Abstract
Cysts residing in benthic nepheloid layers (BNLs) documented in the Gulf of Maine have been proposed as a possible source of inoculum for annual blooms of a toxic dinoflagellate in the region. Herein we present a spatially extensive data set of the distribution and thickness of benthic nepheloid layers in the Gulf of Maine and the abundance and inventories of suspended Alexandrium fundyense cysts within these near-bottom layers. BNLs are pervasive throughout the gulf and adjacent Bay of Fundy with maximum layer thicknesses of 50–60 m observed. Mean BNL thickness is 30 m in the eastern gulf and Bay of Fundy, and 20 m in the western gulf. Cyst densities in the near-bottom particle resuspension layers varied by three orders of magnitude across the gulf with maxima of 105 cysts m−3. An important interconnection of elevated BNL cyst densities is observed between the Bay of Fundy, the Maine Coastal Current and the south-central region of the gulf. BNL cyst inventories estimated for the eastern and western gulf are each on the order of 1015 cysts, whereas the BNL inventory in the Bay of Fundy is on the order of 1016 . Although BNL cyst inventories in the eastern and western gulf are 1–2 orders of magnitude smaller than the abundance of cysts in the upper 1 cm of sediment in those regions, BNL and sediment-bound cyst inventories are comparable in the Bay of Fundy. The existence of widespread BNLs containing substantial cyst inventories indicates that these near-bottom layers represent an important source of germinating A. fundyense cysts in the region.
Keywords: Benthic nepheloid layer, Suspended particulate matter, Gulf of Maine, Alexandrium fundyense, Cysts
1. Introduction
The marine benthic boundary layer (BBL) is the region encompassing the uppermost surface sediments and the overlying water column that is impacted by the presence of the sediment-water interface (Boudreau and Jorgensen, 2001). Bottom stress-generated turbulence levels are enhanced in the BBL on micro-to macro-scales depending on the depth and strength of mixing and current flow (Dade et al., 2001). It is within the BBL that near-bottom particle resuspension layers or benthic nepheloid layers (BNLs) develop, representing a distinctive zone of increased suspended particle concentration (McCave, 1985; Hill and McCave, 2001). BNLs are commonly observed on continental margins and at the base of continental slopes (Eittreim et al., 1976; Newberger and Caldwell, 1981; Spinrad and Zaneveld, 1982; McCave, 1983; Spinrad et al., 1983; Dickson and McCave, 1986; Spinrad, 1986; McCave, 1986; Durrieu de Mandron et al., 1990; Townsend et al., 1992). The thickness of the BNL as well as its particle size spectra and composition vary as a function of local and regional fluid flow, bottom sediment type, particulate export from overlying waters, and local biological community composition (Agrawal and Traykovski, 2001; Boss et al., 2001; Chang et al., 2001; Gardner et al., 2001; Hill et al., 2001). Significant biological and particulate geochemical activity in the BNL relative to the overlying, less turbid water is indicated by the high particle concentration, enhanced aggregation rates, elevated biological activity and bio-mass, plus high concentrations of organic matter in the near-bottom layer (Wishner and Meise-Munns, 1984; Lampitt, 1985; Gowing and Wishner, 1986; McCave, 1986; Smith et al., 1987; Wainright, 1987; Dortch et al., 1988; Mayer et al., 1988; Gardner and Walsh, 1990; Townsend et al., 1992; Ransom et al., 1997, 1998; Rutgers van der Loeff et al., 2002). In shallow coastal settings, BNLs may extend from the sediment-water interface to the surface ocean with potentially significant impacts on upper water-column nutrient dynamics, phytoplankton bloom activity, and higher trophic level production (Flint and Rabalais, 1981; Fanning et al., 1982; Kirn et al., 2005).
Throughout the Gulf of Maine, BNLs have been identified by numerous CTD/transmissometer profiles collected over different seasons and years and thus they appear to be permanent to semipermanent features in the region (Spencer and Sachs, 1970; Spinrad, 1986; Dortch et al., 1988; Townsend et al., 1992; Pilskaln et al., 1998). Identification of BNLs in the gulf has been based on the transition from relatively particle-free water at mid-depths where beam attenuation is approximately 0.5 m −1 to an underlying, near-bottom layer where beam attenuation increases by > 40% (reciprocal % light transmission decreases from ~90% to ~75% or less; Spinrad, 1986; Townsend et al., 1992; Kirn et al., 2005; Pilskaln et al., 1998). BNL heights are reported to vary from 10 to 30 m and are associated with substantially elevated concentrations of labile organic compounds, total particulate organic carbon (POC) and particulate organic nitrogen (PON) as well as an enhanced abundance of microbes, nanoplankton and zooplankton biomass relative to the overlying, low particle density water (Dortch et al., 1988; Townsend et al., 1992; Pilskaln et al., 1998; Hayashi and Pilskaln, 2011; Pilskaln et al., 2014). Results from time-series sediment traps placed above and within the BNLs at various gulf sites clearly document the persistent intensity of bottom resuspension in the gulf. Highly lithogenic resuspension fluxes in gulf BNLs, with seasonally variable organic and biomineral content, are up to several orders of magnitude greater than the particle mass fluxes measured above the BNLs (Pilskaln et al., 1998; Hayashi and Pilskaln, 2011; Pilskaln, 2009; Pilskaln et al., 1998).
Several important studies in the Gulf of Maine have speculated that sediment-bound Alexandrium fundyense cysts are resus-pended into the overlying BNLs (Anderson et al., 2005; Kirn et al., 2005). A. fundyense is the toxic dinoflagellate responsible for “red tides” or harmful algal blooms (HABs) that cause shellfish to become dangerously toxic after they filter the organism from the water during feeding and concentrate the potent neurotoxin that it produces in their tissues (Anderson, 1997, 1998). The A. fundyense life history includes a resting cyst that sinks down from the water column to the underlying sediments and remains dormant for most of the year, germinating in the spring to initiate annually recurrent blooms (Anderson et al., 2005). Our working hypothesis is that the blooms are initiated via resuspension of mature cysts from the upper sediments, with input to the BNLs (Anderson et al., 2005; Kirn et al., 2005). Resuspended cysts may be maintained in the near-bottom particle resuspension layers for an unknown period. Regardless of the cyst BNL residence time, the BNLs likely constitute a suspended cyst reservoir that places mature cysts in favorable germination conditions (Kirn et al., 2005; Anderson et al., 2005).
The purpose of this communication is to report on the Gulf of Maine and Bay of Fundy distribution and thickness of benthic particle resuspension layers, provide estimates of the inventory of A. fundyense cysts within the benthic resuspension layers, compare the BNL cyst inventory to that in the underlying surface sediments, and determine if there is a relationship between BNL thickness and suspended cyst abundance. Using these results, we examine the potential movement of cyst-rich BNLs along the northern New England coast and to the deep central and southern regions of the gulf.
2. Methods
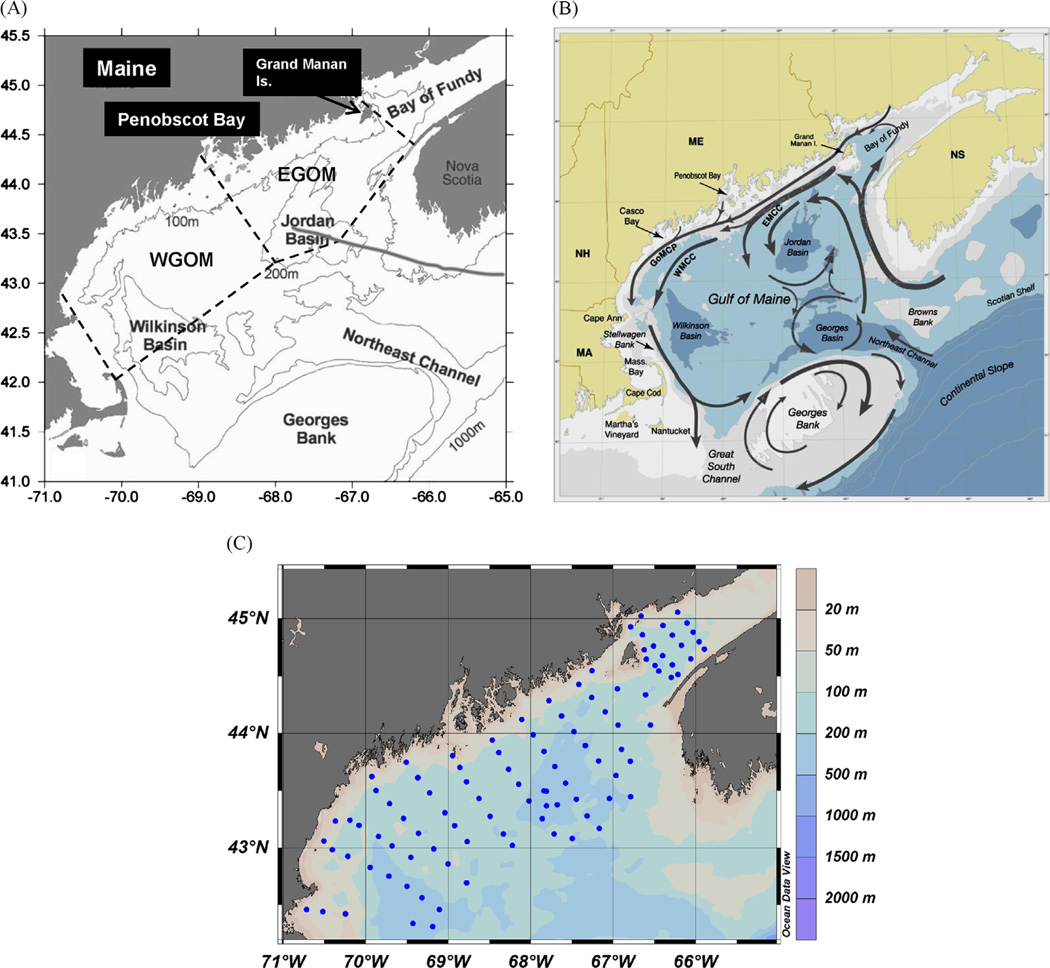
In October 2004, we conducted a 10 d cruise (CH15604) aboard the R/V Cape Hatteras to identify and map the vertical and geographical extent of the near-bottom resuspension layer or BNL, obtain concentrations of near-bottom suspended A. fundyense cysts, and collect surface sediments for cyst counts. Full-depth CTD/transmissometer profiles with 1 m vertical resolution were completed at 97 stations extending from the edge of Massachusetts Bay, up to the Bay of Fundy, and out to the deep offshore Wilkinson and Jordan Basin (Fig. 1). Thirty four stations were completed in the eastern Gulf of Maine (EGOM), 40 in the western Gulf of Maine (WGOM) and 23 in the Bay of Fundy (BOF; Fig. 1). Station bottom depths ranged from 50 to 290 m. The data were obtained with a rosette-mounted Sea-Bird CTD, Sea Tech transmissometer (25 cm path length, 660 nm) and Chelsea Instruments chlorophyll a fluorometer. All data were analyzed using the standard Sea-Bird SeaSoft software program which provides beam attenuation due to particles only (e.g., attenuation due to water and dissolved organic material is removed; Bishop, 1986). The top of the BNL at each CTD station was approximated at the depth at which the beam attenuation values increased by > 40% over the overlying, mid-depth particle minimum zone. At 14 of our CTD stations, casts were completed to collect 10 L water samples at 6 depths for suspended particle mass (SPM) and POC determination. Samples were obtained 1–5 m below the surface, below the base of the mixed layer, within the particle minimum zone underlying the mixed layer, and at 2–3 depths within the BNL (10–20 m above the bottom). All sampling depths were determined on the CTD/transmissometer down-cast and water samples were collected on the up-cast. Onboard, in-line vacuum filtration of the samples onto pre-combusted, pre-weighed GFF filters was completed with an average of 5–6 L filtered per depth sample. SPM (as mgL−1) was calculated by dividing the filtered sample dry weight by the total volume of water filtered; POC content of suspended particles was obtained by coulometer analysis of filtered samples following standard acid fuming to remove carbonate.
Fig. 1.
(A) Gulf of Maine bathymetry with major physiographic features; dashed lines indicate the general boundaries between the three major Gulf subdomains from west to east: western Gulf of Maine (WGOM), eastern Gulf of Maine (EGOM), and Bay of Fundy (BOF) (Anderson et al., 2013). (B) Schematic circulation diagram for major currents in the Gulf of Maine (from Anderson et al., 2005; EMCC=Eastern Maine Coastal Current; WMCC=Western Maine Coastal Current; GOMCP=Gulf of Maine Coastal Plume). (C) October 2004 CTD/transmissometer profile stations (blue dots) discussed in text. USGS Gulf of Maine bathymetry was used (Poppe et al., 2003, http://pubs.usgs.gov/of/2003/of03-1/index.htm).
To quantify the inventory of near-bottom suspended A. fundyense cysts, large volume water samples were collected at depths ranging from 2 to 50 mab (meters above bottom; mean depth sampled=21 mab), depending on the presence and thickness of a BNL. Using the CTD/transmissometer down-cast profiles, we identified the depth of the near-bottom resuspension zone and on the up-cast, we tripped three 10 L Niskin bottles at two depths within the BNL (below the top of the layer and near the bottom) to obtain samples from which cyst abundance (# cysts L−1) could be determined. At stations where we did not observe a well-defined benthic resuspension layer/BNL, we obtained samples at 3–5 and 10–20 mab. Each single-depth, 30 L water sample was sieved through a 20 µm Nylon mesh sieve with filtered seawater (< 20 µm) to concentrate the cysts. The cysts and particles remaining on the sieve were then resuspended to 45 ml in a 50 ml centrifuge tube and fixed with formalin (5% final) in the field. Onshore, samples were further processed using standard cyst processing protocols (Anderson et al., 2003, 2005). Samples were first re-sieved and resuspended to 45 ml in filtered seawater removed the formalin. Secondly, the samples were sonified for 60 s; the disaggregated sample was sieved sequentially through a 100 µm mesh to remove detritus and the filtrate was passed through a 20 µm sieve to retain the 20–100 µm cyst fraction. The sample was then resuspended into 14 ml of filtered seawater in a 15 ml tube. Finally, for primuline staining of the cysts, the sample was centrifuged (3000 × g for 10 min), the seawater was removed by aspiration, and the resulting pellet of centrifuged cysts was resuspended into cold methanol and stored for at least 24 h. The sample was centrifuged again, the methanol removed, and the pellet resuspended in 10 ml of distilled water. After centrifugation, 2 ml of primuline stock (2 mg ml−1) was added directly to the pellet and incubated at 4 °C for 1 h. The stained sample was centrifuged, excess primuline removed, and the pellet resuspended in a final known volume of distilled water (usually 5–10 ml). One milliliter of the processed sample was loaded into a Sedgewick-Rafter chamber and the green fluorescently-stained cysts were counted at 10x with a Zeiss epi-fluorescence microscope (excitation=450–490 nm BP; emission = 510 nm LP). All cyst data reported in this study represent intact cysts; empty A. fundyense cysts resulting from cyst death or germination were not enumerated.
Concentrations of A. fundyense cysts were determined from the cyst counts obtained from the large-volume, BNL water samples collected at each station. We calculated mean cyst abundance over the depth interval that was sampled at each station, regardless of the presence of a well-defined BNL. Due to sampling irregularities, the farthest above-bottom depth sampled for cysts within the BNLs did not consistently represent the top of the BNL. Thus our calculated inventory of suspended cysts in the BNL per station is conservative as it is based on the depth interval sampled within a station’s BNL, which was less than the total BNL thickness at approximately 40% of the stations.
3. Results
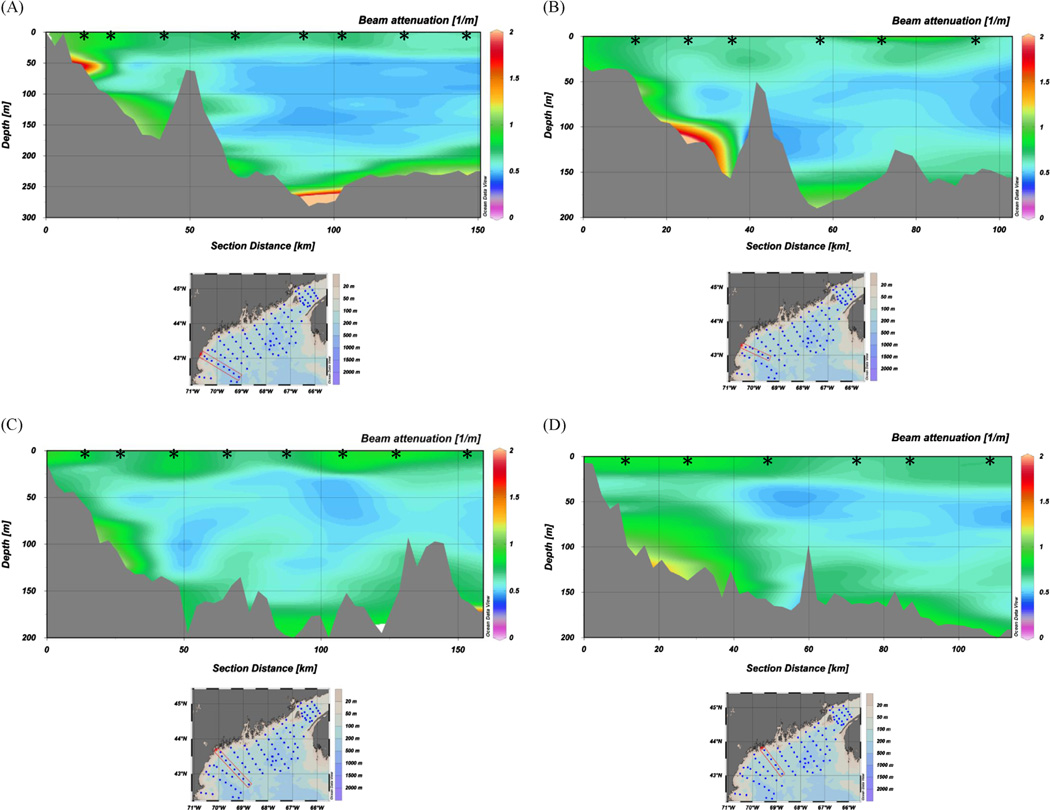
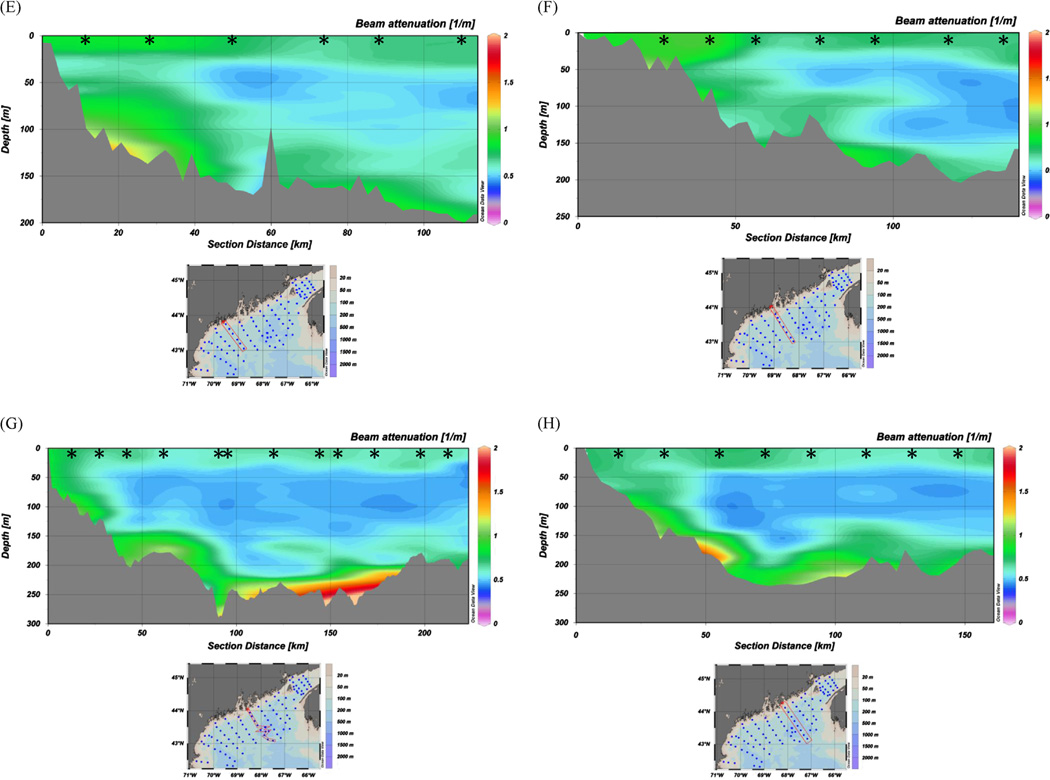
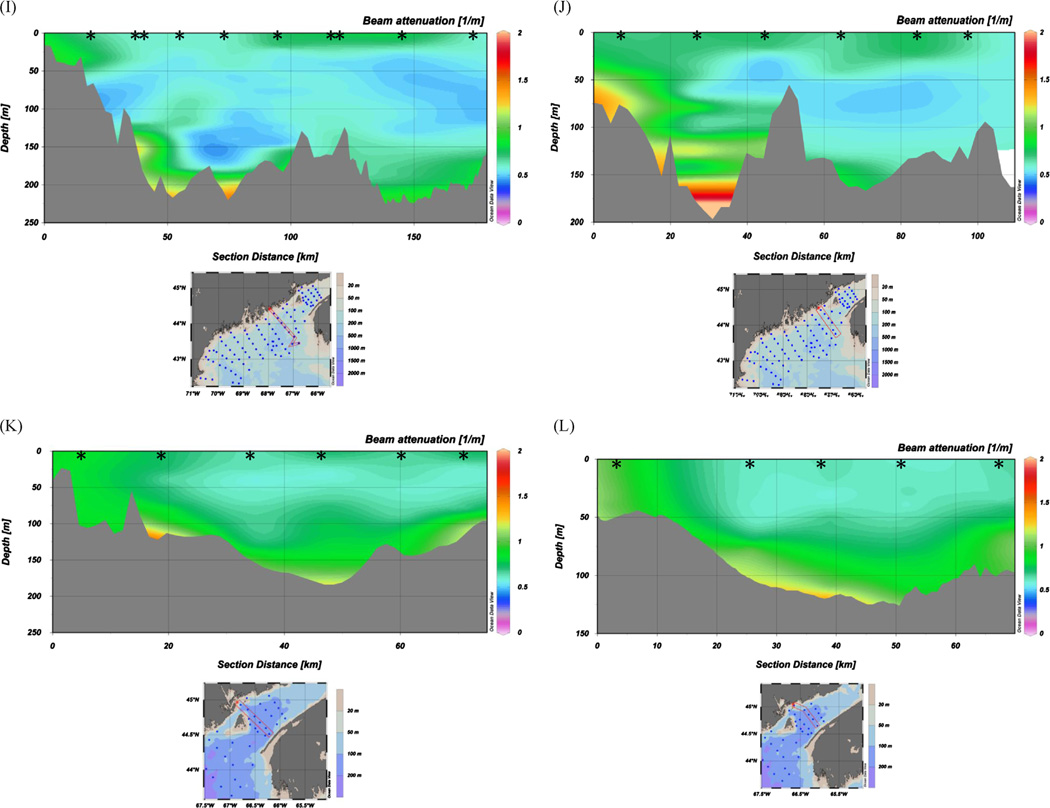
Pervasive BNLs exhibiting substantial variability and structure with depth are revealed by the transect profiles of beam attenuation (hereafter beam-c) plotted and presented in color contoured sections in Figs. 2 and 3. To produce the contoured sections, we used Ocean Data View (ODV 4-version 4.3.9) which included the DIVA (Data-Interpolating Variational Analysis) software tool to perform spatial interpolation on a finite element grid. DIVA is especially useful in situations where bathymetry is highly variable over the data collection horizontal scale. Our interpolation grid field ( = 160 per mille of full horizontal by 50 per mille of full vertical) produced a grid cell size of 24 km (horizontal) × 15 m (vertical), resulting in greater smoothing in the horizontal direction due to the large length scale. However, the most important and variable beam-c features as a function of depth throughout the gulf are clearly represented in Figs. 2 and 3. We incorporated Gulf of Maine bathymetry from Poppe et al. (2003) into ODV for the contour sections. Figs. 2 and 3 show values of beam-c ranging from < 0.5 m−1 ( > 85% transmission) in the relatively less turbid, midwater depths, to 0.8–5.0 m−1 (< 75% transmission) in the near-bottom BNL Near-surface and sub-surface chlorophyll maximum regions display beam-c values of 0.75–1.0 m−1. Maximum beam-c values of > 1.0 are primarily seen in basins or topographically low regions in the gulf (e.g., lime-green to red regions within ~ 50 m of the bottom in Fig. 2 and Fig. 3 panels). SPM values in the BNLs and near-surface chlorophyll maxima were > 1.0 mg L−1. By comparison, the mid-depth particle minimum zones displayed SPM values of ~0.5 mg L−1. POC content of suspended particles was 90 to > 100 µg L−1 in the BNLs and in the near-surface filtered samples; mid-depth particle minimum filtered samples showed substantially less POC content of 40–50 µg L−1.
Fig. 2.
Representative onshore-offshore transects of beam-c from October 2004 CTD/transmissometer profiles: (A–D) far western Gulf of Maine, (E–H) western into eastern Gulf of Maine, (I–L) far eastern Gulf of Maine and Bay of Fundy. Transect shown in each panel is denoted by red rectangle on associated station map; left side of each contour panel is inshore, right is offshore. Vertical profile stations are denoted by black stars. USGS Gulf of Maine bathymetry was used (Poppe et al., 2003, http://pubs.usgs.gov/of/2003/of03-1/index.htm).
Fig. 3.
(A–D) Southwest to northeast contoured beam-c sections across Gulf of Maine, October 2004. Sections move progressively offshore from (A) to (D). CTD station locations are denoted by black stars.
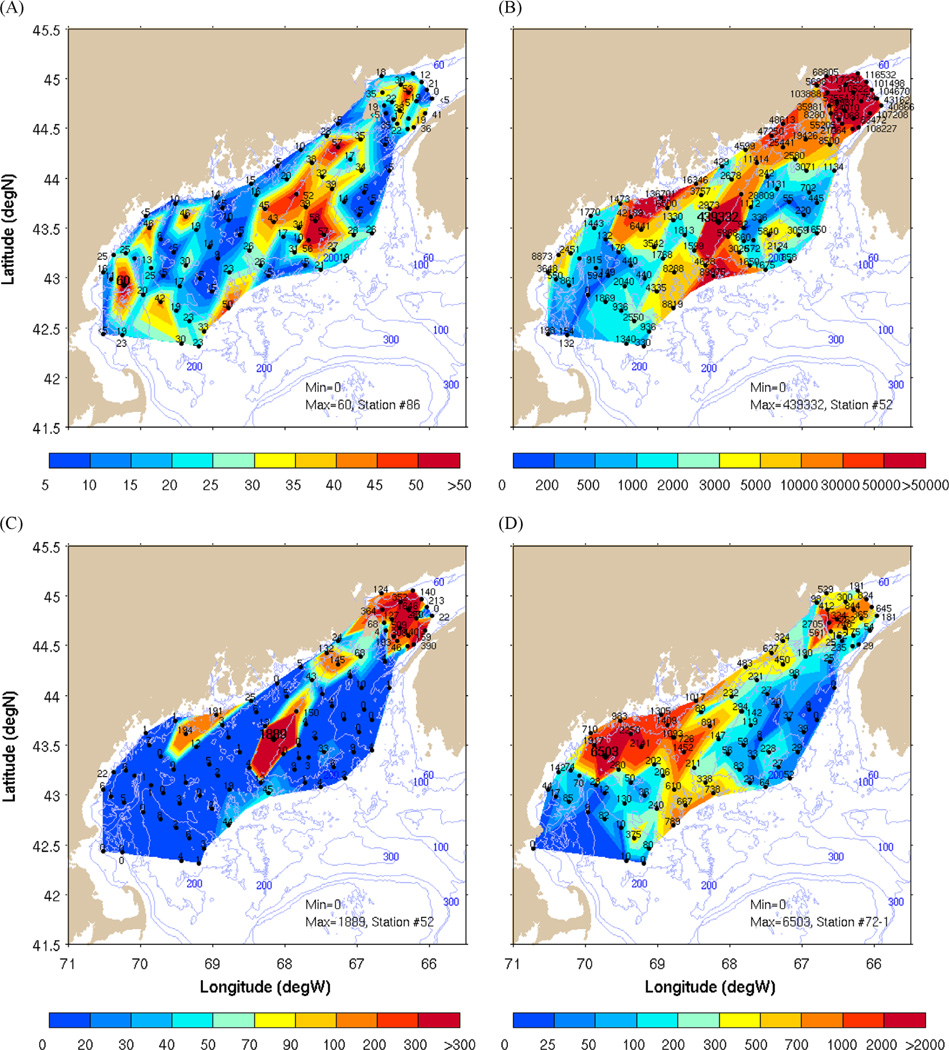
Map views of Gulf of Maine and BOF BNL thickness, BNL suspended cyst data and 0–1 cm sedimentary cyst abundances are shown in Fig. 4. The data for each station (shown as black dots in Fig. 4) were gridded onto a high resolution (0.01° latitude) mesh using linear interpolation within Matlab’s “meshgrid” algorithm. Overall, higher beam-c values in the benthic boundary region characterized the eastern gulf as compared to the western gulf region (Fig. 4A). The thickest BNLs of 45–60 m were found within the BOF, in the offshore eastern gulf/Jordan Basin region, and in topographically low areas of the western gulf (Fig. 4A). BNLs of < 5–20 m thick were prevalent along the coast at bottom depths of < 100 m, in the area between the Scotian Shelf and eastern Jordan Basin and in a region extending offshore in a southwesterly direction from Penobscot Bay (Fig. 4A). Mean thickness of the BNLs was 24 m in the western gulf, 32 m in the eastern gulf and 27 m in the BOF with the eastern gulf displaying greater areal extent of thick BNLs than the western domain. One station in the BOF revealed no BNL (Fig. 4A).
Fig. 4.
(A) Thickness of the BNLs in the Gulf of Maine determined from October 2004 CTD/transmissometer survey data at 97 stations. (B) BNL suspended A. fundyense cyst abundance from large volume water samples, October 2004. (C) Vertically integrated, # cysts cm−2 in BNL at each station. (D) 0–1 cm sedimentary cyst abundance map for 2004 presented as # cysts cm2 (from Anderson et al., 2014).
BNL suspended cysts (concentrations presented as # m−3 in Fig. 4B) were found at all 2004 stations with the exception of one station in northwestern Wilkinson Basin. However, stations adjacent to this site (< 20 km) all showed cysts in the BNL filtered samples (Fig. 4B). Additionally, cysts were found in near-bottom suspended particle samples from stations with minimal evidence of a BNL. The overall pattern shows highest suspended cyst concentrations from the BOF into the far northeastern gulf, south and southwest of Grand Manan Island and Eastport, Maine (ME). Offshore of Corea, ME the high suspended cyst concentration region extends southward along the western edge of Jordan Basin (JB) in a tongue reaching out to the deep central gulf where maximum values of 4 × 105 cysts m−3 were observed. Another region of elevated, near-bottom suspended cyst abundances (104−105 cysts m−3) is found off Penobscot Bay and to the southwest. Smaller areas of moderate suspended cyst abundance values are seen to the south of Casco Bay and at the southern end of JB. Of particular interest relative to these data is the existence of large, permanent sedimentary cyst beds in the BOF and off the central Maine coast/Penobscot Bay where cyst concentrations of ~105−106 cysts m−2 within the upper 1 cm have been consistently reported from benthic surveys (Martin and Wildish, 1994; Anderson et al., 2005, 2014). These seedbeds have been proposed as the primary sources of the inoculum for the annual A. fundyense bloom in the gulf (Anderson et al., 2005; McGillicuddy et al., 2005; Anderson et al., 2014).
If the majority of suspended matter (i.e., cysts, lithogenic particles, siliceous tests, etc.) in the BNLs is delivered via sediment resuspension (Kirn et al., 2005; Pilskaln et al., 2014), and we hypothesize that the particles are proportionately distributed within the BNLs, it follows that thicker BNLs would likely exhibit higher suspended particle (and cyst) inventories. Comparison of the contoured BNL thickness map and the suspended cyst concentration in the BNL (Fig. 4A and B) indicates that there no obvious covariance between BNL thickness and BNL cyst abundance. Linear regression analysis shows that stations overall with BNLs and suspended cysts (n=97) give r=0.18 (p=0.08). Even though there are areas where both parameters were elevated such as along the western edge of JB and in the BOF, there are regions where the BNL was relatively thin and near-bottom suspended cyst abundance was high. The latter situation was observed at the stations to the south and southeast of Grand Manan Island and in the region extending offshore and to the southwest of western Penobscot Bay (Fig. 4A and B).The western gulf displays a slightly lower r value of 0.06 (n=40, p=0.71) than the eastern gulf stations (r=0.20, n=34, p=0.25). The BOF stations displayed the highest r value of 0.54, (n=23, p=0.01) between the two parameters which is significant at the 95% confidence level. Fig. 4(C) is the vertically integrated cysts per cm2 within the BNL which allows comparison to the underlying, 0–1 cm sedimentary cyst abundance per cm−2 in Fig. 4D (from Anderson et al. (2014)/cyst maps).
We calculated BNL median cyst concentrations for each sub-domain from the Fig. 4B data set. Due to the large range of BNL cyst concentrations across the gulf and BOF, we have based our calculations in Table 1 on median rather than mean cyst concentration for each subregion as the latter can be significantly skewed by a single value that is extremely large or small. Table 1 also presents the integrated cysts per m−2 in each subdomain using mean BNL thickness values for each region calculated from data shown in Fig. 4A, and the total subdomain BNL cyst inventories and underlying surface sediment cyst inventories (latter from Anderson et al. (2014)/cyst maps). Even though the BOF area is 2–3 times smaller than that of the WGOM and EGOM, it exhibits the largest BNL cyst concentrations (# m−3, Table 1, column 2) and largest BNL cyst inventory (Table 1, column 5). Interestingly, the smallest BNL cyst concentrations and inventory is represented by the largest subdomain area, the WGOM, which also has the greatest total sediment reservoir of cysts. WGOM and EGOM show essentially identical BNL cyst inventories whereas the west has a 4-fold greater sedimentary cyst inventory (Table 1).
Table 1.
2004 BNL cyst concentrations and inventories for Gulf of Maine subdomains compared to surface sediment (0–1 cm) cyst inventories.
| Sub-domain (area) (km2) |
Median BNL cyst concentration (# m−3) |
Mean BNL thickness (m) |
Cysts per BNL (# m−2) |
BNL cyst inventory per subdomain |
0–1 cm sedimentary cyst inventory per subdomain* |
|---|---|---|---|---|---|
| WGOM (3.1 × 104*) | 1.6 × 103 | 24 | 0.4 × 105 | 0.1 × 1016 | 17.2 × 1016 |
| EGOM (2.1 × 104*) | 2.1 × 103 | 32 | 0.7 × 105 | 0.1 × 1016 | 4.4 × 1016 |
| BOF (0.9 × 104*) | 9.4 × 104 | 27 | 25.4 × 105 | 2.3 × 1016 | 2.3 × 1016 |
Subdomains defined and surface sediment cyst inventories provided in Anderson et al., 2014.
4. Discussion
4.1. Gulf of Maine BBL particle resuspension and BNL connections: implications for cyst transport and bloom initiation
Gulf-wide, full-depth beam attenuation data sets clearly document the abundance of BNLs within the bottom boundary layer. Elevated SPM and POC content of BNL samples additionally distinguish the layer from immediately overlying waters with much lower particle abundance. Multiple forces affect fluid and particle motion in the gulf benthic boundary region (e.g., seasonally large storm waves, energetic tidal flow and strong coastal currents) and inevitably impact the geographic development and persistence of BNLs. Nonetheless, BNLs are a prominent and distinct feature in the gulf. Deciphering potential transport links between BNLs that overly principal sedimentary cyst beds and the rest of the gulf may be important to include in bloom forecasting applications such as that presented by He et al. (2008), Li et al. (2009) and McGillicuddy et al. (2011).
Of particular interest is the observed connection between the BOF and eastern gulf BNLs (Figs. 2–6) and high suspended cyst abundance (Fig. 4B and C). A near-bottom conduit of particles and cysts from the BOF into the Maine Coastal Current (MCC) is indicated by our data in which a high BNL cyst concentration region (> 1000 cysts m−3) extends from the BOF down to offshore Penobscot Bay, excluding the one lower-abundance station (429 cysts m−3) just off the coast from Corea, ME (Fig. 4B). There are striking similarities in the BNL suspended cyst patterns of Fig. 4(B and C) and the 0–1 cm sedimentary cyst map in Fig. 4(D) in terms of focused, high-value areas and connections between some of these areas. Based on these results, we suggest that southwesterly MCC transport of BOF cysts along the Maine coast provides an important contribution to the cyst inventories in the BNLs located to the southwest and downstream of the BOF. A similar northeast-to-southwest pattern has been observed and modeled in the propagation from the BOF to midcoast Maine of annual A. fundyense blooms via the MCC (Anderson et al., 2005; McGillicuddy et al., 2005). However, numerical model simulations of suspended cyst transport suggest the possibility of more modest advective length scales (Aretxabaleta et al. 2014).
An additional significant feature of the suspended cyst map (Fig. 4B) is the prominent southerly extension and increase in cyst concentration offshore along the western edge of JB and into the deep central gulf. The pattern is very similar to and thus likely related to the observed offshore branching of the MCC that contributes to the cyclonic JB gyre (Pettigrew et al., 1998; Brooks and Townsend, 1989; Anderson et al., 2005; Pettigrew et al., 2005) and perhaps is a conduit towards Georges Bank. Large to moderate-sized blooms of A. fundyense have occurred in the spring-summer on Georges Bank, although it is not clear what provides the initial inoculum for the blooms that proliferate within its retentive gyre (McGillicuddy et al., 2014). Based on the above observations and the BNL-cyst distribution data presented here, it is suggested that the southerly extension of the MCC may periodically be associated with a high abundance of suspended cysts in the underlying BNLs. If the cysts are eventually carried farther south to the northern side of Georges Bank, strong tidal mixing and upwelling (Franks and Chen, 1996; Chen and Beardsley, 1998; Townsend and Thomas, 2001; Hu et al., 2008) could bring those cysts to the surface where favorable nutrient and light conditions would allow them to bloom. Obviously, this pathway of suspended cyst transport from the central gulf to Georges Bank is not resolved by the present data and a comprehensive assessment of that potential awaits further study.
4.2. Benthic nepheloid layer and surface sediment cyst inventories: how they compare
Our maximum BNL cyst concentrations of 105 cysts m−3 (median=104 cysts m−3, Table 1) were an order of magnitude greater than that reported in Kirn et al. (2005). Differences between BNL cyst concentrations reported in the two studies reflect several factors. Firstly, interannual variability in sedimentary cyst abundance clearly occurs (Anderson et al., 2005, 2014/cyst maps), thus leading to variations in the potential contribution of cysts to the BNLs via resuspension. Secondly, significantly different BNL water sample sizes were collected, with the present study using 90 L samples and Kirn et al., 2005 study obtaining 30 L samples. No replicates were collected and filtered for cysts in either study due to station time constraints. Finally, the two studies were conducted in different seasons. Kirn et al. (2005) collected BNL water samples in February, April and June which precedes the late summer-fall encystment period when the maximum delivery of cysts from the overlying water column to the BNL occurs (a, 2013). The Kirn et al. early spring-summer data likely represent cysts that have remained suspended or have been resuspended into the BNL in the winter-spring with minimal input from the upper water column of newly formed cysts. A significant increase in the ratio of empty/intact cysts between February and April in their BNL samples suggests excystment of the BNL cysts as a result of the cysts’ endogenous clock plus increasing temperatures and light levels (Anderson and Keafer, 1987; Kirn et al., 2005). By comparison, the fall BNL cyst concentrations reported in the present study may also result from bloom termination, cyst formation and settling at the end of the summer, coupled with input from resuspension.
An objective of our study was to answer the following questions: are the A. fundyense cyst numbers in the BNL what we might expect from resuspension of the cysts in the underlying sediments, and how do the BNL and sedimentary cyst inventories compare as potential sources of bloom inoculum? Bottom resuspension has been identified as a significant and consistent source of cysts to the gulf BNLs (Kirn et al., 2005; Pilskaln et al., 2014). Time-series sediment trap data obtained above and within the gulf BNLs indicate that cyst delivery from the overlying water column is highly seasonal but on an annual basis cannot account for the cyst fluxes measured within the BNLs and thus bottom resuspen-sion must make a substantial contribution (Pilskaln et al., 2014). Kirn et al. (2005) concluded from their measurements of near-bottom, suspended and sedimentary cyst abundances that resus-pended sedimentary cysts may be an important source of the annual A. fundyense vegetative population.
The primary time period of cyst formation, settling out of the water column, delivery to the BNL and (presumably) to the underlying sediments occurs in the late summer-fall, following the annual spring-summer bloom as documented by many years of cyst surveys and time-series sediment trap studies (Anderson et al., 2014/cyst maps; Pilskaln et al., 2014). Newly formed cysts must undergo a mandatory, 2–6 months maturation and dormancy after which the activation of their endogenous clocks leads to germination if they are exposed to favorable temperature and oxygen conditions (Anderson, 1980; Anderson and Keafer, 1987). However cysts potentially may remain dormant for years if buried deep enough and in low-oxygen content, shelf sediments (Anderson, 19 80; Anderson and Keafer, 1987). These life-cycle components are especially important to the fate of A. fundyense cysts deposited in the organic-rich, upper sediments in the Gulf of Maine that accumulate at relatively high rates of 1–3 mm year−1 and are dysoxic to anoxic below approximately 0.5–1 cm (Hulbert and Given, 1975; Faas and Nittrouer, 1976; Bothner et al., 1981; Hines et al., 1991; Christensen, 19 89; Keafer et al., 1992; Anderson et al., 2005; Keigwin unpublished communication).
To examine the potential contribution of suspended cysts to bloom initiation, we need to compare cyst concentrations within the BNL and the sedimentary cyst reservoirs. Additionally, we need to consider the mean thickness of the BNLs over which resus-pended cysts are distributed, the sediment depth over which the majority of mature, germination-ready cysts are found, and estimates of Gulf of Maine sediment erosion depths due to natural physical processes such as shear stresses from currents and waves. Multi-year sedimentary cyst densities for the Gulf of Maine and BOF are reported by Anderson et al. (2005, 2014)/cyst maps. Gulf-wide, 0–1 cm sedimentary cyst densities range from zero to 106–107 cysts m−2 with the maxima observed within major cyst seedbed areas in the BOF, offshore of Penobscot-Casco Bays and occasionally on the eastern edge of JB (Anderson et al., 2005, 2014). It is within the 0–1 cm sediment depth horizon that the greatest abundance of viable cysts is concentrated in comparison to the immediately underlying sediments (Anderson et al., 2005). Hypothetically, if all the cysts within the top 1 cm of the seedbed sediments were resuspended into the overlying water column to a depth that approximates the mean gulf BNL thickness (i.e., ~30 m), we might reasonably expect 105–106 cysts m−3 within the overlying BNLs. These values are remarkably similar to the maximum suspended cyst densities of 105 cysts m−3 that we report in the current study from the BOF, eastern JB/deep central gulf and the region to the southwest offshore Penobscot Bay (i.e., the dark red areas in Fig. 4B). Assuming that the majority of the mature cysts in the BNL are derived from the underlying sediments, then once resuspended, they have a greater probability of being transported up into the water column where they would encounter germination-favorable light, temperature and oxygen conditions as compared to those cysts that are sediment-bound. In this scenario, BNL cysts could represent a highly significant, potential contributor to the bloom inoculum in the gulf.
Resuspension provides a mechanism to release sedimentary-bound cysts into the overlying, oxygenated BNLs and bottom waters where germination-favorable conditions exist (Anderson et al., 2005). In the Gulf of Maine, sediment resuspension is primarily driven by bottom stress resulting from storm-generated waves, strong tidal flows and well-defined subtidal flows (e.g. the Maine Coastal Current, Jordan and Wilkinson Basin gyres; Greenberg, 1979; Brown, 1984; Moody et al., 1984; Brown and Irish, 1992; Lynch et al., 1996; Pettigrew et al., 2005; Pilskaln et al., 2014). Storm-wave resuspension is most prevalent in the shallow regions of the gulf near the coast (Butman et al., 2008). Tidal flow energy and its likely impact on the bottom boundary region decreases in strength from east to west (Bigelow, 1927; Garrett, 1972; Xue et al., 2000). In the deep BNLs of Jordan and Wilkinson Basins that are inside the peripheries of the cyclonic gyres centered over the basins, mean tidal flows of 10–20 cm s-1 dominate smaller residual (mean) flows (Pilskaln et al., 2013a). In the BOF where a massive A. fundyense seedbed exists, model simulations of the BOF cyclonic gyre reveal inter- and intra-annual variability in particle retention and advective interaction with the adjacent Gulf of Maine circulation (Aretxabaleta et al., 2008, 2009). Theoretically, it follows that such variability might also impact the resuspension and transport of cysts from the BOF seedbed into the gulf. Butman et al. (2014) conducted sediment erosion experiments on small, 10.7 cm diameter cores obtained from seven gulf stations (70– > 200 m bottom depth) and used the results into a one-dimensional model to estimate sediment resuspension and transport. Aretxabalets et al. (2014) used the erosion results to explore transport of sediment during single erosion events. The major A. fundyense seedbeds in the gulf and BOF are dominated by fine silt and clay sediments (Anderson et al., 2005; 2014; Butman et al., 2014). For simulations at known cyst seedbed stations, the resuspension suggested that about 1 mm of sediment is mobilized in winter and spring, with interannual variability in the frequency of resuspension events (Butman et al., 2014). The simulations also showed no resuspension in Wilkinson basin and negligible surface sediment resuspension in Jordan Basin where clay and fine silt sediments are predominant. The surprisingly low, 1 mm erosion depth reported by Butman et al. may be due in part to edge effects of the relatively small cylinder used in the erosion experiments as compared to that employed by others (e.g., Gust and Mueller, 1997). In similar studies using a different mechanism to generate stress (oscillating plunger vs. rotating plate), a significant shear stress reduction near the cylinder wall has been shown (Grant et al., in press) which could impact the total erosion rate determined over a fairly small experimental surface area. Another issue concerns the magnitude of the near-bottom velocities used to predict the stress. Although the model velocities (from Chen et al., 2003, Finite Volume Coastal Ocean Model, FVCOM) used by Butman et al. (2014) are consistent with those reported by Pilskaln et al. (2014) for 255 m (25 mab) in Jordan Basin, the near-bottom velocities used for the stress computation are attenuated in a bottom boundary layer. To our knowledge, there are no near-bottom velocity profiles in the Gulf of Maine that can be used to quantitatively evaluate the shear predicted by FVCOM. In any case, for the sake of comparison, if we consider that only the top 1 mm of surface sediment in the seedbed areas is resuspended, the estimated cyst concentration in the BNLs would be 103–104 cysts m−3 which matches or exceeds 87% of our measured BNL cyst concentrations. However, approximately 1 cm of sediment resuspension would be required to provide the highest BNL suspended cyst densities of 105 cysts m−3 observed in particular in the BOF BNLs (Fig. 4B).
Spatially scaling up all of our BNL suspended cyst and sedimentary cyst data, we are able to directly compare the total BNL cyst versus sediment cyst inventories in each subdomain (Table 1). Looking at the last two columns in Table 1, the BNL cyst reservoirs estimated for the WGOM and EGOM subdomains are equivalent and 1–2 orders of magnitude smaller than the corresponding subdomain 0–1 cm cyst reservoirs. If we consider the resuspension of only the upper 0–1 mm sedimentary cyst inventories (projected from the 0–1 cm values) as a source of BNL cysts, as suggested in Kirn et al. (2005), then all the BNL cyst and sediment inventories begin to significantly converge. Most notable in the last two columns of Table 1 are the total BNL cyst and 0–1 cm sediment cyst inventories for the BOF. These two cyst reservoirs are of equal size and thus significance as a source of cysts for blooms and potential export to adjacent downstream regions.
The conceptual model of A. fundyense dynamics presented in Anderson et al. (2005) suggests that blooms are initiated by germinating cysts from the noted subregional seedbeds. In this context, the BOF serves as the critical A. fundyense incubator, retaining enough cells to contribute to the seedbed but also supplying cells that propagate to the west and south into the gulf via the MCC (Anderson et al., 2005). Our study adds to and enhances the conceptual model by providing substantial support for the benthos-to-water-column connection in bloom generation via sediment resuspension and the existence of a large BNL cyst reservoir, particularly in the Bay of Fundy (Table 1).
5. Conclusions
Extensive benthic nepheloid layers are present in the Gulf of Maine and contain substantial A. fundyense cyst concentrations that vary by several orders of magnitude (e.g., 102–105 cysts m−3) over distances of 15–30 km. BNL cyst inventories within the Bay of Fundy and the eastern and western gulf regions were calculated to be on the order of 1015–1016 cysts per sub-region. A general northeast to southwest trend of decreasing mean BNL thickness and cyst concentrations was observed. Our data indicate that BNL cysts are transported from the Bay of Fundy to the central coast of Maine via the MCC and to the south-central gulf via the southward branching component of the MCC. The latter is an important finding relative to the potential source of cysts that could fuel A. fundyense blooms in the Georges Bank region. We estimate that the gulf BNLs represent an important cyst reservoir in the near-bottom waters of up to 105–106 cysts m−2 in particular regions that is at least as significant as the 0–1 cm surface sediment cyst pool in terms of providing bloom inoculum. Specifically, if only the upper 1 mm of sedimentary cysts were viable for germination, the potential contribution of BNL versus sedimentary cysts to bloom initiation would shift toward the BNL. Greater refinement and increased certainty of these estimates requires much more effort in determining the age of cysts in the BNLs and the uppermost sediments, as well as the germination success rate in these two environments. Additionally, modeling of the potential and probable lateral movement of the gulf BNLs will go a long way towards increasing understanding of bloom occurrences in south-central areas as well as the potential impact of shelf BNLs on sedimentation and particle resuspension on the adjacent slope.
Acknowledgments
We are extremely grateful to Captain D. Ogus and the crew of the R/V Cape Hatteras for making the October 2004 cruise a success (and for tolerating our World Series obsession), and we thank J. Brown, C. Falkner, S. McQuilken, K. Norton and B. Tupper for assistance at sea and/or in the laboratory. R. Signell provided Gulf of Maine bathymetry and V. Kosnyrev assisted in the graphics production. This work was supported by NOAA Grants NA04NOS4780274 (ECOHAB-GOM/Cyst) and NA06NOS4780245 (GOMTOX). Additional support for DMA and DJM was provided by the Woods Hole Center for Oceans and Human Health through National Science Foundation Grants OCE-0430724 and OCE-0911031 and OCE-1314642 and National Institute of Environmental Health Sciences Grants 1P50-ES01274201 and 1P01ES021923-01. This is the Ecology and Oceanography of Harmful Algal Blooms Program contribution number XXXX.
References
- Agrawal YC, Traykovski P. Particles in the bottom boundary layer: concentration and size dynamics through events. J. Geophys. Res. 2001;106:9533–9542. [Google Scholar]
- Anderson DM. Effects of temperature conditioning on development and germination of Gonyaulax tamarensis (Dinophyeae) hynozygote. J. Phycol. 1980;16:166–172. [Google Scholar]
- Anderson DM. Bloom dynamics of toxic Alexandrium species in the northeast US. Limnol Oceanogr. 1997;42:1009–1022. [Google Scholar]
- Anderson DM. Physiology and bloom dynamics of toxic Alexandrium species, with emphasis on life cycle transitions. In: Anderson DM, Cembella AD, Hallegraeff GM, editors. Physiological Ecology of Harmful Algal Blooms. Vol. 41. Berlin, Germany: Springer; 1998. pp. 29–48. [Google Scholar]
- Anderson DM, Keafer BA. An endogenous annual clock in the toxic dinoflagellate Gonyaulax tamarensis. Nature. 1987;325:616–617. doi: 10.1038/325616a0. [DOI] [PubMed] [Google Scholar]
- Anderson DM, Fukuyo Y, Matsuoka K. Hallegraeff GM, Anderson DM, Cembella AD, editors. Cyst methodologies. Manual on Harmful Marine Microalgae, Monographs on Oceanographic Methodology. 2003;11:165–190. UNESCO. [Google Scholar]
- Anderson DM, Keafer BA, Kleindinst JL, McGillicuddy DJ, Jr, Martin JL, Norton K, Pilskaln CH, Smith JL, Sherwood CR, Butman B. Alexandrium fundyense cysts in the Gulf of Maine: long-term time series of abundance and distribution, and linkages to past and future blooms. Deep-Sea Res. II. 2014;103:6–26. doi: 10.1016/j.dsr2.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Stock CA, Keafer BA, Bronzino Nelson A, Thompson B, McGilli-cuddy DJ, Jr, Keller M, Matrai PA, Martin J. Alexandrium fundyense cyst dynamics in the Gulf of Maine. Deep-Sea Res. II. 2005;52(19–21):2522–2542. [Google Scholar]
- Aretxabalets AL, Butman B, Signell RP, Dalyander S, Sherwood CS, McGilli-cuddy DJ., Jr Near-bottom circulation and dispersion of sediment containing Alexandrium fundyense cysts in the Gulf of Maine during 2010–2011. Deep-Sea Res. II. 2014;103:96–111. doi: 10.1016/j.dsr2.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aretxabaleta AL, McGillicuddy DJ, Jr, Smith KW, Lynch DR. Model simulations of the Bay of Fundy Gyre: 1. Climatological results. J. Geophys. Res. 2008;113:C10027. doi: 10.1029/2007JC004480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aretxabaleta AL, McGillicuddy DJ, Jr, Smith KW, Lynch DR. Model simulations of the Bay of Fundy Gyre: 2. Hindcasts for 2005–2007 reveal interannual variability in retentiveness. J. Geophys. Res. 2009;114:C09005. doi: 10.1029/2008JC004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow HB. Physical oceanography of the Gulf of Maine. Fish. Bull. 1927;40(511–1027):1927. [Google Scholar]
- Bishop JKB. The correction and suspended particulate matter calibration of Sea Tech transmissometer data. Deep-Sea Res. I. 1986;33:121–134. [Google Scholar]
- Boss E, Pegau WS, Gardner WD, Zaneveld JRV, Barnard AH, Twardowski MS, Chang GC, Dickey TD. Spectral particulate attenuation and particle size distribution in the bottom boundary layer of a continental shelf. J. Geophys. Res. 2001;106:9509–9509. 516. [Google Scholar]
- Bothner MH, Spiker EC, Johnson PP, Rendigs RR, Aruscavage PJ. Geochemical evidence for modern sediment accumulation on the continental shelf off Southern New England. J. Sediment. Petrol. 1981;51:281–292. [Google Scholar]
- Boudreau BP, Jorgensen BB. Introduction. In: Boudreau BP, Jorgensen BB, editors. The Benthic Boundary Layer: Transport Processes and Biogeochemistry. New York, NY: Oxford University Press; 2001. pp. 1–3. [Google Scholar]
- Brooks DA, Townsend DW. Variability of the coastal current and nutrient pathways in the eastern Gulf of Maine. J. Mar. Res. 1989;47:303–321. [Google Scholar]
- Brown WS. A comparison of Georges Bank, Gulf of Maine and New England Shelf tidal dynamics. J. Phys. Oceanogr. 1984;14:145–167. [Google Scholar]
- Brown WS, Irish JD. The annual evolution of geostrophic flow in the Gulf of Maine: 1986–1987. J. Phys. Oceanogr. 1992;22:445–473. [Google Scholar]
- Butman B, Sherwood CR, Dalyander PS. Northeast storms ranked wind stress and wave-generated bottom stress observed in Massachusetts Bay, 1990–2006. Cont. Shelf Res. 2008;28:1231–1245. [Google Scholar]
- Butman B, Aretxabaleta AL, Dickhudt PJ, Dalyander PS, Sherwood CR, Anderson DM, Keafer BA, Signell RP. Investigating the importance of sediment resuspension in Alexandrium fundyense cyst population dynamics in the Gulf of Maine. Deep-Sea Res. II. 2014;103:79–95. doi: 10.1016/j.dsr2.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GC, Dickey TD, Williams AJ. Sediment resuspension over a continental shelf during Hurricanes Edouard and Hortense. J. Geophys. Res. 2001;106:9517–9531. [Google Scholar]
- Chen C, Beardsley RC. Tidal mixing and cross-frontal particle exchange over a finite amplitude asymmetric bank: a model study with application to Georges Bank. J. Mar. Res. 1998;56:1163–1201. [Google Scholar]
- Chen C, Lui H, Beardsley RC. An unstructured, finite-volume, three-dimensional, primitive equation ocean model: application to coastal ocean and estuaries. J. Atmos. Oceanic Technol. 2003;20:159–186. [Google Scholar]
- Christensen JP. Sulfate reduction and carbon oxidation rates in continental shelf sediments, an examination of off-shelf carbon transport. Cont. Shelf Res. 1989;9:223–246. [Google Scholar]
- Dade WB, Hogg AJ, Boudreau BP. Physics of flow above the sediment-water interface. In: Boudreau BP, Jorgensen BB, editors. The Benthic Boundary Layer: Transport Processes and Biogeochemistry. New York, NY: Oxford University Press; 2001. pp. 4–43. [Google Scholar]
- Dickson RR, McCave IN. Nepheloid layers on the continental slope west of Porcupine Bank. Deep-Sea Res. I. 1986;33:791–818. [Google Scholar]
- Dortch Q, Townsend DW, Spinrad RS, Mayer LM. The role of the nepheloid layers in benthic-pelagic coupling. NOAA-NURP Res. Rept. 1988:181–204. 883: Benthic productivity and marine resources in the Gulf of Maine. [Google Scholar]
- Durrieu de, Mandron X, Nyffeler F, Godet CH. Hydrographic structure and nepheloid spatial distribution in the Gulf of Lions continental margin. Cont. Shelf Res. 1990;10:915–929. [Google Scholar]
- Eittreim SL, Thorndike EM, Sullivan L. Turbidity distribution in the Atlantic Ocean. Deep-Sea Res. I. 1976;23:1115–1137. [Google Scholar]
- Faas RW, Nittrouer CA. Postdepositional facies development in the finegrained sediments of the Wilkinson Basin, Gulf of Maine. J. Sediment. Petrol. 1976;46:337–344. [Google Scholar]
- Fanning KA, Carder KL, Betzer PR. Sediment resuspension by coastal waters: a potential mechanism for nutrient recycling on the ocean’s margins. Deep-Sea Res. I. 1982;29:953–965. [Google Scholar]
- Flint RW, Rabalais NN, editors. Environmental Studies of a Marine Ecosystem: South Texas Outer Continental Shelf. University of Texas Press; 1981. [Google Scholar]
- Franks PJS, Chen C. Plankton production in tidal fronts: a model of Georges Bank in summer. J. Mar. Res. 1996;54:63l–651. [Google Scholar]
- Gardner WD, Walsh ID. Distribution of macroaggregates and fine-grained particles across a continental margin and their potential role in fluxes. Deep-Sea Res. I. 1990;37:401–411. [Google Scholar]
- Gardner WD, Blakey JC, Walsh ID, Richardson MJ, Pegau S, Zaneveld JRV, Roesler C, Gregg MC, MacKinnon JA, Sosik HM, Williams AJ. Optics, particles, stratification, and storms on the New England continental shelf. J. Geophys. Res. 2001;106:9473–9497. [Google Scholar]
- Garrett C. Tidal resonance in the Bay of Fundy and Gulf of Maine. Nature. 1972;238:441–443. [Google Scholar]
- Gowing MM, Wishner KF. Trophic relationships of deep-sea calanoid copepods from the benthic boundary layer of the Santa Catalina Basin, California. Deep-Sea Res. I. 1986;33:939–961. [Google Scholar]
- Grant J, Walker TR, Hill PS, Lintern DG. BEAST—a portable device for quantification of erosion in natural intact sediment cores. Methods Oceanogr. 2013 (in press) [Google Scholar]
- Greenberg DA. A numerical model investigation of tidal phenomena in the Bay of Fundy and Gulf of Maine. Mar. Geod. 1979;2:161–187. [Google Scholar]
- Gust G, Mueller V. Interfacial hydrodynamics and entrainment functions of currently used erosion devices. In: Burt , Parker , Watts , editors. Cohesive Sediments. Wallingford, U.K: 1997. pp. 149–174. [Google Scholar]
- Hayashi K, Pilskaln CH. New Hampshire, USA: Program of Abstracts; Aug, 2011. Geochemical composition and particle flux dynamics in the Gulf of Maine benthic nepheloid layer; Gordon Research Conference in Chemical Oceanography; p. 6. 2011. [Google Scholar]
- He R, McGillicuddy DJ, Jr, Keafer BA, Anderson DM. Historic 2005 toxic bloom of Alexandrium fundyense in the western Gulf of Maine: 2. Coupled biophysical numerical modeling. J. Geophys. Res. 2008;113:C07040. doi: 10.1029/2007JC004602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill PS, McCave IN. Suspended particle transport in benthic boundary layers. In: Boudreau BP, Jorgensen BB, editors. The Benthic Boundary Layer: Transport Processes and Biogeochemistry. New York, NY: Oxford University Press; 2001. pp. 78–103. [Google Scholar]
- Hill PS, Voulgaris G, Trowbridge JH. Controls on floc size in a continental shelf bottom boundary layer. J. Geophys. Res. 2001;106:9543–9549. [Google Scholar]
- Hines ME, Bazylinski DA, Tugel JB, Lyons WB. Anaerobic microbial biogeochemistry in sediments from two basins in the Gulf of Maine: evidence for iron and manganese reduction. Estuarine, Coastal Shelf Sci. 1991;32:313–324. [Google Scholar]
- Hu S, et al. Tidal pumping and nutrient fluxes on Georges Bank: a process-oriented modeling study. J. Mar. Syst. 2008;74:528–544. [Google Scholar]
- Hulbert MH, Given DE. Geotechnical and chemical property relationships for Wilkinson Basin, Gulf of Maine, sediments. J. Sediment. Petrol. 1975;45:504–512. [Google Scholar]
- Keafer BA, Buesseler KO, Anderson DM. Burial of living dinoflagellate cysts in estuarine and nearshore sediments. Mar. Micropaleontol. 1992;20:147–161. [Google Scholar]
- Kirn SL, Townsend DW, Pettigrew NR. Suspended Alexandrium spp. hypnozygote cysts in the Gulf of Maine. Deep-Sea Res. II. 2005;52(19–21):2543–2559. [Google Scholar]
- Lampitt RS. Evidence of the seasonal deposition of detritus to the deep-sea floor and its subsequent resuspension. Deep-Sea Res. I. 1985;32:885–897. [Google Scholar]
- Li Y, He R, McGillicuddy DJ, Anderson DM, Keafer BA. Investigation of the 2006 Alexandrium fundyense bloom in the Gulf of Maine: in-situ observations and numerical modeling. Cont. Shelf Res. 2009;29:2069–2082. doi: 10.1016/j.csr.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch DR, Ip JTC, Naimie CE, Werner FE. Comprehensive coastal circulation model with application to the Gulf of Maine. Cont. Shelf Res. 1996;16:875–906. [Google Scholar]
- Martin JL, Wildish DJ. Forbes JR, editor. Temporal and spatial dynamics of Alexandrium fundyense cysts during 1981–1983 and 1992 in the Bay of Fundy. Proceedings of the Fourth Canadian Workshop on Harmful Marine Algae. 1994;45(11):1986–1975. Canadian Technical Report of Fisheries and Aquatic Sciences. [Google Scholar]
- Mayer LM, Macko SA, Cammen L. Provenance, concentrations and nature of sedimentary organic nitrogen in the Gulf of Maine. Mar. Chem. 1988;25:291–304. [Google Scholar]
- McCave IN. Particulate size spectra, behavior, and origin of nepheloid layers over the Nova Scotian continental rise. J. Geophys. Res. 1983;88:7647–7666. [Google Scholar]
- McCave IN. Mechanics of deposition of fine-grained sediments from nepheloid layers. Geo-Mar. Lett. 1985;4:243–245. [Google Scholar]
- McCave IN. Local and global aspects of the bottom nepheloid layers in the world ocean. Neth. J. Sea Res. 1986;20:167–181. [Google Scholar]
- McGillicuddy DJ, Jr, Anderson DM, Lynch DR, Townsend DW. Mechanisms regulating the large-scale seasonal fluctuations in Alexandrium fundyense populations in the Gulf of Maine: results from a physical-biological model. Deep-Sea Res. II. 2005;52:2698–2714. [Google Scholar]
- McGillicuddy DJ, Townsend DW, He R, Keafer BA, Kleindinst JL, Li Y, Manning JP, Mountain DG, Thomas MA, Anderson DM. Suppression of the 2010 Alexandrium fundyense bloom by changes in physical, biological, and chemical properties of the Gulf of Maine. Limnol. Oceanogr. 2011;56(6):2411–2426. doi: 10.4319/lo.2011.56.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillicuddy DJ, Jr, Townsend DW, Keafer BA, Thomas MA, Anderson DM. Georges Bank: a leaky incubator of Alexandrium fundyense blooms. Deep-Sea Res. II. 2014;103:163–173. doi: 10.1016/j.dsr2.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody JA, Butman B, Beardsley RC, Brown WS, Diafuku P, Irish JD, Mayer DA, Mofjeld HO, Petrie B, Ramp S, Smith P, Wright WR. Atlas of tidal elevation and current observa tions on the Northeast American Continental Shelf and Slope. U.S. Geol. Survey Bull. 1984;1661:122. [Google Scholar]
- Newberger PA, Caldwell DSR. Mixing and the bottom nepheloid layer. Mar. Geol. 1981;41:321–336. [Google Scholar]
- Pettigrew NR, Townsend DW, Xue H, Wallinga JP, Brickley PJ, Hetland RD. Observations of the eastern Maine coastal current and its offshore extensions in 1994. J. Geophys. Res. 1998;103:623–30. 639. [Google Scholar]
- Pettigrew NR, Churchill JH, Janzen CD, Mangum LJ, Signell RP, Thomas AC, Townsend DW, Wallinga JP, Xue X. The kinematic and hydrographic structure of the Gulf of Maine Coastal Current. Deep-Sea Res. II. 2005;52(19–21):2369–2391. [Google Scholar]
- Pilskaln CH. Gulf of Maine Symposium—Advancing Ecosystem Research for the Future of the Gulf. New Brunswick, Canada: Program of Abstracts; 2009. Seasonal and interannual biogeochemical particle flux dynamics in the Gulf of Maine; p. 84. [Google Scholar]
- Pilskaln CH, Churchill JH, Mayer LM. Resuspension of sediment by bottom trawling in the Gulf of Maine and potential geochemical consequences. J. Conserv. Biol. 1998;12:1223–1230. [Google Scholar]
- Pilskaln CH, Anderson DM, McGillicuddy DJ, Jr, Keafer BA, Hayashi K, Norton K. Spatial and temporal variability of Alexandrium cyst fluxes in the Gulf of Maine relationship to seasonal particle export and resuspension. Deep-Sea Res. II. 2014;103:40–54. doi: 10.1016/j.dsr2.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe LJ, Paskevich VF, Williams SJ, Hastings ME, Kelley JT, Belknap DF, Ward LG, FitzGerald DM, Larsen PF. Surficial sediment data from the Gulf of Maine, Georges Bank, and vicinity: a GIS compilation. U.S. Geological Survey open-file report. 2003:03–001. [Google Scholar]
- Ransom B, Bennett RH, Baerwald R, Shea KF. TEM of in situ organic matter on continental margins: occurrence and “monolayer” hypothesis. Mar. Geol. 1997;138:1–9. [Google Scholar]
- Ransom B, Shea KF, Burkett PJ, Bennett RH, Baerwald R. Comparison of pelagic and nepheloid layer marine snow: implications for carbon cycling. Mar. Geol. 1998;150:39–50. [Google Scholar]
- Rutgers van der Loeff MM, Meyer R, Rachor E. Resuspension and particle transport in the benthic nepheloid layer in and near Fram Strait in relation to faunal abundances and 234Th depletion. Deep-Sea Res. I. 2002;49:1941–1958. [Google Scholar]
- Smith KL, Carlucci AF, Jahnke RA, Craven DB. Organic carbon mineralization in the Santa Catalina Basin: benthic boundary layer metabolism. Deep-Sea Res. I. 1987;34:185–211. [Google Scholar]
- Spencer DW, Sachs PL. Some aspects of the distribution, chemistry and mineralogy of suspended matter in the Gulf of Maine. Mar. Geol. 1970;9:117–136. [Google Scholar]
- Spinrad RJ. An optical study of the water masses of the Gulf of Maine. J. Geophys. Res. 1986;91:1007–1018. [Google Scholar]
- Spinrad RW, Zaneveld JRV. An analysis of the optical features of the near-bottom and bottom nepheloid layers in the area of the Scotian Rise. J. Geophys. Res. 1982;87:9553–9561. [Google Scholar]
- Spinrad RW, Zaneveld JRV, Kitchen JC. A study of the optical characteristics of the suspended particles in the nepheloid layer of the Scotian Rise. J. Geophys. Res. 1983;88:7641–7645. [Google Scholar]
- Townsend DW, Mayer LM, Dortch Q, Spinrad RW. Vertical structure and biological activity in the bottom nepheloid layer of the Gulf of Maine. Cont. Shelf Res. 1992;12:367–387. [Google Scholar]
- Townsend DW, Thomas AC. Winter-spring transition of phytoplankton chlorophyll and inorganic nutrients on Georges Bank. Deep-Sea Res. II. 2001;48:199–214. [Google Scholar]
- Wainright S. Stimulation of heterotrophic microplankton production by resuspended marine sediments. Science. 1987;238:1710–1712. doi: 10.1126/science.238.4834.1710. [DOI] [PubMed] [Google Scholar]
- Wishner KF, Meise-Munns CJ. In situ grazing rates of deep-sea benthic boundary layer zooplankton. Mar. Biol. 1984;84:65–74. [Google Scholar]
- Xue H, Chai F, Pettigrew NR. A model study of seasonal circulation in the Gulf of Maine. J. Phys. Oceanogr. 2000;30:1111–1135. [Google Scholar]