diet. The fat diets, like the protein diets and starvation, greatly increased plasma leucine (119 +/- 13 vs. 222 +/- 15 microM, P less than 0.01) and beta-hydroxybutyrate (0.12 +/- 0.02 vs. 4.08 +/- 0.43 mM, P less than 0.01) concentrations, and significantly decreased plasma glucose (96 +/- 4 vs. 66 +/- 3 mg/dl, P less than 0.01) and insulin (18 +/- 4 vs. 9 +/- 1 microU/ml, P less than 0.05) concentrations. These changes did not occur, or were greatly attenuated, when subjects consumed carbohydrate alone or in combination with fat or protein. We conclude that during brief caloric restriction, dietary lipid and protein, unlike carbohydrate, do not diminish the catabolism of branched-chain amino acids and the decrease in branched-chain amino acid oxidation is associated with protein sparing.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adibi S. A. Alteration in the urinary excretion rate of amino acids and nitrogen by dietary means in obese and normal human subjects. J Lab Clin Med. 1971 Feb;77(2):278–289. [PubMed] [Google Scholar]
- Adibi S. A., Drash A. L. Hormone and amino acid levels in altered nutritional states. J Lab Clin Med. 1970 Nov;76(5):722–732. [PubMed] [Google Scholar]
- Adibi S. A. Influence of dietary deprivations on plasma concentration of free amino acids of man. J Appl Physiol. 1968 Jul;25(1):52–57. doi: 10.1152/jappl.1968.25.1.52. [DOI] [PubMed] [Google Scholar]
- Adibi S. A. Interrelationships between level of amino acids in plasma and tissues during starvation. Am J Physiol. 1971 Sep;221(3):829–838. doi: 10.1152/ajplegacy.1971.221.3.829. [DOI] [PubMed] [Google Scholar]
- Adibi S. A., Mercer D. W. Protein digestion in human intestine as reflected in luminal, mucosal, and plasma amino acid concentrations after meals. J Clin Invest. 1973 Jul;52(7):1586–1594. doi: 10.1172/JCI107335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi S. A. Metabolism of branched-chain amino acids in altered nutrition. Metabolism. 1976 Nov;25(11):1287–1302. doi: 10.1016/s0026-0495(76)80012-1. [DOI] [PubMed] [Google Scholar]
- Adibi S. A. Roles of branched-chain amino acids in metabolic regulation. J Lab Clin Med. 1980 Apr;95(4):475–484. [PubMed] [Google Scholar]
- Adibi S. A., Stanko R. T., Morse E. L. Modulation of leucine oxidation and turnover by graded amounts of carbohydrate intake in obese subjects. Metabolism. 1982 Jun;31(6):578–588. doi: 10.1016/0026-0495(82)90096-8. [DOI] [PubMed] [Google Scholar]
- Block K. P., Harper A. E. Valine metabolism in vivo: effects of high dietary levels of leucine and isoleucine. Metabolism. 1984 Jun;33(6):559–566. doi: 10.1016/0026-0495(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Buse M. G., Reid S. S. Leucine. A possible regulator of protein turnover in muscle. J Clin Invest. 1975 Nov;56(5):1250–1261. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua B., Siehl D. L., Morgan H. E. Effect of leucine and metabolites of branched chain amino acids on protein turnover in heart. J Biol Chem. 1979 Sep 10;254(17):8358–8362. [PubMed] [Google Scholar]
- Felig P., Owen O. E., Wahren J., Cahill G. F., Jr Amino acid metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):584–594. doi: 10.1172/JCI106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Hughes W. A., Halestrap A. P. The regulation of branched-chain 2-oxo acid dehydrogenase of liver, kidney and heart by phosphorylation. Biochem J. 1981 May 15;196(2):459–469. doi: 10.1042/bj1960459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson S. M., Cree T. C., Harper A. E. Regulation of leucine and alpha-ketoisocaproate metabolism in skeletal muscle. J Biol Chem. 1978 Nov 25;253(22):8126–8133. [PubMed] [Google Scholar]
- Matthews D. E., Schwarz H. P., Yang R. D., Motil K. J., Young V. R., Bier D. M. Relationship of plasma leucine and alpha-ketoisocaproate during a L-[1-13C]leucine infusion in man: a method for measuring human intracellular leucine tracer enrichment. Metabolism. 1982 Nov;31(11):1105–1112. doi: 10.1016/0026-0495(82)90160-3. [DOI] [PubMed] [Google Scholar]
- Odessey R., Goldberg A. L. Oxidation of leucine by rat skeletal muscle. Am J Physiol. 1972 Dec;223(6):1376–1383. doi: 10.1152/ajplegacy.1972.223.6.1376. [DOI] [PubMed] [Google Scholar]
- Paul H. S., Adibi S. A. Leucine oxidation and protein turnover in clofibrate-induced muscle protein degradation in rats. J Clin Invest. 1980 Jun;65(6):1285–1293. doi: 10.1172/JCI109791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul H. S., Adibi S. A. Leucine oxidation in diabetes and starvation: effects of ketone bodies on branched-chain amino acid oxidation in vitro. Metabolism. 1978 Feb;27(2):185–200. doi: 10.1016/0026-0495(78)90164-6. [DOI] [PubMed] [Google Scholar]
- Paul H. S., Adibi S. A. Role of ATP in the regulation of branched-chain alpha-keto acid dehydrogenase activity in liver and muscle mitochondria of fed, fasted, and diabetic rats. J Biol Chem. 1982 May 10;257(9):4875–4881. [PubMed] [Google Scholar]
- Paxton R., Harris R. A. Regulation of branched-chain alpha-ketoacid dehydrogenase kinase. Arch Biochem Biophys. 1984 May 15;231(1):48–57. doi: 10.1016/0003-9861(84)90361-8. [DOI] [PubMed] [Google Scholar]
- Rennie M. J., Edwards R. H., Halliday D., Matthews D. E., Wolman S. L., Millward D. J. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond) 1982 Dec;63(6):519–523. doi: 10.1042/cs0630519. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]


