Abstract
TLR9 is a cellular DNA-receptor, which is widely expressed in breast and other cancers. Although synthetic TLR9-ligands induce cancer cell invasion in vitro, the role of TLR9 in cancer pathophysiology has remained unclear. We show here that living cancer cells uptake DNA from chemotherapy-killed cancer cells. We discovered that such DNA induces TLR9- and cathepsin-mediated invasion in living cancer cells. To study whether this phenomenon contributes to treatment responses, triple negative, human MDA-MB-231 breast cancer cells stably expressing control or TLR9 siRNA were inoculated orthotopically into nude mice. The mice were treated with vehicle or doxorubicin. The tumor groups exhibited equal decreases in size in response to doxorubicin. However, while the weights of vehicle-treated mice were similar, mice bearing control siRNA tumors became significantly more cachectic in response to doxorubicin, as compared with similarly treated mice bearing TLR9 siRNA tumors, suggesting a TLR9-mediated inflammation at the site of the tumor. In conclusion, our findings propose that DNA released from chemotherapy-killed cancer cells has significant influence on TLR9-mediated biological effects in living cancer cells. Through these mechanisms, tumor TLR9 expression may affect treatment responses to chemotherapy.
Keywords: Triple negative breast cancer, Toll-like receptor-9, Invasion, DNA, Inflammation
Introduction
Toll like receptor-9 (TLR9) is an innate immune system effector and a cellular DNA-receptor that recognizes microbial and vertebrate DNA.[1,2] Stimulation of TLR9 induces a NF-κB-mediated rapid inflammation, characterized by increased expression of various interleukins and cytokines, and eventually, this inflammation results in the activation of the adaptive immune system and clearance of the invading pathogens.[2] A similar inflammatory response, mediated via TLRs, also takes place in sterile tissue damage.[3–5]
It is now clear that in addition to the cells of the immune system, TLR9 is also widely expressed in various cancer cell lines and in clinical cancer specimens, including breast, prostate, brain, gastric and esophageal cancers.[6–14] In addition to inducing the expression of inflammatory cytokines in TLR9-expressing cancer cells, synthetic TLR9 ligands, which structurally mimic bacterial DNA, also induce invasion in various cancer cells in vitro. Such invasion is mediated through matrix metalloproteinase-13 (MMP-13) activation and down-regulation of tissue inhibitor of matrix metalloproteinases-3 (TIMP-3).[6,11,12] Despite this invasive effect, the significance of TLR9 in the pathophysiology of breast or any cancer has remained unclear. It is also not known what is the physiological TLR9-ligand for such invasion in cancer.
We demonstrated recently, that although widely expressed in all clinical subtypes of breast cancer, TLR9 expression has significant, prognostic significance only in triple negative breast tumors that lack the expression of estrogen (ER), progesterone (PR) and HER2-receptors. More specifically, low tumor TLR9 expression upon diagnosis is associated with a significantly shortened disease-free-specific survival.[7,9] Although we demonstrated that low TLR9-triple negative breast cancer cells become highly invasive in hypoxic conditions, it is currently unclear whether this mechanism contributes to the poor survival of the breast cancer patients that have a similar disease.
The aim of this study was to investigate whether TLR9 expression affects treatment response to chemotherapeutic agents in triple negative breast cancer cells. Furthermore, since host-derived DNA that is derived from dying cells has been shown to induce TLR9-mediated inflammation in living cells, [5,15] we hypothesized that by analogy, DNA from dead cancer cells could serve as an endogenous TLR9-ligand in living cancer cells. LL-37 is a multifunctional, cathelicidin-class antimicrobial peptide, and the only known cathelicidin that is expressed in human tissues.[16,17] It can transfer extra-cellular DNA into mammalian cells and it is also expressed in breast cancers.[16,18,19] Since LL-37 has been shown to promote DNA uptake and mediate endogenous DNA effects on TLR9 in non-cancerous cells, we also investigated the role of LL-37 in these processes in cancer cells.[15,16]
Materials and Methods
Chemicals
The ready-cast Matrigel invasion inserts were from BD Biosciences (Bedford, MA), the MMP-inhibitor GM6001 from Enzo Life Sciences (Farmingdale, NY), the MMP-9 and -13 inhibitors and MMP-inhibitor III were from Calbiochem (EMD Millipore, Billerica, MA). The cathepsin K inhibitor I was bought from EMD Millipore (Billerica, MA). Doxorubicin, taxol and cis-platinum were bought from Sigma (St. Louis, MO). LL-37 was bought from Anaspec (Fremont, CA).
Cell culture
The parental, human triple negative MDA-MB-231 breast cancer and human D54MG glioblastoma cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco BRL, Life Technologies, Paisley, UK) supplemented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 100 IU/ml penicillin/streptomycin and non-essential amino acids (all from Gibco BRL, Life Technologies). Human estrogen receptor expressing (ER+) T47-D breast cancer cells were cultured in RPMI, supplemented with 20% heat-inactivated fetal bovineserum, 100 IU/ml penicillin/streptomycin, 2 mM L-glutamine and with 10 μg/ml insulin (Sigma, St. Louis, MO). The stable control siRNA and TLR9 siRNA MDA-MB-231, T47-D and D54MG cells have been described in detail previously.[9,20] The regular culture medium of these cells was supplemented with neomycin (G418, Invitrogen).[9,20] All cell cultures were done in incubators in a 37°C atmosphere of 5% CO2/95% air.
DNA isolation and complexing with LL-37
MDA-MB-231, T47-D and D54MG cells were treated for 72 h with 10−4 M doxorubicin in serum-free medium. For some experiments, DNA was isolated also from taxol- (10−6 M, 7 days in serum-free medium) or cis-platinum-treated (10−4 M, 7 days in normal growth medium) MDA-MB-231 cells. After these treatments, cell viability was analyzed with Trypan Blue, using the TC10 automated cell counter (Biorad). The supernatants were collected and combined with the trypsinized cells from the bottoms of the same flasks, spun down and DNA was isolated from the pellets, using the QIAamp DNA blood mid kit (Qiagen). The DNA that was isolated from the doxorubicin-treated cells is referred to as dox DNA. Intact DNA was isolated with a similar method from corresponding, steady state, proliferating cells that were cultured in normal growth medium. Cell specific dox DNA or intact DNAs were used in the experiments. DNA concentrations were measured with NanoDrop (Thermo Scientific, Wilmington, DE, USA). For the experiments where DNA was used in LL-37 complexes, DNA (1 μg) and LL-37 (10 μg) were first added to a sterile Eppendorf tube and kept at 37 °C for ~ 30–60 min, after which the formed complexes were added to the cells in the various assays.
DNA labeling and immunofluorescence detection of DNA up-take into MDA-MB-231 cells
The isolated DNAs were labeled with Alexa Fluor 488 using a Random Primers DNA labeling system and AREStm DNA labeling kit (Invitrogen), according to the manufacturer’s instructions. The reaction products were purified with PureLink™ PCR Purification Kit (Invitrogen). The labeled DNAs, alone or in LL-37 complexes were then added to the cell culture medium of viable MDA-MB-231 cells growing on glass coverslips for 15 min. The cells were then fixed with 4% paraformaldehyde for 10 min and permeabilized with 0.025% saponin for 30 min at 4 °C. Nonspecific antibody binding sites were blocked with 2% BSA, 5% Goat serum and 0.025% saponin in PBS at RT and stained for TLR9, using a previously described protocol.[6] Detection of TLR9 was done with Alexa Fluor 555 (Invitrogen). Nuclei were stained with TO-PRO-3-3 iodide (Molecular probes). The samples were examined with LSM510 confocal microscope (ZeissGmbH, Jena, Germany).
RNA isolation and quantitative RT-PCR
Control or TLR9 siRNA MDA-MB-231 cells were treated for 24 h with dox DNA or intact DNA, alone or in complex with LL-37 (1:10, w/w). Total RNA was then isolated from the cells using the Trizol reagent (Invitrogen) and purified with RNeasy mini kits (Qiagen). All TaqMan reagents for the qRT-PCR experiments were purchased from Applied Biosystems. cDNA was synthesized from 0.2 μg of total RNA, using Multiscribe reverse transcriptase and random hexamers. Quantification of TLR9 mRNA expression was done with a primer & probe set that was purchased from Applied Biosystems. For all qRT-PCR assays, a standard amplification program was used (1 cycle of 50 °C for 2 min, 1 cycle of 95 °C for 10 min, 40 cycles of 95 °C for 15 s and 60 °C for 1 min). After normalization with RPLPO expression levels for each cDNA, a relative quantification of target cDNA was performed using 2-Δct values.
Next Generation RNA Sequencing (RNA-seq) and data analysis
RNA-seq was performed from 3 vehicle-treated and 3 dox DNA-treated (100 ng/ml, 24 h, in serum-free culture medium) MDA-MB-231 cell samples. Total RNA was extracted with a Qiagen RNeasy Kit (Valencia, CA) following the manufacturer’s instructions, including an on-column DNase I digestion. Total RNA quality was assessed using an Agilent 2100 bioanalyzer and an Agilent RNA 6000 Nano Kit (Agilent Technologies, Santa Clara, CA). The Ribo-Zero Magnetic Kit (Epicentre, Madison, Wisconsin) was used for an rRNA depletion step, and the samples were concentrated with an RNeasy MinElute Cleanup Kit (Qiagen). The RNA-Seq libraries were prepared with the ScriptSeq v2 RNA-Seq Library Preparation Kit (Epicentre). Library validation was accomplished with Agilent DNA 1000 (Agilent Technologies) and Illumina Library Quantification (Kapa Biosystems, Woburn, MA) Kits. The DNA samples were sequenced using HiSeq 2000 (Illumina) at the UAB Core. The raw fastq files were aligned to the human genome (hg19) in Galaxy instance installed at University of Alabama at Birmingham (UAB, http://galaxy.uabgrid.uab.edu). Pre-alignment was conducted to determine if trimming is needed based on reads quality score. The BAM files were generated following RNA-seq workflow of tophat [21], cufflinks and cuffcompare. [22]. These BAM files were loaded into Partek Genomics Suite 6.6 (Saint Louis, MO) for further analysis. Briefly, the reads per kilobase of exon model per million mapped reads (RPKM)-normalized reads [23] were calculated and the expression levels of genes were estimated.[23–25] The differential expressions were determined by 2 group Student’s t-test. A gene list was then created after false discovery rate (FDR) p value correction by Benjamini and Hochberg method. Further functional analysis was conducted by using Ingenuity Pathway Analysis (IPA, Redwood City, CA).
Zymograms
The cells were cultured on 12-well plates until confluent, after which they were rinsed with sterile PBS and further cultured for 24 h in serum-free media (500 μL per well), with or without the various DNAs (1 μg) or LL-37 (10 μg) alone, or in complex (DNA: LL-37, 1:10 w/w). After the supernatants were collected, they were concentrated with centrifugal filter device (Millipore, cut-off size 3 kDa, cat # UFC5-003-24). Same amount of protein (20–30 μg) was added per lane to the zymogram gels (10% gelatin, Biorad). The gels were then run, re-naturated, developed and stained, using Biorad zymogram buffers, according to the manufacturer’s recommendations. Proteomic analysis of the proteolytic bands in the zymograms was carried out as previously described.[26]
In vitro invasion assays
For the Matrigel-invasion assays, the cells were plated at the density of 2 × 104 cells per upper well in 400 μl of 2% FBS-containing culture medium. The various DNAs (1 μg), alone or in complex with LL-37 (10 μg) were added to the upper wells. When indicated, MMP-inhibitors (GM6001 or MMP-inhibitor III, 2 – 50 μM) or the cathepsin K inhibitor I (2 – 50 μM) were also added to both upper and lower wells, with the indicated concentrations. The cells were allowed to invade for 18 h, after which the inserts were removed and stained with the Hema 3 Stain set (Fisher Diagnostics, Middletown, PA), according to the manufacturer’s recommendation. The number of invaded cells was counted from representative microscopic fields using a 40X objective. [6,11,12]
Cell viability assays
The cells were plated at the density of 10,000 cells/well in 96-well plates in 100 μl of normal culture medium, and cultured for 24 h with dox DNA/LL-37 (1:10, w/w) complexes or with vehicle. Cell viability was assessed after the MTS-reagent was added for the final 0.5 hours of the experimental cultures as recommended by the manufacturer (CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay, Promega, Madison, WI).
Animal studies
The MDA-MB-231 control or TLR9 siRNA cells (5 × 105 cells in 100 μL in PBS, n = 80, with 20 mice in each arm, data combined from two independent experiments), were inoculated into the mammary fat pads of four-week-old, athymic nude mice (Athymic nude/nu Foxn1 mice, Harlan), as previously described.[9] Starting on day 2 after tumor cell inoculation, the mice were divided into vehicle and doxorubicin (5 mg/kg) treatment groups. The mice were treated for a total of 4 times (on day 2 and every 5 days after that), during the experiment, which was terminated on day 21 after the tumor cells were inoculated. Tumor diameters were measured throughout the experiment and tumor volumes were calculated using the formula V = (π/6)(d1 × d2)3/2, where d1 and d2 are the perpendicular tumor diameters.[27] After 3 weeks, the mice were sacrificed and the tumors were dissected and measured. The mice were also weighed throughout the experiment. The animals were maintained under controlled pathogen-free environmental conditions. Animal welfare was monitored daily for clinical signs. The body composition of some mice in both doxorubicin-treated groups were analyzed after sacrifice with dual-energy X-ray absorptiometry, as previously described in detail.[28] The experimental procedures were reviewed and approved by the UAB Institutional Animal Care and Use Committee (IACUC).
Statistical analysis
The results are given as mean ± sd, unless otherwise stated. Student’s t test or Mann-Whitney test was used to calculate statistically significant differences between the various study groups.
Results
Labeled DNAs, which were first isolated from steady state proliferating, MDA-MB-231 cells (intact DNA) or from cells that had been killed with doxorubicin (dox DNA, chemotherapy effects on cell viability are shown in Suppl. Fig. 1.), were added to the cell culture medium of living MDA-MB-231 cells. Uptake of the labeled DNAs into the living MDA-MB-231 cells was visualized by confocal microscope. Both intact and dox DNAs were rapidly (within 15 min) taken up into the living cells (Figs. 1a–b). The cathelicidin peptide LL-37 has been shown to increase cellular DNA uptake.[16] Complexing with LL-37 was also required for TLR9-recognition of self-DNA in dendritic cells. [15] Since LL-37 is also expressed in breast and other cancers, [18,29] we asked whether complexing with LL-37 affects DNA uptake also in cancer cells. Interestingly, LL-37 complexed DNA (both intact and dox) accumulated as large clusters with very restricted localization inside the cells. These clusters were shown to partially co-localize with TLR9 positive structures. In contrast, un-complexed DNA was more randomly distributed in vesicles that also co-localized with TLR9. (Figs. 1c–d). In conclusion, these results suggested that complexing with LL-37 indeed enhances cellular up-take of both intact and dox DNAs (Figs. 1c–d).
Figure 1.
Alexa Fluor 488-labeled DNA (green) that was first isolated from proliferating (intact DNA) or doxorubicin-treated MDA-MB-231 cells (dox DNA), was added alone, or in complex with LL-37 to the cell culture medium of intact MDA-MB-231 cells. The cells were fixed 15 min after DNA addition and DNA uptake was assessed with confocal microscope. TLR9 was visualized with Alexa Fluor 555-conjugated antibodies (red), nuclei were stained with To-Pro-3 and pseudo-colored with blue. In merged images co-localization is shown as yellow. Scale bar 5 μm.
We have previously shown that synthetic TLR9-ligands (CpG-sequence containing oligonucleotides, such as ODN M362) induce TLR9-mediated invasion in cancer cells in vitro. [6,11,12] To investigate whether cancer cell-derived DNAs affect cellular invasion, we added the DNAs, alone or complexed with LL-37 to various cancer cells in invasion assays. While LL-37, or DNA alone had no effects, dox DNA/LL-37 complexes (1:10, w/w) were demonstrated to induce a significant increase in invasion in parental MDA-MB-231 cells (Fig. 2a). This invasion was not due to changes in cell numbers, as demonstrated by the lack of effect on cell viability by such complexes (Suppl. Fig. 2.). Similar effects on invasion were also detected in parental human T47-D breast cancer and in D54MG glioblastoma cells (Fig. 2b) and also with DNA that was isolated from MDA-MB-231 cells that were first killed with taxol or cis-platinum (Fig. 2c). To investigate the role of TLR9 in this response, the invasion assays were repeated with stable control siRNA or TLR9 siRNA cells. Dox DNA/LL-37 complexes induced invasion in control siRNA cells, but not in TLR9 siRNA cells, thus suggesting that the pro-invasive effect wasTLR9 -mediated (Fig. 2d).
Figure 2.
Parental A) MDA-MB-231 cells (n=3, mean ± S.D., * p<0.05 vs. vehicle) or B) T47-D and D54MG cells (n=6 – 10, mean ± S.E.M., * p<0.05, *** p<0.001 vs. corresponding control) were treated with DNA (1 μg) derived from proliferating of doxorubicin-killed cells, alone or in complex with LL-37 (10 μg) and the cells were allowed to invade through Matrigel-coated invasion inserts. Cells that invaded through the membranes were counted microscopically. C) MDA-MB-231 cells were also treated with LL-37-complexes in which DNA was isolated from taxol or cis-platinum-treated MDA-MB-231 cells (n=4, mean ± S.E.M., ** p<0.01 vs. vehicle). D) Similar studies were conducted also with stable control and TLR9 siRNA cells (n=3 – 14, mean ± S.E.M., * p <0.05 vs. vehicle-treatment, # p < 0.05 vs. corresponding control siRNA cells).
ODN M362 (a synthetic TLR9-ligand) induces cell invasion that is mediated via TIMP-3 suppression and MMP-13 activation in cancer cells.[6,11,12] To investigate the role of TIMP-3 and MMPs in the pro-invasive effects of the cell-derived DNAs, we studied their effects on matrix metalloproteinase (MMP-2, -9 and -13) and TIMP-3 mRNA expression with qRT-PCR. While LL-37 or intact DNA alone or in complex with LL-37 had no effects, treatment with dox DNA alone induced a significant up-regulation of MMP-2, MMP-9 and MMP-13 mRNA expression in the control siRNA cells and significantly less so, also in the TLR9 siRNA cells. Complexing with LL-37 did not change dox DNA effects on MMP-2 and MMP-13 mRNAs in either cell line. However, complexing the dox DNA with LL-37 slightly suppressed MMP-9 mRNA expression, as compared with dox DNA alone in both control and TLR9 siRNA cells (Supplementary Figs. 3a–c). Finally, while other treatments had no effect, dox DNA alone induced a significant reduction in TIMP-3 mRNA expression in both control and TLR9 siRNA cells at a similar level. This effect on TIMP-3 mRNA was significantly enhanced by complexing the dox DNA with LL-37 in the control siRNA cells, but not in TLR9 siRNA cells, (Supplementary Fig. 3d). Taken together, these results suggested that while DNA from intact cells is inert in this regard, DNA from doxorubicin-killed cancer cells increases MMP-2, -9 and -13 mRNA expression through TLR9 in living cancer cells. Furthermore, the only pro-invasive effect that was enhanced by complexing dox DNA with LL-37 was the further decrease in TIMP-3 mRNA expression, and also that was TLR9-mediated. These results suggest that MMP activation may mediate the dox DNA/LL-37-induced invasion downstream of TLR9. However, wide-spectrum MMP inhibitors (GM6001 or MMP-inhibitor III), or specific MMP-9 or MMP-13 inhibitors did not inhibit such invasion in MDA-MB-231 cells when tested with various concentrations (Supplementary Fig. 4, data shown only for the highest MMP-inhibitor III concentration).
We then analyzed the proteolytic effects of the various DNA-treatments with zymography. Unlike previously detected with the synthetic TLR9-ligand ODN M362, [12,30] we did not detect an induction of MMP-13 activity or changes in MMP-2 or -9 activity by the cell derived DNAs. Both intact DNA and even more strongly, dox DNA-treatment, however, induced the formation of high molecular weight (~ 100 kDa) proteolytic band in the zymograms. This band was of a higher molecular weight than those the typical MMP-2 and MMP-9 bands detected on MDA-MB-231 zymograms.[12,30] Such high molecular weight band was not induced by LL-37 alone, and its appearance was not obviously enhanced by complexing the DNAs with LL-37. Unlike the ODN M362 induced MMP-13 bands,[12,30] these bands were unaffected when the zymograms were developed in the presence of MMP-9 inhibitor or a general MMP-inhibitor (GM6001). Similar bands were also observed after DNA-treatment of control siRNA MDA-MB-231 cells, but not in the TLR9 siRNA cells (Supplementary Fig. 5). Furthermore, a similar pattern of proteolytic bands was induced by supernatants of parental T47-D cells, or from control siRNA T47-D and control siRNA D54MG cells, but not by those of TLR9 siRNA T47-D or D54-MG cells, suggesting that their formation is TLR9-mediated (Supplementary Fig. 6a–b). To identify the protein responsible for the proteolysis, we analyzed the dox DNA/LL-37 induced high molecular weight band from the zymograms of D54MG cells with proteomics. No amino acid sequences corresponding with MMPs were discovered. Instead, the found amino acid sequences suggested the presence of cathepsins in the dox DNA/LL-37-induced proteolytic band (data not shown). Based on these findings, and also because cathepsin K has been shown to be important in TLR9-signaling, [31] we tested the effect of cathepsin K- inhibitor I on the dox DNA/LL-37-complex-induced invasion of parental MDA-MB-231 cells. In line with the proteomics findings, the dox DNA/LL-37-complex-induced invasion was completely blocked with 10 μM cathepsin K-inhibitor I (Fig. 3). This effect was achieved with inhibitor concentrations that did not affect cell viability (Supplementary Fig. 7). In conclusion, these results suggest that despite the invasion promoting effects on the MMP/TIMP-3 mRNAs, dox DNA/LL-37-complex induced invasion is not MMP, but rather cathepsin-mediated in viable cancer cells.
Figure 3.
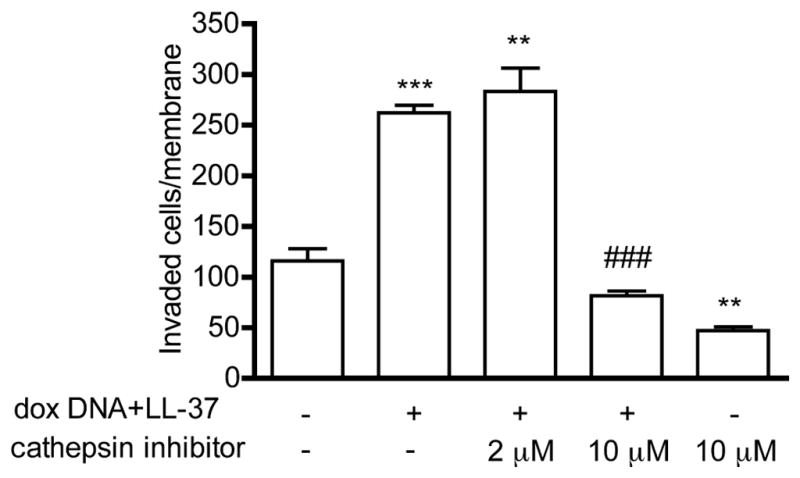
Parental MDA-MB-231 cells were treated with dox DNA-LL-37-complexes (1:10, w/w) in invasion assays, in the presence of vehicle or 2 or 10 uM cathepsin K inhibitor, as indicated. Cells that invaded through the membranes were counted microscopically. Data is expressed as mean ± S.E.M., n = 3, ** p <0.01, *** p<0.001 vs. vehicle-treated cells, ### p<0.001 vs. dox DNA/LL-37-complex-treated cells.
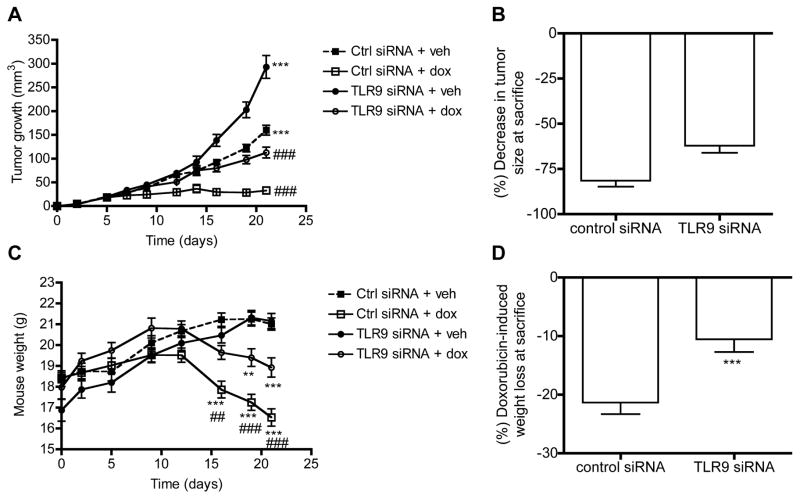
We next investigated whether such DNA-induced invasion had a role in treatment responses. More specifically, since local tumor growth is the sum of proliferation and local invasion, we hypothesized that if invasion-inducing DNA-structures are released upon chemotherapy-treatment and subsequently internalized by viable cells of the same tumor, then TLR9-expressing (control siRNA) tumors might shrink less in response to treatment. This is because, the described DNA-induced and TLR9-mediated local invasion could contribute to the local growth of such tumors. As a corollary, tumors with reduced TLR9 expression (TLR9 siRNA tumors), thus lacking this invasive mechanism, might respond better to treatment in vivo. To study this, the control and TLR9 siRNA MDA-MB-231 cells were inoculated into the mammary fat pads of nude mice. The mice were then divided into treatment-groups and starting on day 2 after tumor cell inoculation, treated with doxorubicin (5 mg/kg) or with the same volume of vehicle. The mice were treated for a total of 4 times during the experiment. As expected based on our earlier studies, the TLR9 siRNA MDA-MB-231 cells grew into significantly larger orthotopic tumors than the control siRNA cells.[9] Doxorubicin-treatment induced a significant reduction in tumor sizes in both mouse groups, as compared with vehicle-treatment. Although the TLR9 siRNA tumors appeared to be shrink less than the control siRNA tumors, the difference was not statistically significant (Fig. 4a–b). This effect was unexpected, since the TLR9 siRNA cells actually exhibited also increased in vitro sensitivity to doxorubicin (Supplementary Fig. 8). Although there were no weight differences between the vehicle-treated mice, the doxorubicin-treated control siRNA tumor-bearing mice lost significantly more weight than the corresponding doxorubicin-treated, TLR9 siRNA tumor-bearing mice (Fig. 4c–d). The weight loss was mostly due to loss of lean tissue mass, suggesting tumor TLR9-mediated and inflammation-induced cachexia (Fig. 5). Since an inflammatory reaction is a hallmark of TLR-activation, we hypothesized that the more pronounced weight loss in the control siRNA tumor-bearing mice might be caused by a vicious cycle, whereby DNA derived from dead cells induces TLR9-mediated release of cachexia-inducing cytokines from living cancer cells (Fig. 6). We initially tested this hypothesis by adding 100 ng of dox DNA to the cell culture medium of parental MDA-MB-231 cells for 24 h and performing RNA-seq analyses, to determine whether such treatment has any effects on the expression of inflammatory mediators. Several genes involved in inflammatory reactions were significantly affected by the dox DNA-treatment, suggesting that tumor-derived DNA can indeed induce the expression of inflammatory proteins in surviving cancer cells (Suppl. Table I.).
Figure 4.
Control or TLR9 siRNA MDA-MB-231 cells were inoculated orthotopically into the mammary fat pads of nude mice. The mice were then treated with vehicle or doxorubicin (5 mg/kg) for a total of 4 times during the experiment. A) Tumor sizes were measured as a function of time, n = 20–40 in each arm, data is expressed as mean ± S.E.M., *** p <0.001 vs. corresponding control siRNA-tumors, ### p< 0.001 vs. corresponding vehicle-treatment. B) Tumor shrinkage is expressed as % decrease in size as compared against the mean tumor sizes of corresponding vehicle-treated tumors in each group at sacrifice. Mean ± S.E.M., n = 36 –38. C) Mouse weights as a function of time, n = 20 in each arm, mean ±S.E.M., ** p < 0.01, *** p < 0.001 vs. corresponding vehicle-treatment, ## p < 0.01, ### p < 0.001 vs. corresponding TLR9 siRNA-group. D) Doxorubicin-induced weight loss at sacrifice, data is expressed as % weight loss as compared with the weights of the corresponding vehicle-treated mice at sacrifice, mean ± S.E.M., *** p< 0.001 vs. control siRNA-group, n=18–19 in each group.
Figure 5.
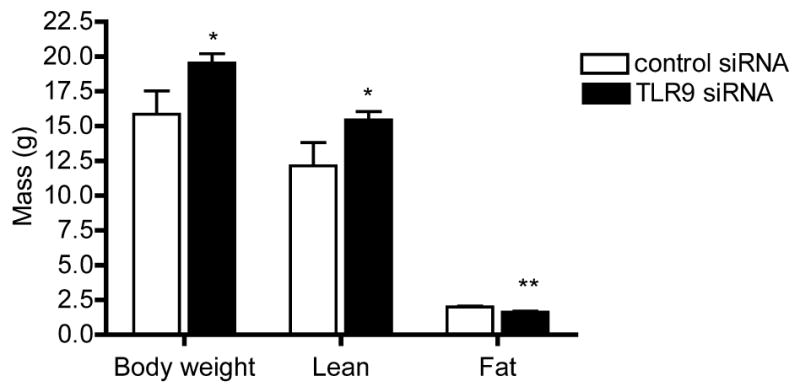
Body composition of doxorubicin-treated mice at sacrifice, as determined by dual-energy X-ray absorptiometry. Data is expressed as mean± S.E.M., n =3 –8, * p<0.05, ** p<0.01 vs. mice bearing TLR9 siRNA cell tumors.
Figure 6.
The proposed mechanism of tumor TLR9 function in anti-tumor immune response during chemotherapy.
Discussion
The innate immune system DNA-receptor TLR9 is widely expressed in various cancer types.[13,14,32] Despite the fact that synthetic TLR9-ligands and bacterial DNA induce invasion in various cancer cells in vitro, [6,11,12,33] the role of TLR9 in cancer pathophysiology has remained unclear. Also, the prognostic significance of TLR9 in cancers appears to be bipartite. In some cancers, such as gliomas and oesophageal adenocarcinomas, high tumor TLR9 expression has been associated with poor survival whereas in others, such as triple negative breast cancer or renal cell carcinoma, low tumor TLR9 expression upon diagnosis predicts poor prognosis.[9,14,32,34] In addition to the actual tumor cells, also the TLR9 expression status of tumor associated fibroblast-like cells has been shown to be of prognostic value in breast cancer.[35] In this context, high TLR9 expression was associated with better prognosis.[35] Furthermore, although self DNA has been shown to be TLR9-ligand in human diseases, such as psoriasis, rheumatoid arthritis and SLE, there is no previous information on whether self DNA could be a TLR9-ligand for cancer, and if so, what are the consequences of such a reaction.[15,36–38]
We demonstrate here for the first time that self DNA that is derived from chemotherapy-treated, dead cancer cells is rapidly taken up into surviving cancer cells, where it then serves as an invasion-inducing TLR9-ligand. Interestingly, in line with observations in psoriasis, complexing these DNAs with LL-37 enhances DNA up-take into living cells and is a requirement for the invasion-inducing effects in cancer cells.[15,16] Whether the LL-37 actually facilitates TLR9-recognition, or only acts as a carrier for the DNA needs to be characterized in further experiments. Such complex formation may, however, also be physiologically relevant as the expression of the multifunctional, antimicrobial peptide LL-37 has also been demonstrated in clinical breast cancer tissues.[18,19] The fact that intact cell-derived DNA does not induce invasion suggests that upon cell death, specific DNA-structures with biological activity are formed, possibly through the action of cell death-activated DNAses [39]. The structure of such DNAs requires further characterization, but the findings by Napirei and coworkers suggest that they may be resistant to extracellular nucleases.[40] Interestingly, doxorubicin as a DNA intercalating drug may induce the formation of such nuclease-resistant structures.[41] Our results suggest, however, that the formation of such pro-invasive DNA structures is not limited to doxorubicin alone, because also other chemotherapeutic drugs had similar effects. We further demonstrated that this invasion is mediated via cathepsins and surprisingly, not via MMPs, which are the mediators for CpG-sequence-containing oligonucleotide-induced invasion.[6,11,12,30] Our findings thus suggest that the DNA-induced up-regulation of MMP mRNAs could be suppressed by specific miRNAs, but this too requires further investigation. Proteolytic cleavage by cathepsins, has been shown to be a prerequisite for TLR9 signaling in cells of the immune system.[42,43] Our new findings further suggest that in addition to facilitating TLR9 activation by nucleic acids, cathepsins may also mediate downstream effects of TLR9 activation. In principle, such DNA-induced and TLR9-mediated cancer cell invasion could represent a novel mechanism of treatment resistance. Our in vivo results, however, suggest that either such invasion-inducing DNA structures are not formed in response to treatment in vivo or that they are not important in this particular cancer model. Furthermore, it is likely that the doxorubicin concentrations in the in vivo tumors are much smaller than those used in the in vitro treatments.[44,45] As a consequence, the in vivo and in vitro doxorubicin exposures may result in different forms of cell death and thereby, also different amounts and structures of DNA released from dying cancer cells. To clarify the in vivo significance of this finding, pre-clinical metastasis models should be used, as well as also cancer cells representing tumors in which high TLR9 expression has been associated with poor prognosis.[14,34] Nevertheless, circulating DNA derived from dead cancer cells has been detected in the plasma samples of cancer patients. Such DNA has also been associated with poor prognosis in patients with metastatic disease. [46,47]
Doxorubicin is a well known inducer of cachexia, and similar observations were made also in the in vivo experiments performed here. [48,49] Surprisingly, however, doxorubicin-induced cachexia was significantly dependent on the tumor TLR9-expression status. Several cytokines have been shown to mediate cachexia, including IL-8, TNF-α and MIC-1.[50–54] On the other hand, dying cells have been shown to release numerous biomolecules, including DNA, that may act as TLR-stimulating and inflammation-inducing endogenous danger signals to alert the innate immune system.[55,56] We hypothesize that the tumor TLR9-dependent, treatment-induced weight loss is actually a surrogate marker for DNA-induced and TLR9-mediated inflammation that takes place at the site of the tumors. More specifically, we predict that the DNA fragments released from dead cancer cells to be taken up by surviving cells of the same tumors, in which TLR9-mediated recognition of the DNA results in inflammation. Although we predict that the TLR9-triggering molecule in this scenario are DNA fragments derived from dead cancer cells, the danger signal that first alerts the innate immune system requires specification in further experiments. This is because, in addition to DNA, also histone proteins and hemozoin have been shown to activate TLR9, and thus it is possible that also other parts of the dying cells contribute to the TLR9-mediated inflammation. [4,57] Our RNA-seq results however, lend support to the hypothesis that DNA from the doxorubicin-killed cancer cells is the mediator for the inflammation.
It has been shown that anthracyclin chemotherapeutics induce an immunogenic form of apoptotic cell death. In response to such immunogenic cell death, an adaptive immune response, which is capable of eradicating residual tumors, develops. [55,56] Our results indirectly suggest that tumor TLR9 expression, at least in triple negative breast cancer cells, is an important regulator of immunogenic response to cell death. The tumor TLR9-mediated inflammatory response at the tumor site may amplify anti-tumor immune response, eradicate microscopic disease and through this mechanism, translate into better treatment responses. This hypothesis is summarized in Fig. 6. We predict that the lack of such immunogenic effect in tumors that have low TLR9 expression indeed contributes to the described poor disease-specific survival in triple negative disease and these patients do not gain the immunogenic benefits from chemotherapy.[9] This hypothesis requires further testing in immune-competent pre-clinical cancer models, but if true, it would mean that low tumor TLR9 triple negative breast cancer patients could benefit from adjuvant immunotherapy that is already approved and available for clinical use.
In conclusion, we show here that DNA fragments from dead cancer cells induce TLR9- and cathepsin-mediated invasion in surviving cancer cells. Furthermore, our results demonstrate that tumor TLR9 expression is an important determinant of cachexia after doxorubicin-treatment. These results imply that DNA fragments derived from dying cancer cells are biologically active and may contribute to treatment responses via tumor TLR9 expression.
Supplementary Material
Acknowledgments
Ms. Christine Pressey is acknowledged for skillful assistance with the qRT-PCR assays. This work was supported by a grant from the Department of Defense (W81XWH-10-1-0308, K.S.S & D.G), Elsa U. Pardee Foundation (K.S.S), Cancer Foundation of Northern Ostrobotnia, Oulu University Scholarship Foundation, and Finnish Medical Foundation (J.H.K), Maud Kuistila Memorial Foundation (J.T), Orion-Farmos Research Foundation (J.T.) and Finnish Cultural Foundation (J.T). The body composition measurement services were provided by the UAB Small Animal Phenotyping Core supported by NIH P30AR046031, P30DK056336, and P60DK079626. The RNA-seq analyses were provided by the Heflin Genomics Core Facility and the Comprehensive Cancer Center Core grant CA13148, the proteomics analysis was provided by the UAB proteomics shared facility, and the CCC grant number P30CA13148-38.
References
- 1.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 3.Hoque R, Malik AF, Gorelick F, Mehal WZ. Sterile inflammatory response in acute pancreatitis. Pancreas. 2012;41:353–357. doi: 10.1097/MPA.0b013e3182321500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang H, Evankovich J, Yan W, Nace G, Zhang L, et al. Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice. Hepatology. 2011;54:999–1008. doi: 10.1002/hep.24501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ilvesaro JM, Merrell MA, Li L, Wakchoure S, Graves D, et al. Toll-like receptor 9 mediates CpG oligonucleotide-induced cellular invasion. Mol Cancer Res. 2008;6:1534–1543. doi: 10.1158/1541-7786.MCR-07-2005. [DOI] [PubMed] [Google Scholar]
- 7.Jukkola-Vuorinen A, Rahko E, Vuopala KS, Desmond R, Lehenkari PP, et al. Toll-like receptor-9 expression is inversely correlated with estrogen receptor status in breast cancer. J Innate Immun. 2009;1:59–68. doi: 10.1159/000151602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandholm J, Kauppila JH, Pressey C, Tuomela J, Jukkola-Vuorinen A, et al. Estrogen receptor-alpha and sex steroid hormones regulate Toll-like receptor-9 expression and invasive function in human breast cancer cells. Breast Cancer Res Treat. 2012;132:411–419. doi: 10.1007/s10549-011-1590-3. [DOI] [PubMed] [Google Scholar]
- 9.Tuomela J, Sandholm J, Karihtala P, Ilvesaro J, Vuopala KS, et al. Low TLR9 expression defines an aggressive subtype of triple-negative breast cancer. Breast Cancer Res Treat. 2012;135:481–493. doi: 10.1007/s10549-012-2181-7. [DOI] [PubMed] [Google Scholar]
- 10.Väisänen MR, Väisänen T, Jukkola-Vuorinen A, Vuopala KS, Desmond R, et al. Expression of toll-like receptor-9 is increased in poorly differentiated prostate tumors. Prostate. 2010;70:817–824. doi: 10.1002/pros.21115. [DOI] [PubMed] [Google Scholar]
- 11.Ilvesaro JM, Merrell MA, Swain TM, Davidson J, Zayzafoon M, et al. Toll like receptor-9 agonists stimulate prostate cancer invasion in vitro. Prostate. 2007;67:774–781. doi: 10.1002/pros.20562. [DOI] [PubMed] [Google Scholar]
- 12.Merrell MA, Ilvesaro JM, Lehtonen N, Sorsa T, Gehrs B, et al. Toll-like receptor 9 agonists promote cellular invasion by increasing matrix metalloproteinase activity. Mol Cancer Res. 2006;4:437–447. doi: 10.1158/1541-7786.MCR-06-0007. [DOI] [PubMed] [Google Scholar]
- 13.Takala H, Kauppila JH, Soini Y, Selander KS, Vuopala KS, et al. Toll-like receptor 9 is a novel biomarker for esophageal squamous cell dysplasia and squamous cell carcinoma progression. J Innate Immun. 2011;3:631–638. doi: 10.1159/000329115. [DOI] [PubMed] [Google Scholar]
- 14.Kauppila JH, Takala H, Selander KS, Lehenkari PP, Saarnio J, et al. Increased Toll-like receptor 9 expression indicates adverse prognosis in oesophageal adenocarcinoma. Histopathology. 2011;59:643–649. doi: 10.1111/j.1365-2559.2011.03991.x. [DOI] [PubMed] [Google Scholar]
- 15.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 16.Sandgren S, Wittrup A, Cheng F, Jonsson M, Eklund E, et al. The human antimicrobial peptide LL-37 transfers extracellular DNA plasmid to the nuclear compartment of mammalian cells via lipid rafts and proteoglycan-dependent endocytosis. J Biol Chem. 2004;279:17951–17956. doi: 10.1074/jbc.M311440200. [DOI] [PubMed] [Google Scholar]
- 17.Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169:3883–3891. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- 18.Heilborn JD, Nilsson MF, Jimenez CI, Sandstedt B, Borregaard N, et al. Antimicrobial protein hCAP18/LL-37 is highly expressed in breast cancer and is a putative growth factor for epithelial cells. Int J Cancer. 2005;114:713–719. doi: 10.1002/ijc.20795. [DOI] [PubMed] [Google Scholar]
- 19.Weber G, Chamorro CI, Granath F, Liljegren A, Zreika S, et al. Human antimicrobial protein hCAP18/LL-37 promotes a metastatic phenotype in breast cancer. Breast Cancer Res. 2009;11:R6. doi: 10.1186/bcr2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandholm J, Tuomela JM, Kauppila JH, Harris KW, Graves D, et al. Hypoxia regulates toll-like receptor-9 expression and invasive function in human brain cancer cells in vitro. Oncol Letters. 2013 doi: 10.3892/ol.2014.2095. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 24.Xing Y, Yu T, Wu YN, Roy M, Kim J, et al. An expectation-maximization algorithm for probabilistic reconstructions of full-length isoforms from splice graphs. Nucleic Acids Res. 2006;34:3150–3160. doi: 10.1093/nar/gkl396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, et al. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kojima K, Bowersock GJ, Kojima C, Klug CA, Grizzle WE, et al. Validation of a robust proteomic analysis carried out on formalin-fixed paraffin-embedded tissues of the pancreas obtained from mouse and human. Proteomics. 2012;12:3393–3402. doi: 10.1002/pmic.201100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wärri AM, Huovinen RL, Laine AM, Martikainen PM, Härkönen PL. Apoptosis in toremifene-induced growth inhibition of human breast cancer cells in vivo and in vitro. J Natl Cancer Inst. 1993;85:1412–1418. doi: 10.1093/jnci/85.17.1412. [DOI] [PubMed] [Google Scholar]
- 28.Johnson MS, Jumbo-Lucioni P, Watts AJ, Allison DB, Nagy TR. Effect of dairy supplementation on body composition and insulin resistance in mice. Nutrition. 2007;23:836–843. doi: 10.1016/j.nut.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Kim JE, Kim HJ, Choi JM, Lee KH, Kim TY, et al. The antimicrobial peptide human cationic antimicrobial protein-18/cathelicidin LL-37 as a putative growth factor for malignant melanoma. Br J Dermatol. 2010;163:959–967. doi: 10.1111/j.1365-2133.2010.09957.x. [DOI] [PubMed] [Google Scholar]
- 30.Nurmenniemi S, Kuvaja P, Lehtonen S, Tiuraniemi S, Alahuhta I, et al. Toll-like receptor 9 ligands enhance mesenchymal stem cell invasion and expression of matrix metalloprotease-13. Exp Cell Res. 316:2676–2682. doi: 10.1016/j.yexcr.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 31.Asagiri M, Hirai T, Kunigami T, Kamano S, Gober HJ, et al. Cathepsin K-dependent toll-like receptor 9 signaling revealed in experimental arthritis. Science. 2008;319:624–627. doi: 10.1126/science.1150110. [DOI] [PubMed] [Google Scholar]
- 32.Ronkainen H, Hirvikoski P, Kauppila S, Vuopala KS, Paavonen TK, et al. Absent Toll-like receptor-9 expression predicts poor prognosis in renal cell carcinoma. J Exp Clin Cancer Res. 2011;30:84. doi: 10.1186/1756-9966-30-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kauppila JH, Karttunen TJ, Saarnio J, Nyberg P, Salo T, et al. Short DNA sequences and bacterial DNA induce esophageal, gastric, and colorectal cancer cell invasion. APMIS. 2012 doi: 10.1111/apm.12016. [DOI] [PubMed] [Google Scholar]
- 34.Wang C, Cao S, Yan Y, Ying Q, Jiang T, et al. TLR9 expression in glioma tissues correlated to glioma progression and the prognosis of GBM patients. BMC Cancer. 10:415. doi: 10.1186/1471-2407-10-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez-Reyes S, Marin L, Gonzalez L, Gonzalez LO, del Casar JM, et al. Study of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasis. BMC Cancer. 10:665. doi: 10.1186/1471-2407-10-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamphier MS, Sirois CM, Verma A, Golenbock DT, Latz E. TLR9 and the recognition of self and non-self nucleic acids. Ann N Y Acad Sci. 2006;1082:31–43. doi: 10.1196/annals.1348.005. [DOI] [PubMed] [Google Scholar]
- 37.Rifkin IR, Leadbetter EA, Busconi L, Viglianti G, Marshak-Rothstein A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 38.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Widlak P, Garrard WT. Roles of the major apoptotic nuclease-DNA fragmentation factor-in biology and disease. Cell Mol Life Sci. 2009;66:263–274. doi: 10.1007/s00018-008-8472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Napirei M, Wulf S, Mannherz HG. Chromatin breakdown during necrosis by serum Dnase1 and the plasminogen system. Arthritis Rheum. 2004;50:1873–1883. doi: 10.1002/art.20267. [DOI] [PubMed] [Google Scholar]
- 41.Tsou KC, Yip KF. Effect of deoxyribonuclease on adriamycin-polynucleotide complexes. Cancer Res. 1976;36:3367–3373. [PubMed] [Google Scholar]
- 42.Park B, Brinkmann MM, Spooner E, Lee CC, Kim YM, et al. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat Immunol. 2008;9:1407–1414. doi: 10.1038/ni.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ewald SE, Engel A, Lee J, Wang M, Bogyo M, et al. Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J Exp Med. 2011;208:643–651. doi: 10.1084/jem.20100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laginha KM, Verwoert S, Charrois GJ, Allen TM. Determination of doxorubicin levels in whole tumor and tumor nuclei in murine breast cancer tumors. Clin Cancer Res. 2005;11:6944–6949. doi: 10.1158/1078-0432.CCR-05-0343. [DOI] [PubMed] [Google Scholar]
- 45.Stallard S, Morrison JG, George WD, Kaye SB. Distribution of doxorubicin to normal breast and tumour tissue in patients undergoing mastectomy. Cancer Chemother Pharmacol. 1990;25:286–290. doi: 10.1007/BF00684887. [DOI] [PubMed] [Google Scholar]
- 46.Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–1665. [PubMed] [Google Scholar]
- 47.Umetani N, Giuliano AE, Hiramatsu SH, Amersi F, Nakagawa T, et al. Prediction of breast tumor progression by integrity of free circulating DNA in serum. J Clin Oncol. 2006;24:4270–4276. doi: 10.1200/JCO.2006.05.9493. [DOI] [PubMed] [Google Scholar]
- 48.Ng B, Wolf RF, Weksler B, Brennan MF, Burt M. Growth hormone administration preserves lean body mass in sarcoma-bearing rats treated with doxorubicin. Cancer Res. 1993;53:5483–5486. [PubMed] [Google Scholar]
- 49.Hajjaji N, Couet C, Besson P, Bougnoux P. DHA effect on chemotherapy-induced body weight loss: an exploratory study in a rodent model of mammary tumors. Nutr Cancer. 2012;64:1000–1007. doi: 10.1080/01635581.2012.714832. [DOI] [PubMed] [Google Scholar]
- 50.Tsai VW, Husaini Y, Manandhar R, Lee-Ng KK, Zhang HP, et al. Anorexia/cachexia of chronic diseases: a role for the TGF-beta family cytokine MIC-1/GDF15. J Cachexia Sarcopenia Muscle. 2012;3:239–243. doi: 10.1007/s13539-012-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wakchoure S, Swain TM, Hentunen TA, Bauskin AR, Brown DA, et al. Expression of macrophage inhibitory cytokine-1 in prostate cancer bone metastases induces osteoclast activation and weight loss. Prostate. 2009;69:652–661. doi: 10.1002/pros.20913. [DOI] [PubMed] [Google Scholar]
- 52.Song B, Zhang D, Wang S, Zheng H, Wang X. Association of interleukin-8 with cachexia from patients with low-third gastric cancer. Comp Funct Genomics. 2009:212345. doi: 10.1155/2009/212345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baumgarten AJ, Fiebig HH, Burger AM. Molecular analysis of xenograft models of human cancer cachexia--possibilities for therapeutic intervention. Cancer Genomics Proteomics. 2007;4:223–231. [PubMed] [Google Scholar]
- 54.Tisdale MJ. Pathogenesis of cancer cachexia. J Support Oncol. 2003;1:159–168. [PubMed] [Google Scholar]
- 55.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner H. Hemozoin: malaria’s “built-in” adjuvant and TLR9 agonist. Cell Host Microbe. 2010;7:5–6. doi: 10.1016/j.chom.2010.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.