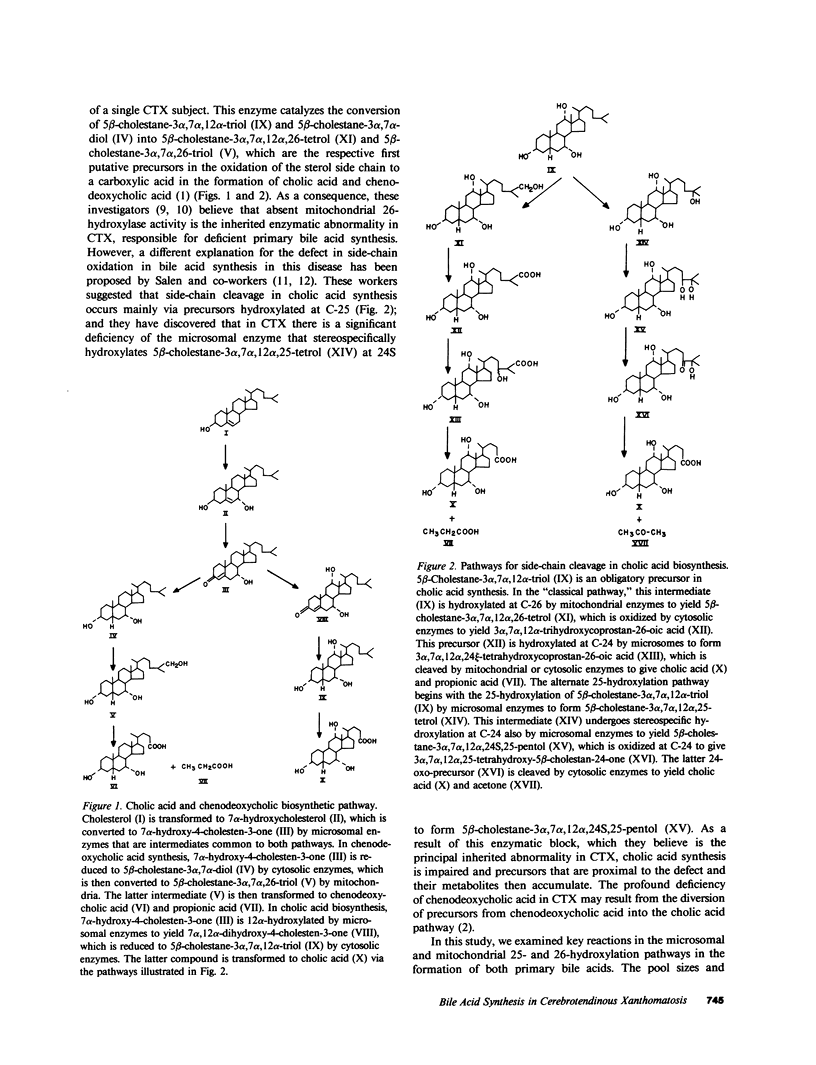
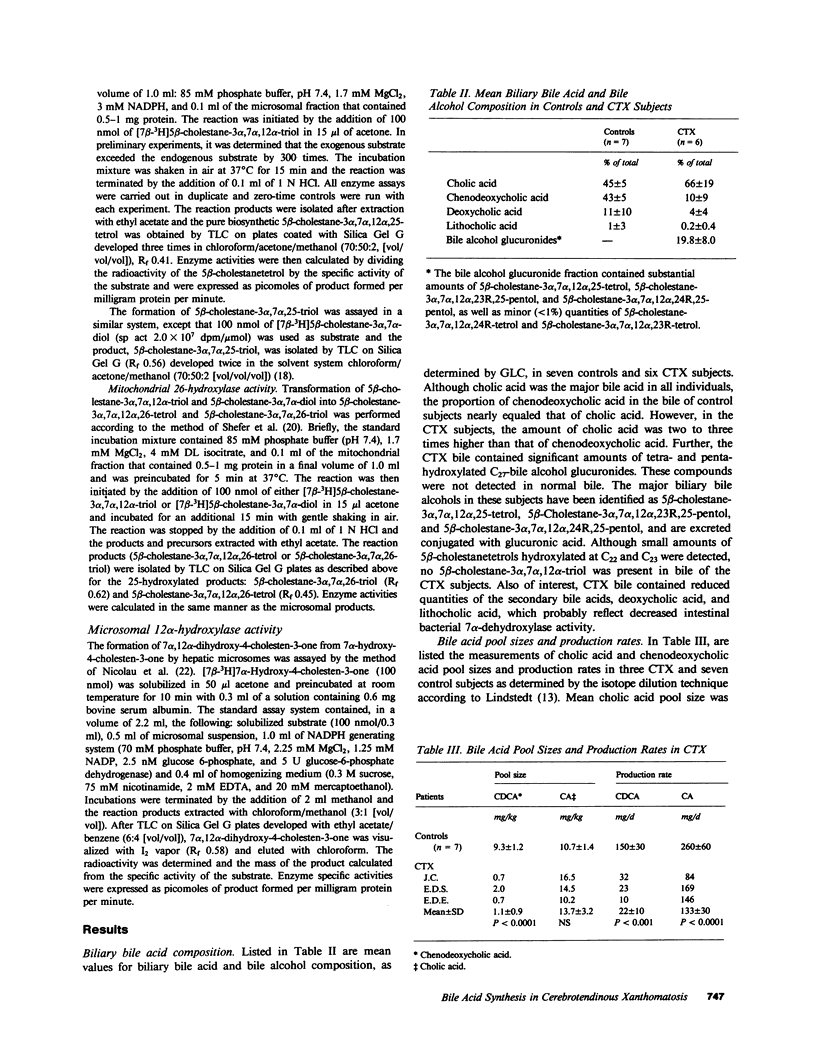
Abstract
To examine the defect in side-chain oxidation during the formation of bile acids in cerebrotendinous xanthomatosis, we measured in vitro hepatic microsomal hydroxylations at C-12 and C-25 and mitochondrial hydroxylation at C-26 and related them to the pool size and synthesis rates of cholic acid and chenodeoxycholic acid as determined by the isotope dilution technique. Hepatic microsomes and mitochondria were prepared from seven subjects with cerebrotendinous xanthomatosis and five controls. Primary bile acid synthesis was markedly reduced in cerebrotendinous xanthomatosis as follows: cholic acid, 133 +/- 30 vs. 260 +/- 60 mg/d in controls; and chenodeoxycholic acid, 22 +/- 10 vs. 150 +/- 30 mg/d in controls. As postulated for chenodeoxycholic acid synthesis, mitochondrial 26-hydroxylation of 5 beta-cholestane-3 alpha, 7 alpha-diol was present in all specimens and was 30-fold more active than the corresponding microsomal 25-hydroxylation. However, mean mitochondrial 26-hydroxylation of 5 beta-cholestane-3 alpha,7 alpha-diol was less active in cerebrotendinous xanthomatosis than in controls: 59 +/- 17 compared with 126 +/- 21 pmol/mg protein per min. As for cholic acid synthesis, microsomal 25-hydroxylation of 5 beta-cholestane-3 alpha,7 alpha,12 alpha-triol was substantially higher in cerebrotendinous xanthomatosis and control preparations (620 +/- 103 and 515 +/- 64 pmol/mg protein per min, respectively) than the corresponding control mitochondrial 26-hydroxylation of the same substrate (165 +/- 25 pmol/mg protein per min). Moreover in cerebrotendinous xanthomatosis, mitochondrial 5 beta-cholestane-3 alpha,7 alpha,12 alpha-triol-26-hydroxylase activity was one-seventh as great as in controls. Hepatic microsomal 12 alpha-hydroxylation, which may be rate-controlling for the cholic acid pathway, was three times more active in cerebrotendinous xanthomatosis than in controls: 1,600 vs. 500 pmol/mg protein per min. These results demonstrate severely depressed primary bile acid synthesis in cerebrotendinous xanthomatosis with a reduction in chenodeoxycholic acid formation and pool size disproportionately greater than that for cholic acid. The deficiency of chenodeoxycholic acid can be accounted for by hyperactive microsomal 12 alpha-hydroxylation that diverts precursors into the cholic acid pathway combined with decreased side-chain oxidation (mitochondrial 26-hydroxylation). However, side-chain oxidation in cholic acid biosynthesis may be initiated via microsomal 25-hydroxylation of 5beta-cholestane-3alpha,7alpha,12alpha-triol was substantially lower in control and cerebrotendinous xanthomatosis liver. Thus, separate mechanisms may exist for the cleavage of the cholesterol side chain in cholic acid and chenodeoxycholic acid biosynthesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berginer V. M., Salen G., Shefer S. Long-term treatment of cerebrotendinous xanthomatosis with chenodeoxycholic acid. N Engl J Med. 1984 Dec 27;311(26):1649–1652. doi: 10.1056/NEJM198412273112601. [DOI] [PubMed] [Google Scholar]
- Björkhem I., Danielsson H., Issidorides C., Kallner A. On the synthesis and metabolism of cholest-4-en-7-alpha-ol-3-one. Bile acids and steroids 156. Acta Chem Scand. 1965;19(9):2151–2154. doi: 10.3891/acta.chem.scand.19-2151. [DOI] [PubMed] [Google Scholar]
- Björkhem I., Fausa O., Hopen G., Oftebro H., Pedersen J. I., Skrede S. Role of the 26-hydroxylase in the biosynthesis of bile acids in the normal state and in cerebrotendinous xanthomatosis. An in vivo study. J Clin Invest. 1983 Jan;71(1):142–148. doi: 10.1172/JCI110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkhem I., Gustafsson J., Johansson G., Persson B. Biosynthesis of bile acids in man. Hydroxylation of the C27-steroid side chain. J Clin Invest. 1975 Mar;55(3):478–486. doi: 10.1172/JCI107954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkhem I., Gustafsson J. Omega-hydroxylation of steriod side-chain in biosynthesis of bile acids. Eur J Biochem. 1973 Jul 2;36(1):201–212. doi: 10.1111/j.1432-1033.1973.tb02902.x. [DOI] [PubMed] [Google Scholar]
- Dayal B., Shefer S., Tint G. S., Salen G., Mosbach E. H. Synthesis of 5beta-cholestane-3alpha, 7alpha, 12alpha, 25-tetrol and 5beta-cholestane-3alpha, 7alpha, 245, 25-pentol. J Lipid Res. 1976 Jan;17(1):74–77. [PubMed] [Google Scholar]
- Hanson R. F., Staples A. B., Williams G. C. Metabolism of 5 beta-cholestane-3 alpha, 7 alpha, 12 alpha, 26-tetrol and 5 beta-cholestane-3 alpha, 7 alpha, 12 alpha, 25-tetrol into cholic acid in normal human subjects. J Lipid Res. 1979 May;20(4):489–493. [PubMed] [Google Scholar]
- Hoshita T., Yasuhara M., Une M., Kibe A., Itoga E., Kito S., Kuramoto T. Occurrence of bile alcohol glucuronides in bile of patients with cerebrotendinous xanthomatosis. J Lipid Res. 1980 Nov;21(8):1015–1021. [PubMed] [Google Scholar]
- Kajer L. E., Williams G. C., Szczepanik P., Silvis S., Hanson R. F. Formation of cholic acid via 3 alpha, 7 alpha,12 alpha-trihydroxy-5 beta-cholestan-26-oic acid in the dog. Biochim Biophys Acta. 1979 Jun 21;573(3):430–435. doi: 10.1016/0005-2760(79)90217-0. [DOI] [PubMed] [Google Scholar]
- Koopman B. J., van der Molen J. C., Wolthers B. G., de Jager A. E., Waterreus R. J., Gips C. H. Capillary gas chromatographic determination of cholestanol/cholesterol ratio in biological fluids. Its potential usefulness for the follow-up of some liver diseases and its lack of specificity in diagnosing CTX (cerebrotendinous xanthomatosis). Clin Chim Acta. 1984 Mar 13;137(3):305–315. doi: 10.1016/0009-8981(84)90119-0. [DOI] [PubMed] [Google Scholar]
- LINDSTEDT S. The turnover of cholic acid in man: bile acids and steroids. Acta Physiol Scand. 1957 Sep 17;40(1):1–9. doi: 10.1111/j.1748-1716.1957.tb01473.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nicolau G., Cohen B. I., Salen G., Mosbach E. H. Studies on the 12alpha and 26-hydroxylation of bile alcohols by rabbit liver microsomes. Lipids. 1976 Feb;11(2):148–152. doi: 10.1007/BF02532665. [DOI] [PubMed] [Google Scholar]
- Oftebro H., Björkhem I., Skrede S., Schreiner A., Pederson J. I. Cerebrotendinous xanthomatosis: a defect in mitochondrial 26-hydroxylation required for normal biosynthesis of cholic acid. J Clin Invest. 1980 Jun;65(6):1418–1430. doi: 10.1172/JCI109806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oftebro H., Björkhem I., Størmer F. C., Pedersen J. I. Cerebrotendinous xanthomatosis: defective liver mitochondrial hydroxylation of chenodeoxycholic acid precursors. J Lipid Res. 1981 May;22(4):632–640. [PubMed] [Google Scholar]
- Salen G. Cholestanol deposition in cerebrotendinous xanthomatosis. A possible mechanism. Ann Intern Med. 1971 Dec;75(6):843–851. doi: 10.7326/0003-4819-75-6-843. [DOI] [PubMed] [Google Scholar]
- Salen G., Grundy S. M. The metabolism of cholestanol, cholesterol, and bile acids in cerebrotendinous xanthomatosis. J Clin Invest. 1973 Nov;52(11):2822–2835. doi: 10.1172/JCI107478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salen G., Shefer S., Cheng F. W., Dayal B., Batta A. K., Tint G. S. Cholic acid biosynthesis: the enzymatic defect in cerebrotendinous xanthomatosis. J Clin Invest. 1979 Jan;63(1):38–44. doi: 10.1172/JCI109275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salen G., Shefer S., Mosbach E. H., Hauser S., Cohen B. I., Nicolau G. Metabolism of potential precursors of chenodeoxycholic acid in cerebrotendinous xanthomatosis (CTX). J Lipid Res. 1979 Jan;20(1):22–30. [PubMed] [Google Scholar]
- Salen G., Shefer S., Setoguchi T., Mosbach E. H. Bile alcohol metabolism in man. Conversion of 5beta-cholestane-3alpha, 7alpha,12alpha, 25-tetrol to cholic acid. J Clin Invest. 1975 Jul;56(1):226–231. doi: 10.1172/JCI108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoguchi T., Salen G., Tint G. S., Mosbach E. H. A biochemical abnormality in cerebrotendinous xanthomatosis. Impairment of bile acid biosynthesis associated with incomplete degradation of the cholesterol side chain. J Clin Invest. 1974 May;53(5):1393–1401. doi: 10.1172/JCI107688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer S., Cheng F. W., Batta A. K., Dayal B., Tint G. S., Salen G. Biosynthesis of chenodeoxycholic acid in man: stereospecific side-chain hydroxylations of 5beta-cholestane-3alpha,7alpha-diol. J Clin Invest. 1978 Sep;62(3):539–545. doi: 10.1172/JCI109158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer S., Cheng F. W., Dayal B., Hauser S., Tint G. S., Salen G., Mosbach E. H. A 25-hydroxylation pathway of cholic acid biosynthesis in man and rat. J Clin Invest. 1976 Apr;57(4):897–903. doi: 10.1172/JCI108366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer S., Dayal B., Tint G. S., Salen G., Mosbach E. H. Identification of pentahydroxy bile alcohols in cerebrotendinous xanthomatosis: characterization of 5beta-cholestane-3alpha, 7alpha, 12alpha, 24xi, 25-pentol and 5beta-cholestane-3alpha, 7alpha, 12alpha, 23xi, 25-pentol. J Lipid Res. 1975 Jul;16(4):280–286. [PubMed] [Google Scholar]
- Wolthers B. G., Volmer M., van der Molen J., Koopman B. J., de Jager A. E., Waterreus R. J. Diagnosis of cerebrotendinous xanthomatosis (CTX) and effect of chenodeoxycholic acid therapy by analysis of urine using capillary gas chromatography. Clin Chim Acta. 1983 Jun 30;131(1-2):53–65. doi: 10.1016/0009-8981(83)90352-2. [DOI] [PubMed] [Google Scholar]



