Abstract
The proper folding of proteins to their functional forms is essential to cellular homeostasis. Perhaps not surprisingly, cells have evolved multiple pathways, some overlapping and others complementary, to resolve misfolded proteins when they arise, ranging from refolding through the action of molecular chaperones to elimination through regulated proteolytic mechanisms. These protein quality control pathways are sufficient, under normal conditions, to maintain a functioning proteome, but in response to diverse environmental, genetic, and/or stochastic events, protein misfolding exceeds the corrective capacity of these pathways, leading to the accumulation of aggregates and ultimately toxicity. Particularly devastating examples of these effects include certain neurodegenerative diseases, such as Huntington’s Disease, which are associated with the expansion of polyglutamine tracks in proteins. In these cases, protein misfolding and aggregation are clear contributors to pathogenesis, but uncovering the precise mechanistic links between the two events remains an area of active research. Studies in the yeast Saccharomyces cerevisiae and other model systems have uncovered previously unanticipated complexity in aggregation pathways, the contributions of protein quality control processes to them, and the cellular perturbations that result from them. Together these studies suggest that aggregate interactions and localization, rather than their size, are the crucial considerations in understanding the molecular basis of toxicity.
Introduction
While the pioneering work of Christian Anfinsen demonstrated that the sequence of amino acids in a polypeptide chain is sufficient to direct its proper folding (Anfinsen, 1967), we now appreciate that the same sequence allows considerable variation in folding trajectory to the native state, including off-pathway alternative states that are often associated with pathogenesis (Jahn and Radford, 2008). These sequence-based challenges to protein folding are also compounded by additional limitations, such as vectoral synthesis, molecular crowding, environmental and metabolic stresses, aging, mutations and synthesis errors, which are specific to the cellular environment (Kim et al., 2013). In the vast majority of cases, cellular quality control pathways act to maintain protein homeostasis (proteostasis) through the reactivation or clearance of aberrantly folded proteins, but in other cases, these pathways become overwhelmed leading to the accumulation of misfolded proteins, the disruption of normal cellular activities, and ultimately disease (Balch et al., 2008).
Many “protein misfolding” diseases are associated with a special group of metastable proteins that can access non-native conformations with a propensity to assemble into β-sheet-rich fibers (Chiti and Dobson, 2006). These complexes, known as amyloid, are characterized by detergent resistance, high thermodynamic stability, and the ability to continually incorporate monomers of the same protein, effectively titrating these species from a productive folding pathway to the native state and thereby self-replicating the amyloid state (Jahn and Radford, 2008). Together, the stability and self-replicating nature of amyloid fibers contributes to their persistence by protecting these complexes from complete disassembly by protein quality control pathways in vivo (Tuite and Serio, 2010).
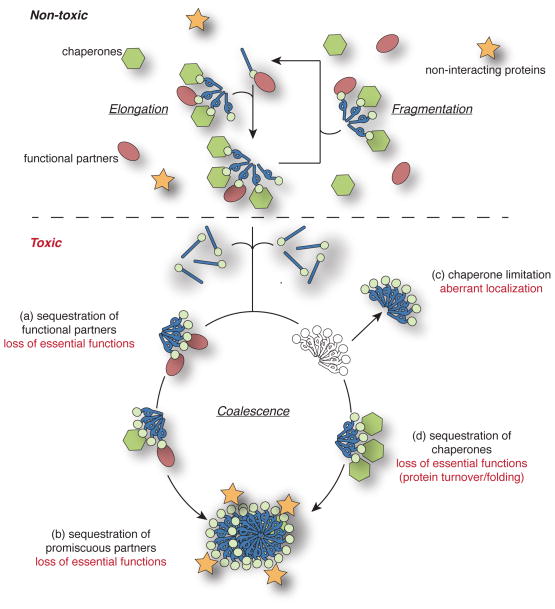
The accumulation of amyloid can be toxic to eukaryotes from yeast to man, but naturally occurring amyloid can also be tolerated benignly and can even contribute functionality, including the regulation of sterol biosynthesis (Suzuki et al., 2012), hormone storage (Maji et al., 2009), organelle biogenesis (Fowler et al., 2006, Berson et al., 2003), memory (Si et al., 2010), nutrient sensing (Brown and Lindquist, 2009), transcription(Wickner, 1994, Du et al., 2008, Patel et al., 2009, Rogoza et al., 2010), and translation (Patino et al., 1996, Paushkin et al., 1996). These observations suggest that the amyloid structure and its assembly intermediates per se are not toxic, but rather that attributes of the constituent proteins themselves and their interactions with their cellular environments specifically mediate toxicity. Studies in many model systems, but particularly in the yeast Saccharomyces cerevisiae, have begun to systematically dissect the impact of both protein-specific and cell-based factors likely to mediate toxicity and of the interactions among these contributors. Here, we synthesize recent work in this area for two classes of proteins: prions, which are transmissible between individuals through either a heritable or infectious route, and polyglutamine (polyQ)-expanded proteins that are non-transmissible but likely to spread among cells within an organism (Aguzzi, 2009, Brundin et al., 2010, Li et al., 2008, Meyer-Luehmann et al., 2006, Ren et al., 2009, Tuite and Serio, 2010). Together, these studies suggest that conditions that create imbalances in aggregation and clearance pathways lead to toxicity by altering aggregate dynamics, localization and resulting interactions (Figure 1).
Figure 1.
Models for Prion and PolyQ Toxicity at High Doses. Natively folded prion/polyQ protein (blue stick and ball) converts to the amyloid form (corkscrew and ball) by associating with and elongating existing aggregates. These complexes are fragmented by chaperones (hexagons) but cannot be cleared under normal conditions. Both natively folded and amyloid-form protein engage in specific interactions with functional partners (ovals). At higher doses (below dotted line), the number of aggregates increases, and these complexes can coalesce into larger aggresomes depending on the properties of the aggregating protein and the availability of cellular factors. Under these conditions, (a) functional partners may be sequestered by mass action and/or chaperone limitations; (b) promiscuous interactions with normally non-binding partners (stars) may arise; (c) aggregates may mislocalize due to chaperone limitations; and/or (d) other cellular functions dependent on chaperone activity such as degradation and folding may become compromised due to their sequestration by aggregates. A color version of this figure is available online.
Sequestration as a Mechanism of Prion Toxicity in Yeast
The yeast Saccharomyces cerevisiae is known to propagate ten endogenous prions,(Aigle and Lacroute, 1975, Alberti et al., 2009, Brown and Lindquist, 2009, Cox, 1965, Derkatch et al., 1997, Du et al., 2008, Patel et al., 2009, Rogoza et al., 2010, Wickner, 1994, Halfmann et al., 2012) with another nearly 20 candidate prions awaiting further characterization (Alberti et al., 2009). Of the confirmed prions, [PSI+] (Cox, 1965), the prion form of the Sup35 protein (Chernoff et al., 1995, Patino et al., 1996, Paushkin et al., 1996, Wickner, 1994), [PIN+]/[RNQ+] (Derkatch et al., 1997), the prion form of the Rnq1 protein (Derkatch et al., 2001, Osherovich and Weissman, 2001, Sondheimer and Lindquist, 2000), and [URE3] (Aigle and Lacroute, 1975), the prion form of the Ure2 protein (Masison and Wickner, 1995, Wickner, 1994), are the most extensively studied. While some self-replicating conformations (variants) of these proteins are toxic, other variants of [PSI+], [URE3], and [RNQ+] are not detrimental to yeast under normal laboratory growth conditions (McGlinchey et al., 2011, Halfmann et al., 2010). Nevertheless, these benign isolates can become toxic in the case of [PSI+] and [RNQ+] with dose-dependent increases in the levels of the Sup35 or Rnq1 proteins, respectively (Douglas et al., 2008, Chernoff et al., 1993, Chernoff et al., 1992, Dagkesamanskaya and Ter-Avanesyan, 1991, Vishveshwara et al., 2009, Zhou et al., 1999). Importantly, toxicity requires the presence of the [PSI+] or [RNQ+] prions, suggesting a transition in the interaction of the underlying Sup35 or Rnq1 amyloid structures, or of their assembly intermediates, with the cellular environment at higher doses (Douglas et al., 2008, Dagkesamanskaya and Ter-Avanesyan, 1991, Vishveshwara et al., 2009, Zhou et al., 1999). Despite their unrelated sequences and targets, parallel mechanisms underlie the toxicity of each protein.
In its non-prion form, Sup35 is a GTPase that functions as the eukaryotic release factor 3 (eRF3) to stimulate both peptidyl-tRNA hydrolysis by, and recycling of, the eukaryotic release factor 1 (eRF1, Sup45) (Stansfield et al., 1995, Zhouravleva et al., 1995, Eyler et al., 2013, Alkalaeva et al., 2006). In its [PSI+] prion form, up to ~90% of Sup35 assembles into SDS-resistant aggregates of heterogeneous size, and this shift in oligomerization is associated with a translation termination defect (Pezza et al., 2009, Kryndushkin et al., 2003, Patino et al., 1996, Paushkin et al., 1996, Tanaka et al., 2006, Derkatch et al., 1996, Cox, 1965, Liebman and Sherman, 1979). The translation termination functions of Sup35, including its interaction with Sup45, are primarily mediated by the C-terminal domain of the protein (amino acids 254–685), while the N-terminus of the protein (amino acids 1–253) supports prion propagation (Ter-Avanesyan et al., 1994, Ito et al., 1998, Paushkin et al., 1997).
The N-terminal prion-determining domain (PrD) is also required for overexpression-mediated toxicity in a [PSI+] strain, suggesting that assembly of the protein into amyloid is required for this effect (Derkatch et al., 1996, Ter-Avanesyan et al., 1993, Vishveshwara et al., 2009). Consistent with this idea, overexpression of the functional domain of Sup35, which cannot be incorporated into aggregates in a [PSI+] strain (Ter-Avanesyan et al., 1994), is sufficient to suppress the toxicity induced by overexpression of the PrD, although it is ineffective in suppressing the toxicity associated with overexpression of full-length Sup35 (Vishveshwara et al., 2009). In this latter case, overexpression of Sup45 is required to suppress toxicity (Vishveshwara et al., 2009, Derkatch et al., 1998, Stansfield et al., 1995, Tank and True, 2009, Gong et al., 2012). Together, these observations suggest that upon overexpression, Sup35 or its PrD sequesters the residual functional pool of Sup35 in a [PSI+] strain. Because Sup45 retains the ability to interact with aggregated Sup35 in a [PSI+] strain (Czaplinski et al., 1998, Paushkin et al., 1997, Gong et al., 2012), overexpression of full-length Sup35 also leads to sequestration of Sup45 (Vishveshwara et al., 2009). In either case, toxicity likely results from a reduction in the availability of these factors to perform their normal functions (Chernoff et al., 1992, Valouev et al., 2002).
In [RNQ+] cells overexpressing Rnq1, toxicity has been linked to a cell cycle arrest in mitosis at the Mad2 spindle checkpoint, resulting from a failure to duplicate the spindle pole body (Treusch and Lindquist, 2012). These effects were linked to Spc42, a core component of the spindle pole body (Bullitt et al., 1997, Treusch and Lindquist, 2012). Spc42 co-localizes with Rnq1 to cytoplasmic foci distinct from the spindle pole body in a [RNQ+] but not in a non-prion [rnq−] strain, and overexpression of Spc42 relieves Rnq1-mediated [RNQ+] toxicity, implicating sequestration once again as the mechanism of toxicity (Treusch and Lindquist, 2012). Unlike Sup35-mediated [PSI+] toxicity however, overexpression of the Rnq1 PrD in a [RNQ+] strain is not toxic (Douglas et al., 2008, Summers et al., 2009b). While these observations suggest a role for the non-prion domain in toxicity, overexpression of this region of the protein does not suppress the toxicity of full-length Rnq1 in a [RNQ+] strain, uncovering an essential interplay between the two regions of the protein (Douglas et al., 2008). Intriguingly, an L94A mutation in the non-prion domain of Rnq1, which causes the protein to assemble into toxic but SDS soluble aggregates in a [rnq−] strain (Douglas et al., 2008), also induces mislocalization of Spc42 (Treusch and Lindquist, 2012). Thus, toxicity in a [RNQ+] strain may require the PrD to drive aggregation and the non-prion domain to mediate Spc42 interaction, both of which are essential for sequestration.
Together, these studies indicate that overexpression of Sup35 or Rnq1 in strains propagating their prion forms alters their interactions with their cellular environments. In the case of Sup35, its normal interaction with Sup45 is enhanced, presumably by mass action, to sequester this cellular factor in a non-functional form (Figure 1a) (Stansfield et al., 1995, Zhouravleva et al., 1995). In the case of Rnq1, the protein is not essential; its deletion has no known effects on yeast growth (Sondheimer and Lindquist, 2000, Strawn and True, 2006), and [RNQ+] strains grow normally when Rnq1 is expressed from its endogenous promoter (Derkatch et al., 1997). Thus, the Rnq1/Spc42 interaction is likely a gain-of-function event that may be explained by the propensity of proteins with high intrinsic disorder to engage in promiscuous interactions at elevated doses (Figure 1b) (Alberti et al., 2009, Cascarina and Ross, 2014, Vavouri et al., 2009). In either case, the imbalance brought about by overexpression converts benign protein aggregates into toxic species.
PolyQ Toxicity in Yeast
Given its experimental manipulability and the presence of endogenous amyloidogenic proteins, Saccharomyces cerevisiae has emerged as a powerful model for studying protein misfolding-related disease mechanisms. Particular effort has been focused on proteins containing polyQ repeats, including variants of the huntingtin (Htt) protein that are associated with Huntington’s Disease (Group, 1993). In the case of Htt, a truncated protein encoded by exon I aggregates in yeast through a process that positively correlates with both the number of glutamines and the expression level of the protein (Krobitsch and Lindquist, 2000, Cao et al., 2001, Dehay and Bertolotti, 2006, Duennwald et al., 2006b). Intriguingly, overexpression of polyQ-expanded Htt in non-prion yeast strains leads to the accumulation of SDS-resistant aggregates of Sup35, Rnq1, and Pub1, another glutamine-rich protein, and polyQ-expanded Htt toxicity can be suppressed by deletions in polyglutamine and asparagine (polyQN)-rich proteins (Giorgini et al., 2005) or by expression antioxidant GPx enzymes which reduce ROS and presumably oxidatively damaged proteins (Mason et al., 2013). Together these observations suggest cellular limitations on the ability to control aggregation of these metastable proteins (Kochneva-Pervukhova et al., 2012, Urakov et al., 2010). A particularly intriguing example of this effect is the toxicity of a synthetic poly-Q protein fused to GFP (pQ56-GFP), which leads to cell-cycle arrest due to compromised assembly of the septin complex at the yeast bud neck (Kaiser et al., 2013). This defect can be suppressed by conditions that limit the number of pQ56 aggregates by promoting the formation of larger complexes, such as deletion or inhibition of Hsp104 or Pho5, or by higher ploidy (Kaiser et al., 2013), which has been similarly shown to alter the accumulation of Sup35 aggregates in a [PSI+] by changing the prion:chaperone ratio (DiSalvo et al., 2011). Although not discussed in this study, two septin family members have been shown to form amyloid fibers in vitro (Garcia et al., 2007, Pissuti Damalio et al., 2012) and to associate with neurofibrillar tanlges in Alzheimer’s Disease (Kinoshita et al., 1998) and cytoplasmic inclusions in Parkinson’s Disease (Ihara et al., 2003), raising the possibility that the limitations on protein quality control pathways in the presence of poly-Q expanded protein aggregates can cause septin aggregation and thereby toxicity.
In addition to cell-based limitations imposed by protein aggregation, more direct pathways to promote toxicity exist. For example, polyQ-expanded Htt aggregation and its associated toxicity are strongly enhanced by overexpression of polyQN-rich proteins or by the presence of the endogenous yeast prions [RNQ+] and [PSI+] (Meriin et al., 2002, Duennwald et al., 2006a, Kochneva-Pervukhova et al., 2012, Gong et al., 2012, Zhao et al., 2012, Gokhale et al., 2005, Giorgini et al., 2005), and this enhancement corresponds to co-localization of the aggregating proteins (Meriin et al., 2003, Duennwald et al., 2006a, Gong et al., 2012). Given the ability of polyQ-expanded Htt to induce aggregation of Sup35 (Kochneva-Pervukhova et al., 2012, Urakov et al., 2010) and the essential function of Sup35 in translation termination (Ter-Avanesyan et al., 1993), several groups explored the possibility of Sup35 sequestration as a mechanism for Htt toxicity in yeast (Gong et al., 2012, Kochneva-Pervukhova et al., 2012, Zhao et al., 2012). In the presence of [RNQ+] alone, polyQ-expanded Htt clearly induced aggregation of Sup35 (Gong et al., 2012, Kochneva-Pervukhova et al., 2012), but expression of the Sup35 functional domain was efficient in suppressing toxicity in one study (Kochneva-Pervukhova et al., 2012) but not in another (Gong et al., 2012). However, in the presence of both [RNQ+] and [PSI+], the toxicity of polyQ-expanded Htt is efficiently suppressed by expression of the functional domain of Sup35 (Gong et al., 2012, Zhao et al., 2012). Thus, while either type of aggregate alone is benign, the combination of polyQ and prion aggregates creates an imbalance presumably between the aggregation assembly pathway and cellular protein quality control pathways that promotes sequestration of Sup35 and thereby toxicity (Figure 1b).
In its native environment, Htt will not encounter a Sup35 homolog with a QN-rich domain (Jean-Jean et al., 1996). Nonetheless, similar types of interactions have been observed in patient-derived tissues and in cell and animal models of polyQ-expansion diseases. In the case of Htt, polyQ-expanded versions of the protein are known to induce co-aggregation of other proteins containing smaller glutamine-rich stretches of amino acids, such as the CREB binding protein (CBP) and the TATA binding protein (TBP), which do not aggregate on their own (McCampbell et al., 2000, Perez et al., 1998, Chai et al., 2002, Kim et al., 2002, Steffan et al., 2000). Notably, these gain-of-function interactions clearly impact the biological outcome of Htt aggregation: Htt toxicity in tissue culture and in mice can be suppressed by overexpression of CBP (Jiang et al., 2006, Nucifora et al., 2001), and the toxic interaction between Htt and human TBP, when reconstituted in yeast, can be suppressed by expression of the non-glutamine-rich yeast TBP (Schaffar et al., 2004). This sequestration model is likely to be more broadly generalizable, as synthetic amyloid-like proteins have interactome sizes that correlate directly with their toxicity in cell culture (Olzscha et al., 2011), and changes in the dosage of nearly 50% of the Htt interactome genetically modifies Htt toxicity in vivo (Kaltenbach et al., 2007). Thus, while there are certainly other potential mechanisms of polyQ-mediated toxicity including proteasome impairment (Bence et al., 2001) and membrane disruption (Arispe et al., 1993, Volles et al., 2001, Kremer et al., 2001), the sequestration and resulting functional titration of essential proteins by amyloid aggregates is a recurring theme in toxicity.
Aggregate Dynamics
Protein misfolding diseases most frequently correlate with the accumulation of aggregates, but studies in many systems suggest that pathogenesis is more accurately a function of the particular type(s) of aggregates present rather than the fraction of protein aggregated (Caughey and Lansbury, 2003, Haass and Selkoe, 2007). The emerging consensus suggests that soluble oligomers rather than high molecular weight complexes are the disease-causing culprits (Cohen et al., 2006, Saudou et al., 1998, Arrasate et al., 2004, Chesebro et al., 2005, Piccardo et al., 2007). However, studies in yeast suggest additional complexity in the link between aggregation and toxicity, particularly in the context of a sequestration model.
In yeast, the toxicity of an N-terminally flag-tagged Htt exon I fragment containing 103 glutamines (FHttQ103) is only observed in a [RNQ+] strain in the absence of an immediately adjacent proline-rich region (Duennwald et al., 2006b, Krobitsch and Lindquist, 2000, Meriin et al., 2002). The toxicity of FHttQ103, however, can be suppressed by co-expression of a Htt exon I fragment containing 25 glutamines (HttQ25), but only if the latter contains the adjacent proline-rich region (HttQ25P) (Duennwald et al., 2006a, Wang et al., 2009). Because HttQ25P cannot aggregate on its own but does co-aggregate with FHttQ103, the proline-rich region likely acts in trans, when incorporated into FHttQ103 aggregates, to alleviate polyQ toxicity in yeast (Duennwald et al., 2006a, Wang et al., 2009).
Intriguingly, while an FHttQ103 variant containing the proline-rich region (FHttQ103P) is not toxic in a [RNQ+] strain (Duennwald et al., 2006a, Wang et al., 2009), this protein is toxic in a [RNQ+] [PSI+] strain, where the pool of functional Sup35 is already depleted (Gong et al., 2012, Zhao et al., 2012). Thus, although FHttQ103 can also induce toxicity through the sequestration of actin assembly proteins (Meriin et al., 2003), the synthetic interaction with [PSI+] suggests that FHttQ103 toxicity, like that of FHttQ103P, can arise through the sequestration of Sup35 but that the latter is less efficient in inactivating Sup35. Indeed, FHttQ103P and Sup35 co-localize in vivo, and FHttQ103P toxicity, like that of FHttQ103, is suppressed by expression of the functional domain of Sup35 (Gong et al., 2012, Zhao et al., 2012). But, the proline-rich region is unlikely to reduce toxicity simply by directly decreasing the affinity of Htt for Sup35 through this binary interaction, as HttQ25P acts dominantly (Duennwald et al., 2006a, Wang et al., 2009). Rather, the available observations suggest that the proline-rich region, either in cis or in trans, mediates its effects by altering the dynamics of Htt aggregates in vivo. Specifically, both FHttQ103 and FHttQ103P form aggregates in a [RNQ+] strain, but the FHttQ103P aggregates are less SDS-resistant, larger in size and fewer in number (Dehay and Bertolotti, 2006, Wang et al., 2009, Duennwald et al., 2006a), attributes which may together restrict the binding promiscuity of aggregates by limiting their available interaction surfaces (Figure 1b). Importantly, the single foci formed by FHttQ103P localize to the spindle pole body and depend on microtubule activity, which suggest that smaller foci might form initially and then coalesce into a larger complex, known as an aggresome (Wang et al., 2009), as is the case in mammalian cells (Figure 1) (Wang et al., 2009, Johnston et al., 1998).
These relationships between aggregate dynamics and their biological outcomes are also observed for the yeast prions, but with the opposite correlation. Under moderate expression levels where the prion state is not toxic, both Sup35 and Rnq1 localize to multiple, highly mobile foci in the cytoplasm of [PSI+] and [RNQ+] strains, respectively (Satpute-Krishnan and Serio, 2005, Sondheimer and Lindquist, 2000). Upon their overexpression to toxic levels, Sup35 and Rnq1 accumulate in single immobile focus that co-localizes with Sup45 or Spc42, respectively, in the cytoplasm (Vishveshwara et al., 2009, Douglas et al., 2008, Kaganovich et al., 2008, Treusch and Lindquist, 2012). Thus, while the ability to sequester essential cellular proteins is impacted by the assembly state of the aggregation-prone proteins, there appears to be no single toxic species based on size. Rather, other properties of each aggregation-prone protein must necessarily determine which species participates in the toxic interactions.
Chaperone Limitations
While the overexpression of prions and polyQ-expanded proteins promotes their assembly into aggregates that are associated with toxicity, cellular quality control pathways exist to counterbalance this propensity (Hartl et al., 2011). Under conditions of normal expression, the accumulation and size of protein aggregates is a function of their assembly, disassembly, and dilution, through either degradation or transmission (Figure 1) (Sindi and Serio, 2009). Upon overexpression, the size of these complexes is likely to increase because assembly is enhanced, and the pathways that counteract this process become inefficient due to the stability of the aggregates and the limited capacity of cellular quality control pathways to clear them (Derdowski et al., 2010, DiSalvo et al., 2011, Sindi and Serio, 2009, Voisine et al., 2010).
Numerous studies have demonstrated that elevating chaperone levels can reduce the accumulation of aggregates of amyloidogenic proteins and reverse toxicity (Broadley and Hartl, 2009, Muchowski and Wacker, 2005), but chaperone proteins are also absolutely required for the accumulation and subcellular localization of these complexes and therefore impact toxicity through other routes. In the case of the yeast prions, a core group of molecular chaperones has been implicated in the disassembly pathway for these aggregates. The AAA+ ATPase Hsp104, which functions as a molecular disaggregase, and its co-chaperones Hsp70 (Ssa1/2) and Hsp40 (Sis1) collaborate to fragment prion aggregates into smaller complexes by extracting monomers (Chernoff et al., 1995, Higurashi et al., 2008, Lum et al., 2004, Ness et al., 2002, Satpute-Krishnan et al., 2007, Tessarz et al., 2008, Tipton et al., 2008, Park et al., 2012). Hsp104 is also required for the accumulation of aggregates containing polyQ-expanded proteins in yeast (Cao et al., 2001, Dehay and Bertolotti, 2006, Kimura et al., 2004, Krobitsch and Lindquist, 2000, Meriin et al., 2002). But, while Hsp104 has no known homolog in metazoans, members of both the Hsp70 and Hsp40 chaperone families have been implicated in the toxicity of polyQ expansions in yeast and in other model systems (Muchowski and Wacker, 2005, Kobayashi and Sobue, 2001), suggesting that these chaperone families retain the ability to recognize amyloidogenic proteins across evolutionary time.
Beyond promoting amyloid accumulation, chaperones impact amyloid interactions and localization. Despite their classification as “misfolded”, amyloid aggregates are actually highly ordered cross β structures (Eisenberg and Jucker, 2012). Nevertheless, in vitro amyloid fibers bind stably to dyes such as ANS, which also recognize molten globules, suggesting the exposure of hydrophobic regions in these structures that may be targeted by chaperones (Stryer, 1965, Schaffar et al., 2004, Serio et al., 2000). Consistent with this idea, the prion forms of Sup35 and Rnq1 form stable, stochiometic complexes with the Hsp70 Ssa1/2 (2:1 ratio) and with the Hsp40 Sis1 (1:1 ratio), respectively, but because these chaperones are more abundant than the prion proteins, their interactions are not detrimental under normal expression conditions (Bagriantsev et al., 2008, Lopez et al., 2003, Sondheimer and Lindquist, 2000). However, at high doses, this balance is perturbed, leading to toxicity through multiple routes.
The toxicity associated with Rnq1 overexpression in a [RNQ+] strain can be suppressed by overexpression of Sis1, with which it forms a stable complex under normal expression conditions (Douglas et al., 2008, Lopez et al., 2003, Sondheimer and Lindquist, 2000). This observation suggests that Sis1 is a limiting factor in suppressing the toxicity of Rnq1 at high doses, and consistent with this idea, depletion of Sis1 promotes the toxicity of Rnq1 at lower doses in a [RNQ+] strain (Douglas et al., 2008, Lopez et al., 2003, Sondheimer and Lindquist, 2000). Intriguingly, an L94A mutation in Rnq1 reduces Sis1 binding, increases the toxicity of excess Rnq1 in a [RNQ+] strain, and promotes Rnq1 toxicity in a non-prion [rnq−] strain, where it now associates with Spc42 (Douglas et al., 2008, Treusch and Lindquist, 2012). Thus, Sis1 binding to Rnq1 may limit its binding promiscuity by shielding a hydrophobic interaction surface, as has been suggested for the suppression of HttQ72 toxicity by small heat shock protein proteins (sHsp) in yeast (Figure 1b) (Cashikar et al., 2005). But, the suppression of toxicity by overexpression of Sis1 can also be explained by the relocalization of Rnq1 to either the nucleus or to a cytoplasmic quality control body, where it is more efficiently assembled into SDS-resistant aggregates and is spatially separated from Spc42 (Figure 1c) (Douglas et al., 2009, Wolfe et al., 2013). In either case, limitations on the availability of Sis1 impact aggregate interactions with their cellular environment.
Chaperones have also been implicated in the toxic interactions between prion and polyQ-expanded Htt. Overexpression of either Ydj1 or Sis1, both members of the Hsp40 family, has no effect on the toxicity of FHttQ103 in a [PSI+] strain. However in a [RNQ+] strain, FHttQ103 toxicity is enhanced by Ydj1 overexpression and reduced by Sis1 overexpression (Gokhale et al., 2005), and these effects correlate directly with changes in the accumulation of large FHttQ103 aggregates (Gokhale et al., 2005). The Hsp40-mediated changes in aggregate dynamics and toxicity is likely mediated by a competition between Rnq1 and FHttQ103 for these factors, as Rnq1 but not Sup35 aggregates have been shown to stably bind to Ydj1 and Sis1 at significant levels (Bagriantsev et al., 2008, Sondheimer et al., 2001, Summers et al., 2009a). Consistent with this idea, the suppression of FHttQ103 toxicity in a [RNQ+] strain by overexpression of Sis1 is dependent on Sis1 co-localization to Rnq1 aggregates (Wolfe et al., 2013), and overexpression of Ydj1 converts SDS-resistant Myc-HttQ53 aggregates to detergent-sensitive aggregates, likely through a direct interaction (Muchowski et al., 2000). Although the precise molecular mechanisms underlying these effects are still unknown, overexpression of Hsp40s ultimately alters aggregate dynamics, which presumably modulates their specific interactions with other cellular components and leads to toxicity.
Sis1 has also been implicated in suppressing the toxicity of polyQ-expanded Htt in the presence of non-prion misfolded proteins. In this case, expression of a myc-tagged Htt exon I fragment containing 96 glutamines and the adjacent proline-rich region (MHttQ96P) becomes toxic upon co-expression of a model misfolded protein (CG*) (Park et al., 2013). As is the case for Rnq1, MHttQ96P binds stably to Sis1, and overexpression of Sis1 is sufficient to reduce its toxicity in the presence of CG*, again suggesting a negative correlation between Sis1 availability and toxicity (Park et al., 2013). Rather than promoting promiscuous polyQ binding however, this Sis1 limitation is associated with an impairment of the 26S-dependent degradation of CG*, which requires its transport into the nucleus (Park et al., 2013). Sis1 shuttles between the nucleus and cytoplasm in response to stress and may mediate transport directly or perhaps indirectly through a modulation of the dynamics of cytoplasmic aggregates containing misfolded proteins (Figure 1c, d) (Park et al., 2013, Malinovska et al., 2012, Summers et al., 2013, Shiber et al., 2013). In either case, the sequestration of Sis1 through its stable interaction with Htt disrupts proteostasis.
The overexpression of polyQ-expanded Htt has also been implicated in the sequestration of other protein quality control factors including Cdc48 and its co-factors Npl4 and Ufd1. This sequestration is associated with defects in ER-associated degradation in yeast and in mammalian cells (Duennwald and Lindquist, 2008). But, these same factors have also been implicated in the assembly of HttQ103P into a single aggresome-like complex, which is associated with reduced toxicity in yeast (Figure 1c) (Wang et al., 2009). As was the case with Sis1, the balance between polyQ-expanded Htt and the Cdc48 system impacts both interactions and localization to mediate toxicity.
More generally, expression of polyQ-expanded proteins promotes the misfolding of non-amyloidogenic proteins with destabilizing mutations in C. elegans (Gidalevitz et al., 2006). These proteins are sufficiently buffered by protein quality control pathways in the absence of the polyQ protein to properly fold and function, suggesting a competition for an overlapping subset of factors (Gidalevitz et al., 2006). Consistent with this idea, the aggregation of a polyQ-expanded protein of intermediate length (40Q) was enhanced in C. elegans lines encoding metastable protein mutants. A component of this effect could result from the titration of co-translational quality control factors, such as the nascent polypeptide-associated complex (NAC), which relocalize from polysomes to polyQ aggregates and induce translational impairment presumably due to widespread protein misfolding (Figure 1d) (Kirstein-Miles et al., 2013).
Together, these studies suggest that while the chaperone-based aspects of protein quality control are insufficient to clear amyloid aggregates once they are established, these highly ordered structures are recognized as aberrant by the same pathways. The binding of chaperones to these complexes modulates their toxicity by shielding hydrophobic surfaces and impacting localization, which together alter their interactions (Figure 1b, c). When the balance between the levels of the aggregation-prone proteins and chaperones is altered by overexpression of the former, chaperones become limiting, exposing the amyloid to promiscuous interactions and jeopardizing other cellular processes that are dependent on chaperone interaction (Figure 1).
Conclusion
Studies in model organisms based on overexpression of prion and polyQ-expanded amyloid proteins have uncovered emerging complexity in the links between protein aggregation and toxicity. While a clear and unquestionable connection between protein misfolding and pathogenesis exists, specific attributes of the proteins and their expression levels promote toxicity through a variety of distinct events that have the common foundation of altered interactions. Despite artificial aspects of these systems, the lessons learned through them are likely to be relevant to pathogenesis under conditions of normal expression. Aggregates accumulate spontaneously in the absence of overexpression, suggesting inherent limitations on protein quality control pathways (Voisine et al., 2010). Moreover, the pathways maintaining proteostasis under normal conditions become impaired with age, creating new thresholds for the acquisition and biological impact of protein aggregates (Broadley and Hartl, 2009, David et al., 2010). Nonetheless, the existence of benign and functional amyloids and the fact that some of these species can be converted to toxic forms under conditions of overexpression suggests that the pathogenic progression of related proteins in mammals may be reversible through manipulations that seek to restore balance, even if these efforts fall short of aggregate clearance (Lindquist and Kelly, 2011).
Acknowledgments
We thank Jeff Laney and members of the Serio and Laney laboratories for helpful discussions.
Footnotes
Declaration of Interest
This work was supported by an award from the National Institutes of Health to TRS (GM069802).
Literature Cited
- AGUZZI A. Cell biology: Beyond the prion principle. Nature. 2009;459:924–5. doi: 10.1038/459924a. [DOI] [PubMed] [Google Scholar]
- AIGLE M, LACROUTE F. Genetical Aspects of [URE3], a Non-Mitochondrial, cytoplasmically Inherited Mutation in Yeast. Molecular and General Genetics. 1975;136:327–335. doi: 10.1007/BF00341717. [DOI] [PubMed] [Google Scholar]
- ALBERTI S, HALFMANN R, KING O, KAPILA A, LINDQUIST S. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell. 2009;137:146–58. doi: 10.1016/j.cell.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALKALAEVA EZ, PISAREV AV, FROLOVA LY, KISSELEV LL, PESTOVA TV. In vitro reconstitution of eukaryotic translation reveals cooperativity between release factors eRF1 and eRF3. Cell. 2006;125:1125–36. doi: 10.1016/j.cell.2006.04.035. [DOI] [PubMed] [Google Scholar]
- ANFINSEN CB. The formation of the tertiary structure of proteins. Harvey Lect. 1967;61:95–116. [PubMed] [Google Scholar]
- ARISPE N, ROJAS E, POLLARD HB. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc Natl Acad Sci U S A. 1993;90:567–71. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARRASATE M, MITRA S, SCHWEITZER ES, SEGAL MR, FINKBEINER S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–10. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- BAGRIANTSEV SN, GRACHEVA EO, RICHMOND JE, LIEBMAN SW. Variant-specific [PSI+] Infection is Transmitted by Sup35 Polymers within [PSI+] Aggregates with Heterogeneous Protein Composition. Mol Biol Cell. 2008 doi: 10.1091/mbc.E08-01-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALCH WE, MORIMOTO RI, DILLIN A, KELLY JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–9. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- BENCE NF, SAMPAT RM, KOPITO RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292:1552–5. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- BERSON JF, THEOS AC, HARPER DC, TENZA D, RAPOSO G, MARKS MS. Proprotein convertase cleavage liberates a fibrillogenic fragment of a resident glycoprotein to initiate melanosome biogenesis. J Cell Biol. 2003;161:521–33. doi: 10.1083/jcb.200302072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROADLEY SA, HARTL FU. The role of molecular chaperones in human misfolding diseases. FEBS Lett. 2009;583:2647–53. doi: 10.1016/j.febslet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- BROWN JC, LINDQUIST S. A heritable switch in carbon source utilization driven by an unusual yeast prion. Genes Dev. 2009;23:2320–32. doi: 10.1101/gad.1839109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUNDIN P, MELKI R, KOPITO R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol. 2010;11:301–7. doi: 10.1038/nrm2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULLITT E, ROUT MP, KILMARTIN JV, AKEY CW. The yeast spindle pole body is assembled around a central crystal of Spc42p. Cell. 1997;89:1077–86. doi: 10.1016/s0092-8674(00)80295-0. [DOI] [PubMed] [Google Scholar]
- CAO F, LEVINE JJ, LI SH, LI XJ. Nuclear aggregation of huntingtin is not prevented by deletion of chaperone Hsp104. Biochim Biophys Acta. 2001;1537:158–66. doi: 10.1016/s0925-4439(01)00068-0. [DOI] [PubMed] [Google Scholar]
- CASCARINA SM, ROSS ED. Yeast prions and human prion-like proteins: sequence features and prediction methods. Cell Mol Life Sci. 2014 doi: 10.1007/s00018-013-1543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASHIKAR AG, DUENNWALD M, LINDQUIST SL. A chaperone pathway in protein disaggregation. Hsp26 alters the nature of protein aggregates to facilitate reactivation by Hsp104. J Biol Chem. 2005;280:23869–75. doi: 10.1074/jbc.M502854200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAUGHEY B, LANSBURY PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–98. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- CHAI Y, SHAO J, MILLER VM, WILLIAMS A, PAULSON HL. Live-cell imaging reveals divergent intracellular dynamics of polyglutamine disease proteins and supports a sequestration model of pathogenesis. Proc Natl Acad Sci U S A. 2002;99:9310–5. doi: 10.1073/pnas.152101299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHERNOFF YO, DERKACH IL, INGE-VECHTOMOV SG. Multicopy SUP35 gene induces de-novo appearance of psi-like factors in the yeast Saccharomyces cerevisiae. Current Genetics. 1993;24:268–70. doi: 10.1007/BF00351802. [DOI] [PubMed] [Google Scholar]
- CHERNOFF YO, INGE-VECHTOMOV SG, DERKACH IL, PTYUSHKINA MV, TARUNINA OV, DAGKESAMANSKAYA AR, TER-AVANESYAN MD. Dosage-dependent translational suppression in yeast Saccharomyces cerevisiae. Yeast. 1992;8:489–99. doi: 10.1002/yea.320080702. [DOI] [PubMed] [Google Scholar]
- CHERNOFF YO, LINDQUIST SL, ONO B, INGE-VECHTOMOV SG, LIEBMAN SW. Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [PSI+] Science. 1995;268:880–4. doi: 10.1126/science.7754373. [DOI] [PubMed] [Google Scholar]
- CHESEBRO B, TRIFILO M, RACE R, MEADE-WHITE K, TENG C, LACASSE R, RAYMOND L, FAVARA C, BARON G, PRIOLA S, CAUGHEY B, MASLIAH E, OLDSTONE M. Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science. 2005;308:1435–9. doi: 10.1126/science.1110837. [DOI] [PubMed] [Google Scholar]
- CHITI F, DOBSON CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–66. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- COHEN E, BIESCHKE J, PERCIAVALLE RM, KELLY JW, DILLIN A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–10. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- COX B. [PSI], a cytoplasmic suppressor of super-suppression in yeast. Heredity. 1965;20:505–521. [Google Scholar]
- CZAPLINSKI K, RUIZ-ECHEVARRIA MJ, PAUSHKIN SV, HAN X, WENG Y, PERLICK HA, DIETZ HC, TER-AVANESYAN MD, PELTZ SW. The surveillance complex interacts with the translation release factors to enhance termination and degrade aberrant mRNAs. Genes & Development. 1998;12:1665–77. doi: 10.1101/gad.12.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGKESAMANSKAYA AR, TER-AVANESYAN MD. Interaction of the yeast omnipotent suppressors SUP1(SUP45) and SUP2(SUP35) with non-Mendelian factors. Genetics. 1991;128:513–20. doi: 10.1093/genetics/128.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVID DC, OLLIKAINEN N, TRINIDAD JC, CARY MP, BURLINGAME AL, KENYON C. Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol. 2010;8:e1000450. doi: 10.1371/journal.pbio.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEHAY B, BERTOLOTTI A. Critical role of the proline-rich region in Huntingtin for aggregation and cytotoxicity in yeast. J Biol Chem. 2006;281:35608–15. doi: 10.1074/jbc.M605558200. [DOI] [PubMed] [Google Scholar]
- DERDOWSKI A, SINDI SS, KLAIPS CL, DISALVO S, SERIO TR. A size threshold limits prion transmission and establishes phenotypic diversity. Science. 2010;330:680–3. doi: 10.1126/science.1197785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DERKATCH IL, BRADLEY ME, HONG JY, LIEBMAN SW. Prions affect the appearance of other prions: the story of [PIN+] Cell. 2001;106:171–82. doi: 10.1016/s0092-8674(01)00427-5. [DOI] [PubMed] [Google Scholar]
- DERKATCH IL, BRADLEY ME, LIEBMAN SW. Overexpression of the SUP45 gene encoding a Sup35p-binding protein inhibits the induction of the de novo appearance of the [PSI+] prion. Proc Natl Acad Sci U S A. 1998;95:2400–5. doi: 10.1073/pnas.95.5.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DERKATCH IL, BRADLEY ME, ZHOU P, CHERNOFF YO, LIEBMAN SW. Genetic and environmental factors affecting the de novo appearance of the [PSI+] prion in Saccharomyces cerevisiae. Genetics. 1997;147:507–19. doi: 10.1093/genetics/147.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DERKATCH IL, CHERNOFF YO, KUSHNIROV VV, INGE-VECHTOMOV SG, LIEBMAN SW. Genesis and variability of [PSI] prion factors in Saccharomyces cerevisiae. Genetics. 1996;144:1375–86. doi: 10.1093/genetics/144.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISALVO S, DERDOWSKI A, PEZZA JA, SERIO TR. Dominant prion mutants induce curing through pathways that promote chaperone-mediated disaggregation. Nat Struct Mol Biol. 2011;18:486–92. doi: 10.1038/nsmb.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS PM, SUMMERS DW, REN HY, CYR DM. Reciprocal efficiency of RNQ1 and polyglutamine detoxification in the cytosol and nucleus. Mol Biol Cell. 2009;20:4162–73. doi: 10.1091/mbc.E09-02-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS PM, TREUSCH S, REN HY, HALFMANN R, DUENNWALD ML, LINDQUIST S, CYR DM. Chaperone-dependent amyloid assembly protects cells from prion toxicity. Proc Natl Acad Sci U S A. 2008;105:7206–11. doi: 10.1073/pnas.0802593105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DU Z, PARK KW, YU H, FAN Q, LI L. Newly identified prion linked to the chromatin-remodeling factor Swi1 in Saccharomyces cerevisiae. Nat Genet. 2008;40:460–5. doi: 10.1038/ng.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUENNWALD ML, JAGADISH S, GIORGINI F, MUCHOWSKI PJ, LINDQUIST S. A network of protein interactions determines polyglutamine toxicity. Proc Natl Acad Sci U S A. 2006a;103:11051–6. doi: 10.1073/pnas.0604548103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUENNWALD ML, JAGADISH S, MUCHOWSKI PJ, LINDQUIST S. Flanking sequences profoundly alter polyglutamine toxicity in yeast. Proc Natl Acad Sci U S A. 2006b;103:11045–50. doi: 10.1073/pnas.0604547103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUENNWALD ML, LINDQUIST S. Impaired ERAD and ER stress are early and specific events in polyglutamine toxicity. Genes Dev. 2008;22:3308–19. doi: 10.1101/gad.1673408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENBERG D, JUCKER M. The amyloid state of proteins in human diseases. Cell. 2012;148:1188–203. doi: 10.1016/j.cell.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EYLER DE, WEHNER KA, GREEN R. Eukaryotic release factor 3 is required for multiple turnovers of peptide release catalysis by eukaryotic release factor 1. J Biol Chem. 2013;288:29530–8. doi: 10.1074/jbc.M113.487090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOWLER DM, KOULOV AV, ALORY-JOST C, MARKS MS, BALCH WE, KELLY JW. Functional amyloid formation within mammalian tissue. PLoS Biol. 2006;4:e6. doi: 10.1371/journal.pbio.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA W, DE ARAUJO AP, LARA F, FOGUEL D, TANAKA M, TANAKA T, GARRATT RC. An intermediate structure in the thermal unfolding of the GTPase domain of human septin 4 (SEPT4/Bradeion-beta) forms amyloid-like filaments in vitro. Biochemistry. 2007;46:11101–9. doi: 10.1021/bi700702w. [DOI] [PubMed] [Google Scholar]
- GIDALEVITZ T, BEN-ZVI A, HO KH, BRIGNULL HR, MORIMOTO RI. Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science. 2006;311:1471–4. doi: 10.1126/science.1124514. [DOI] [PubMed] [Google Scholar]
- GIORGINI F, GUIDETTI P, NGUYEN Q, BENNETT SC, MUCHOWSKI PJ. A genomic screen in yeast implicates kynurenine 3-monooxygenase as a therapeutic target for Huntington disease. Nat Genet. 2005;37:526–31. doi: 10.1038/ng1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOKHALE KC, NEWNAM GP, SHERMAN MY, CHERNOFF YO. Modulation of prion-dependent polyglutamine aggregation and toxicity by chaperone proteins in the yeast model. J Biol Chem. 2005;280:22809–18. doi: 10.1074/jbc.M500390200. [DOI] [PubMed] [Google Scholar]
- GONG H, ROMANOVA NV, ALLEN KD, CHANDRAMOWLISHWARAN P, GOKHALE K, NEWNAM GP, MIECZKOWSKI P, SHERMAN MY, CHERNOFF YO. Polyglutamine toxicity is controlled by prion composition and gene dosage in yeast. PLoS Genet. 2012;8:e1002634. doi: 10.1371/journal.pgen.1002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROUP THSDCR. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–83. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- HAASS C, SELKOE DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–12. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- HALFMANN R, ALBERTI S, LINDQUIST S. Prions, protein homeostasis, and phenotypic diversity. Trends Cell Biol. 2010;20:125–33. doi: 10.1016/j.tcb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALFMANN R, WRIGHT JR, ALBERTI S, LINDQUIST S, REXACH M. Prion formation by a yeast GLFG nucleoporin. Prion. 2012;6:391–9. doi: 10.4161/pri.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTL FU, BRACHER A, HAYER-HARTL M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–32. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- HIGURASHI T, HINES JK, SAHI C, ARON R, CRAIG EA. Specificity of the J-protein Sis1 in the propagation of 3 yeast prions. Proc Natl Acad Sci U S A. 2008;105:16596–601. doi: 10.1073/pnas.0808934105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IHARA M, TOMIMOTO H, KITAYAMA H, MORIOKA Y, AKIGUCHI I, SHIBASAKI H, NODA M, KINOSHITA M. Association of the cytoskeletal GTP-binding protein Sept4/H5 with cytoplasmic inclusions found in Parkinson’s disease and other synucleinopathies. J Biol Chem. 2003;278:24095–102. doi: 10.1074/jbc.M301352200. [DOI] [PubMed] [Google Scholar]
- ITO K, EBIHARA K, NAKAMURA Y. The stretch of C-terminal acidic amino acids of translational release factor eRF1 is a primary binding site for eRF3 of fission yeast. RNA. 1998;4:958–72. doi: 10.1017/s1355838298971874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAHN TR, RADFORD SE. Folding versus aggregation: polypeptide conformations on competing pathways. Arch Biochem Biophys. 2008;469:100–17. doi: 10.1016/j.abb.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEAN-JEAN O, LE GOFF X, PHILIPPE M. Is there a human [psi]? Comptes Rendus de l Academie des Sciences - Serie Iii, Sciences de la Vie. 1996;319:487–92. [PubMed] [Google Scholar]
- JIANG H, POIRIER MA, LIANG Y, PEI Z, WEISKITTEL CE, SMITH WW, DEFRANCO DB, ROSS CA. Depletion of CBP is directly linked with cellular toxicity caused by mutant huntingtin. Neurobiol Dis. 2006;23:543–51. doi: 10.1016/j.nbd.2006.04.011. [DOI] [PubMed] [Google Scholar]
- JOHNSTON JA, WARD CL, KOPITO RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–98. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAGANOVICH D, KOPITO R, FRYDMAN J. Misfolded proteins partition between two distinct quality control compartments. Nature. 2008;454:1088–95. doi: 10.1038/nature07195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAISER CJ, GROTZINGER SW, ECKL JM, PAPSDORF K, JORDAN S, RICHTER K. A network of genes connects polyglutamine toxicity to ploidy control in yeast. Nat Commun. 2013;4:1571. doi: 10.1038/ncomms2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALTENBACH LS, ROMERO E, BECKLIN RR, CHETTIER R, BELL R, PHANSALKAR A, STRAND A, TORCASSI C, SAVAGE J, HURLBURT A, CHA GH, UKANI L, CHEPANOSKE CL, ZHEN Y, SAHASRABUDHE S, OLSON J, KURSCHNER C, ELLERBY LM, PELTIER JM, BOTAS J, HUGHES RE. Huntingtin interacting proteins are genetic modifiers of neurodegeneration. PLoS Genet. 2007;3:e82. doi: 10.1371/journal.pgen.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM S, NOLLEN EA, KITAGAWA K, BINDOKAS VP, MORIMOTO RI. Polyglutamine protein aggregates are dynamic. Nat Cell Biol. 2002;4:826–31. doi: 10.1038/ncb863. [DOI] [PubMed] [Google Scholar]
- KIM YE, HIPP MS, BRACHER A, HAYER-HARTL M, HARTL FU. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem. 2013;82:323–55. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- KIMURA Y, KOITABASHI S, KAKIZUKA A, FUJITA T. The role of pre-existing aggregates in Hsp104-dependent polyglutamine aggregate formation and epigenetic change of yeast prions. Genes Cells. 2004;9:685–96. doi: 10.1111/j.1356-9597.2004.00759.x. [DOI] [PubMed] [Google Scholar]
- KINOSHITA A, KINOSHITA M, AKIYAMA H, TOMIMOTO H, AKIGUCHI I, KUMAR S, NODA M, KIMURA J. Identification of septins in neurofibrillary tangles in Alzheimer’s disease. Am J Pathol. 1998;153:1551–60. doi: 10.1016/S0002-9440(10)65743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRSTEIN-MILES J, SCIOR A, DEUERLING E, MORIMOTO RI. The nascent polypeptide-associated complex is a key regulator of proteostasis. EMBO J. 2013;32:1451–68. doi: 10.1038/emboj.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOBAYASHI Y, SOBUE G. Protective effect of chaperones on polyglutamine diseases. Brain Res Bull. 2001;56:165–8. doi: 10.1016/s0361-9230(01)00593-7. [DOI] [PubMed] [Google Scholar]
- KOCHNEVA-PERVUKHOVA NV, ALEXANDROV AI, TER-AVANESYAN MD. Amyloid-mediated sequestration of essential proteins contributes to mutant huntingtin toxicity in yeast. PLoS ONE. 2012;7:e29832. doi: 10.1371/journal.pone.0029832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREMER JJ, SKLANSKY DJ, MURPHY RM. Profile of changes in lipid bilayer structure caused by beta-amyloid peptide. Biochemistry. 2001;40:8563–71. doi: 10.1021/bi010417x. [DOI] [PubMed] [Google Scholar]
- KROBITSCH S, LINDQUIST S. Aggregation of huntingtin in yeast varies with the length of the polyglutamine expansion and the expression of chaperone proteins. Proc Nat Acad Sci USA. 2000;97:1589–94. doi: 10.1073/pnas.97.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRYNDUSHKIN DS, ALEXANDROV IM, TER-AVANESYAN MD, KUSHNIROV VV. Yeast [PSI+] prion aggregates are formed by small Sup35 polymers fragmented by Hsp104. J Biol Chem. 2003;278:49636–43. doi: 10.1074/jbc.M307996200. [DOI] [PubMed] [Google Scholar]
- LI JY, ENGLUND E, HOLTON JL, SOULET D, HAGELL P, LEES AJ, LASHLEY T, QUINN NP, REHNCRONA S, BJORKLUND A, WIDNER H, REVESZ T, LINDVALL O, BRUNDIN P. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–3. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- LIEBMAN SW, SHERMAN F. Extrachromosomal psi+ determinant suppresses nonsense mutations in yeast. Journal of Bacteriology. 1979;139:1068–71. doi: 10.1128/jb.139.3.1068-1071.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDQUIST SL, KELLY JW. Chemical and biological approaches for adapting proteostasis to ameliorate protein misfolding and aggregation diseases: progress and prognosis. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a004507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOPEZ N, ARON R, CRAIG EA. Specificity of class II Hsp40 Sis1 in maintenance of yeast prion [RNQ+] Mol Biol Cell. 2003;14:1172–81. doi: 10.1091/mbc.E02-09-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUM R, TKACH JM, VIERLING E, GLOVER JR. Evidence for an unfolding/threading mechanism for protein disaggregation by Saccharomyces cerevisiae Hsp104. J Biol Chem. 2004;279:29139–46. doi: 10.1074/jbc.M403777200. [DOI] [PubMed] [Google Scholar]
- MAJI SK, PERRIN MH, SAWAYA MR, JESSBERGER S, VADODARIA K, RISSMAN RA, SINGRU PS, NILSSON KP, SIMON R, SCHUBERT D, EISENBERG D, RIVIER J, SAWCHENKO P, VALE W, RIEK R. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science. 2009;325:328–32. doi: 10.1126/science.1173155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALINOVSKA L, KROSCHWALD S, MUNDER MC, RICHTER D, ALBERTI S. Molecular chaperones and stress-inducible protein-sorting factors coordinate the spatiotemporal distribution of protein aggregates. Mol Biol Cell. 2012;23:3041–56. doi: 10.1091/mbc.E12-03-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASISON DC, WICKNER RB. Prion-inducing domain of yeast Ure2p and protease resistance of Ure2p in prion-containing cells. Science. 1995;270:93–5. doi: 10.1126/science.270.5233.93. [DOI] [PubMed] [Google Scholar]
- MASON RP, CASU M, BUTLER N, BREDA C, CAMPESAN S, CLAPP J, GREEN EW, DHULKHED D, KYRIACOU CP, GIORGINI F. Glutathione peroxidase activity is neuroprotective in models of Huntington’s disease. Nat Genet. 2013;45:1249–54. doi: 10.1038/ng.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCAMPBELL A, TAYLOR JP, TAYE AA, ROBITSCHEK J, LI M, WALCOTT J, MERRY D, CHAI Y, PAULSON H, SOBUE G, FISCHBECK KH. CREB-binding protein sequestration by expanded polyglutamine. Hum Mol Genet. 2000;9:2197–202. doi: 10.1093/hmg/9.14.2197. [DOI] [PubMed] [Google Scholar]
- MCGLINCHEY RP, KRYNDUSHKIN D, WICKNER RB. Suicidal [PSI+] is a lethal yeast prion. Proc Natl Acad Sci U S A. 2011;108:5337–41. doi: 10.1073/pnas.1102762108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERIIN AB, ZHANG X, HE X, NEWNAM GP, CHERNOFF YO, SHERMAN MY. Huntington toxicity in yeast model depends on polyglutamine aggregation mediated by a prion-like protein Rnq1. J Cell Biol. 2002;157:997–1004. doi: 10.1083/jcb.200112104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERIIN AB, ZHANG X, MILIARAS NB, KAZANTSEV A, CHERNOFF YO, MCCAFFERY JM, WENDLAND B, SHERMAN MY. Aggregation of expanded polyglutamine domain in yeast leads to defects in endocytosis. Mol Cell Biol. 2003;23:7554–65. doi: 10.1128/MCB.23.21.7554-7565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER-LUEHMANN M, COOMARASWAMY J, BOLMONT T, KAESER S, SCHAEFER C, KILGER E, NEUENSCHWANDER A, ABRAMOWSKI D, FREY P, JATON AL, VIGOURET JM, PAGANETTI P, WALSH DM, MATHEWS PM, GHISO J, STAUFENBIEL M, WALKER LC, JUCKER M. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–4. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- MUCHOWSKI PJ, SCHAFFAR G, SITTLER A, WANKER EE, HAYER-HARTL MK, HARTL FU. Hsp70 and hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc Natl Acad Sci U S A. 2000;97:7841–6. doi: 10.1073/pnas.140202897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUCHOWSKI PJ, WACKER JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- NESS F, FERREIRA P, COX BS, TUITE MF. Guanidine hydrochloride inhibits the generation of prion “seeds” but not prion protein aggregation in yeast. Mol Cell Biol. 2002;22:5593–605. doi: 10.1128/MCB.22.15.5593-5605.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NUCIFORA FC, JR, SASAKI M, PETERS MF, HUANG H, COOPER JK, YAMADA M, TAKAHASHI H, TSUJI S, TRONCOSO J, DAWSON VL, DAWSON TM, ROSS CA. Interference by huntingtin and atrophin-1 with cbp-mediated transcription leading to cellular toxicity. Science. 2001;291:2423–8. doi: 10.1126/science.1056784. [DOI] [PubMed] [Google Scholar]
- OLZSCHA H, SCHERMANN SM, WOERNER AC, PINKERT S, HECHT MH, TARTAGLIA GG, VENDRUSCOLO M, HAYER-HARTL M, HARTL FU, VABULAS RM. Amyloid-like aggregates sequester numerous metastable proteins with essential cellular functions. Cell. 2011;144:67–78. doi: 10.1016/j.cell.2010.11.050. [DOI] [PubMed] [Google Scholar]
- OSHEROVICH LZ, WEISSMAN JS. Multiple Gln/Asn-rich prion domains confer susceptibility to induction of the yeast [PSI+] prion. Cell. 2001;106:183–94. doi: 10.1016/s0092-8674(01)00440-8. [DOI] [PubMed] [Google Scholar]
- PARK SH, KUKUSHKIN Y, GUPTA R, CHEN T, KONAGAI A, HIPP MS, HAYER-HARTL M, HARTL FU. PolyQ proteins interfere with nuclear degradation of cytosolic proteins by sequestering the Sis1p chaperone. Cell. 2013;154:134–45. doi: 10.1016/j.cell.2013.06.003. [DOI] [PubMed] [Google Scholar]
- PARK YN, MORALES D, RUBINSON EH, MASISON D, EISENBERG E, GREENE LE. Differences in the curing of [PSI+] prion by various methods of Hsp104 inactivation. PLoS ONE. 2012;7:e37692. doi: 10.1371/journal.pone.0037692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATEL BK, GAVIN-SMYTH J, LIEBMAN SW. The yeast global transcriptional co-repressor protein Cyc8 can propagate as a prion. Nat Cell Biol. 2009;11:344–9. doi: 10.1038/ncb1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATINO MM, LIU JJ, GLOVER JR, LINDQUIST S. Support for the prion hypothesis for inheritance of a phenotypic trait in yeast. Science. 1996;273:622–6. doi: 10.1126/science.273.5275.622. [DOI] [PubMed] [Google Scholar]
- PAUSHKIN SV, KUSHNIROV VV, SMIRNOV VN, TER-AVANESYAN MD. Propagation of the yeast prion-like [PSI+] determinant is mediated by oligomerization of the SUP35-encoded polypeptide chain release factor. EMBO J. 1996;15:3127–34. [PMC free article] [PubMed] [Google Scholar]
- PAUSHKIN SV, KUSHNIROV VV, SMIRNOV VN, TER-AVANESYAN MD. Interaction between yeast Sup45p (eRF1) and Sup35p (eRF3) polypeptide chain release factors: implications for prion-dependent regulation. Mol Cell Biol. 1997;17:2798–805. doi: 10.1128/mcb.17.5.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEREZ MK, PAULSON HL, PENDSE SJ, SAIONZ SJ, BONINI NM, PITTMAN RN. Recruitment and the role of nuclear localization in polyglutamine-mediated aggregation. J Cell Biol. 1998;143:1457–70. doi: 10.1083/jcb.143.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEZZA JA, LANGSETH SX, RAUPP YAMAMOTO R, DORIS SM, ULIN SP, SALOMON AR, SERIO TR. The NatA acetyltransferase couples Sup35 prion complexes to the [PSI+] phenotype. Mol Biol Cell. 2009;20:1068–80. doi: 10.1091/mbc.E08-04-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICCARDO P, MANSON JC, KING D, GHETTI B, BARRON RM. Accumulation of prion protein in the brain that is not associated with transmissible disease. Proc Natl Acad Sci U S A. 2007;104:4712–7. doi: 10.1073/pnas.0609241104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PISSUTI DAMALIO JC, GARCIA W, ALVES MACEDO JN, DE ALMEIDA MARQUES I, ANDREU JM, GIRALDO R, GARRATT RC, ULIAN ARAUJO AP. Self assembly of human septin 2 into amyloid filaments. Biochimie. 2012;94:628–36. doi: 10.1016/j.biochi.2011.09.014. [DOI] [PubMed] [Google Scholar]
- REN PH, LAUCKNER JE, KACHIRSKAIA I, HEUSER JE, MELKI R, KOPITO RR. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol. 2009;11:219–25. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGOZA T, GOGINASHVILI A, RODIONOVA S, IV, ANOV M, VIKTOROVSKAYA O, RUBEL A, VOLKOV K, MIRONOVA L. Non-Mendelian determinant [ISP+] in yeast is a nuclear-residing prion form of the global transcriptional regulator Sfp1. Proc Natl Acad Sci U S A. 2010;107:10573–7. doi: 10.1073/pnas.1005949107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATPUTE-KRISHNAN P, LANGSETH SX, SERIO TR. Hsp104-Dependent Remodeling of Prion Complexes Mediates Protein-Only Inheritance. PLoS Biol. 2007;5:e24. doi: 10.1371/journal.pbio.0050024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATPUTE-KRISHNAN P, SERIO TR. Prion protein remodelling confers an immediate phenotypic switch. Nature. 2005;437:262–5. doi: 10.1038/nature03981. [DOI] [PubMed] [Google Scholar]
- SAUDOU F, FINKBEINER S, DEVYS D, GREENBERG ME. Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell. 1998;95:55–66. doi: 10.1016/s0092-8674(00)81782-1. [DOI] [PubMed] [Google Scholar]
- SCHAFFAR G, BREUER P, BOTEVA R, BEHRENDS C, TZVETKOV N, STRIPPEL N, SAKAHIRA H, SIEGERS K, HAYER-HARTL M, HARTL FU. Cellular toxicity of polyglutamine expansion proteins: mechanism of transcription factor deactivation. Mol Cell. 2004;15:95–105. doi: 10.1016/j.molcel.2004.06.029. [DOI] [PubMed] [Google Scholar]
- SERIO TR, CASHIKAR AG, KOWAL AS, SAWICKI GJ, MOSLEHI JJ, SERPELL L, ARNSDORF MF, LINDQUIST SL. Nucleated Conformational Conversion and the Replication of Conformational Information by a Prion Determinant. Science. 2000;289:1317–21. doi: 10.1126/science.289.5483.1317. [DOI] [PubMed] [Google Scholar]
- SHIBER A, BREUER W, BRANDEIS M, RAVID T. Ubiquitin conjugation triggers misfolded protein sequestration into quality control foci when Hsp70 chaperone levels are limiting. Mol Biol Cell. 2013;24:2076–87. doi: 10.1091/mbc.E13-01-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SI K, CHOI YB, WHITE-GRINDLEY E, MAJUMDAR A, KANDEL ER. Aplysia CPEB can form prion-like multimers in sensory neurons that contribute to long-term facilitation. Cell. 2010;140:421–35. doi: 10.1016/j.cell.2010.01.008. [DOI] [PubMed] [Google Scholar]
- SINDI SS, SERIO TR. Prion dynamics and the quest for the genetic determinant in protein-only inheritance. Curr Opin Microbiol. 2009;12:623–30. doi: 10.1016/j.mib.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONDHEIMER N, LINDQUIST S. Rnq1: an epigenetic modifier of protein function in yeast. Mol Cell. 2000;5:163–72. doi: 10.1016/s1097-2765(00)80412-8. [DOI] [PubMed] [Google Scholar]
- SONDHEIMER N, LOPEZ N, CRAIG EA, LINDQUIST S. The role of Sis1 in the maintenance of the [RNQ+] prion. EMBO J. 2001;20:2435–42. doi: 10.1093/emboj/20.10.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANSFIELD I, JONES KM, KUSHNIROV VV, DAGKESAMANSKAYA AR, POZNYAKOVSKI AI, PAUSHKIN SV, NIERRAS CR, COX BS, TER-AVANESYAN MD, TUITE MF. The products of the SUP45 (eRF1) and SUP35 genes interact to mediate translation termination in Saccharomyces cerevisiae. EMBO J. 1995;14:4365–73. doi: 10.1002/j.1460-2075.1995.tb00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEFFAN JS, KAZANTSEV A, SPASIC-BOSKOVIC O, GREENWALD M, ZHU YZ, GOHLER H, WANKER EE, BATES GP, HOUSMAN DE, THOMPSON LM. The Huntington’s disease protein interacts with p53 and CREB-binding protein and represses transcription. Proc Natl Acad Sci U S A. 2000;97:6763–8. doi: 10.1073/pnas.100110097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAWN LA, TRUE HL. Deletion of RNQ1 gene reveals novel functional relationship between divergently transcribed Bik1p/CLIP-170 and Sfi1p in spindle pole body separation. Curr Genet. 2006;50:347–66. doi: 10.1007/s00294-006-0098-6. [DOI] [PubMed] [Google Scholar]
- STRYER L. The interaction of a naphthalene dye with apomyoglobin and apohemoglobin. A fluorescent probe of non-polar binding sites. J Mol Biol. 1965;13:482–95. doi: 10.1016/s0022-2836(65)80111-5. [DOI] [PubMed] [Google Scholar]
- SUMMERS DW, DOUGLAS PM, CYR DM. Prion propagation by Hsp40 molecular chaperones. Prion. 2009a;3:59–64. doi: 10.4161/pri.3.2.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUMMERS DW, DOUGLAS PM, REN HY, CYR DM. The type I Hsp40 Ydj1 utilizes a farnesyl moiety and zinc finger-like region to suppress prion toxicity. J Biol Chem. 2009b;284:3628–39. doi: 10.1074/jbc.M807369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUMMERS DW, WOLFE KJ, REN HY, CYR DM. The Type II Hsp40 Sis1 cooperates with Hsp70 and the E3 ligase Ubr1 to promote degradation of terminally misfolded cytosolic protein. PLoS ONE. 2013;8:e52099. doi: 10.1371/journal.pone.0052099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUKI G, SHIMAZU N, TANAKA M. A yeast prion, Mod5, promotes acquired drug resistance and cell survival under environmental stress. Science. 2012;336:355–9. doi: 10.1126/science.1219491. [DOI] [PubMed] [Google Scholar]
- TANAKA M, COLLINS SR, TOYAMA BH, WEISSMAN JS. The physical basis of how prion conformations determine strain phenotypes. Nature. 2006;442:585–9. doi: 10.1038/nature04922. [DOI] [PubMed] [Google Scholar]
- TANK EM, TRUE HL. Disease-associated mutant ubiquitin causes proteasomal impairment and enhances the toxicity of protein aggregates. PLoS Genet. 2009;5:e1000382. doi: 10.1371/journal.pgen.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TER-AVANESYAN MD, DAGKESAMANSKAYA AR, KUSHNIROV VV, SMIRNOV VN. The SUP35 omnipotent suppressor gene is involved in the maintenance of the non-Mendelian determinant [PSI+] in the yeast Saccharomyces cerevisiae. Genetics. 1994;137:671–6. doi: 10.1093/genetics/137.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TER-AVANESYAN MD, KUSHNIROV VV, DAGKESAMANSKAYA AR, DIDICHENKO SA, CHERNOFF YO, INGE-VECHTOMOV SG, SMIRNOV VN. Deletion analysis of the SUP35 gene of the yeast Saccharomyces cerevisiae reveals two non-overlapping functional regions in the encoded protein. Mol Microbiol. 1993;7:683–92. doi: 10.1111/j.1365-2958.1993.tb01159.x. [DOI] [PubMed] [Google Scholar]
- TESSARZ P, MOGK A, BUKAU B. Substrate threading through the central pore of the Hsp104 chaperone as a common mechanism for protein disaggregation and prion propagation. Mol Microbiol. 2008;68:87–97. doi: 10.1111/j.1365-2958.2008.06135.x. [DOI] [PubMed] [Google Scholar]
- TIPTON KA, VERGES KJ, WEISSMAN JS. In vivo monitoring of the prion replication cycle reveals a critical role for Sis1 in delivering substrates to Hsp104. Mol Cell. 2008;32:584–91. doi: 10.1016/j.molcel.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREUSCH S, LINDQUIST S. An intrinsically disordered yeast prion arrests the cell cycle by sequestering a spindle pole body component. J Cell Biol. 2012;197:369–79. doi: 10.1083/jcb.201108146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUITE MF, SERIO TR. The prion hypothesis: from biological anomaly to basic regulatory mechanism. Nat Rev Mol Cell Biol. 2010;11:823–833. doi: 10.1038/nrm3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- URAKOV VN, VISHNEVSKAYA AB, ALEXANDROV IM, KUSHNIROV VV, SMIRNOV VN, TER-AVANESYAN MD. Interdependence of amyloid formation in yeast: Implications for polyglutamine disorders and biological functions. Prion. 2010:4. doi: 10.4161/pri.4.1.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALOUEV IA, KUSHNIROV VV, TER-AVANESYAN MD. Yeast polypeptide chain release factors eRF1 and eRF3 are involved in cytoskeleton organization and cell cycle regulation. Cell Motil Cytoskeleton. 2002;52:161–73. doi: 10.1002/cm.10040. [DOI] [PubMed] [Google Scholar]
- VAVOURI T, SEMPLE JI, GARCIA-VERDUGO R, LEHNER B. Intrinsic protein disorder and interaction promiscuity are widely associated with dosage sensitivity. Cell. 2009;138:198–208. doi: 10.1016/j.cell.2009.04.029. [DOI] [PubMed] [Google Scholar]
- VISHVESHWARA N, BRADLEY ME, LIEBMAN SW. Sequestration of essential proteins causes prion associated toxicity in yeast. Mol Microbiol. 2009;73:1101–14. doi: 10.1111/j.1365-2958.2009.06836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOISINE C, PEDERSEN JS, MORIMOTO RI. Chaperone networks: tipping the balance in protein folding diseases. Neurobiol Dis. 2010;40:12–20. doi: 10.1016/j.nbd.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLLES MJ, LEE SJ, ROCHET JC, SHTILERMAN MD, DING TT, KESSLER JC, LANSBURY PT., JR Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson’s disease. Biochemistry. 2001;40:7812–9. doi: 10.1021/bi0102398. [DOI] [PubMed] [Google Scholar]
- WANG Y, MERIIN AB, ZAARUR N, ROMANOVA NV, CHERNOFF YO, COSTELLO CE, SHERMAN MY. Abnormal proteins can form aggresome in yeast: aggresome-targeting signals and components of the machinery. FASEB J. 2009;23:451–63. doi: 10.1096/fj.08-117614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WICKNER RB. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science. 1994;264:566–9. doi: 10.1126/science.7909170. [DOI] [PubMed] [Google Scholar]
- WOLFE KJ, REN HY, TREPTE P, CYR DM. The Hsp70/90 cochaperone, Sti1, suppresses proteotoxicity by regulating spatial quality control of amyloid-like proteins. Mol Biol Cell. 2013;24:3588–602. doi: 10.1091/mbc.E13-06-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAO X, PARK YN, TODOR H, MOOMAU C, MASISON D, EISENBERG E, GREENE LE. Sequestration of Sup35 by aggregates of huntingtin fragments causes toxicity of [PSI+] yeast. J Biol Chem. 2012;287:23346–55. doi: 10.1074/jbc.M111.287748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU P, DERKATCH IL, UPTAIN SM, PATINO MM, LINDQUIST S, LIEBMAN SW. The yeast non-Mendelian factor [ETA+] is a variant of [PSI+], a prion- like form of release factor eRF3. EMBO J. 1999;18:1182–1191. doi: 10.1093/emboj/18.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOURAVLEVA G, FROLOVA L, LE GOFF X, LE GUELLEC R, INGE-VECHTOMOV S, KISSELEV L, PHILIPPE M. Termination of translation in eukaryotes is governed by two interacting polypeptide chain release factors, eRF1 and eRF3. EMBO J. 1995;14:4065–72. doi: 10.1002/j.1460-2075.1995.tb00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]