Abstract
Given the complexity of the brain, characterizing relations among distributed brain regions is likely essential to describing the neural instantiation of posttraumatic stress symptoms. This study examined patterns of functional connectivity among key brain regions implicated in the pathophysiology of posttraumatic stress disorder (PTSD) in 35 trauma-exposed adults using an emotion-word Stroop task. PTSD symptom severity (particularly hyperarousal symptoms) moderated amygdala-mPFC coupling during the processing of unpleasant words, and this moderation correlated positively with reported real-world impairment and amygdala reactivity. Reexperiencing severity moderated hippocampus-insula coupling during pleasant and unpleasant words. Results provide evidence that PTSD symptoms differentially moderate functional coupling during emotional interference and underscore the importance of examining network connectivity in research on PTSD. They suggest that hyperarousal is associated with negative mPFC-amygdala coupling and that reexperiencing is associated with altered insula-hippocampus function, patterns of connectivity that may represent separable indicators of dysfunctional inhibitory control during affective processing.
Keywords: hyperarousal, reexperiencing, amygdala, hippocampus, medial prefrontal cortex
The complexity of the human brain makes characterizing relations among distributed neural networks central to advancing research on the etiology and treatment of psychiatric symptoms (Morris & Cuthbert, 2012). Methodological advances have made it possible to move beyond modular models of neural dysfunction toward an understanding of how distinct regions work in concert to produce psychiatric symptoms (Menon, 2011). To begin to characterize these relationships in PTSD, the present study examined patterns of functional connectivity among brain regions associated with the pathophysiology of PTSD symptoms in trauma-exposed individuals.
A widely studied model of neural dysfunction in PTSD (Rauch, Shin, & Phelps, 2006; Rauch, Shin, Whalen, & Pitman, 1998) posits that hyperactivation in amygdala and inadequate top-down regulation by medial prefrontal cortex (mPFC) and hippocampus cause deficits in fear conditioning, habituation, and extinction. Hyperactivity in amygdala may promote hypervigilance to threat-related stimuli and heightened fear acquisition, given its role in evaluating threat and ambiguity (Anderson & Phelps, 2001). Furthermore, animal models indicate that amygdala and mPFC are highly interconnected (McDonald, 1991), and mPFC plays a critical role in successful execution and maintenance of fear extinction (Morgan, Romanski, & LeDoux, 1993), potentially through top-down regulation of amygdala (Phelps, Delgado, Nearing, & LeDoux, 2004). Other research suggests that amygdala inhibits mPFC (Garcia, Vouimba, Baudry, & Thompson, 1999), calling into question the directionality of the regulation deficits associated with mPFC-amygdala coupling. Hippocampus is also connected with amygdala and encodes contextual information during fear conditioning (Phillips & LeDoux, 1992). Impaired amygdala-hippocampus connectivity may result in poor consolidation of trauma-related memories and contribute to overgeneralization of perceived threat to safe contexts (Wilker & Kolassa, 2013).
Functional magnetic resonance imaging (fMRI) research largely supports a model of abnormal amygdala, hippocampus, and mPFC function (Shin, Rauch, & Pitman, 2006; Shin et al., 2005). Hyperactivation in amygdala and hypoactivation in prefrontal cortex, particularly mPFC, correlate with PTSD severity (Shin et al., 2006) and trauma exposure (Patel et al., 2012). There is also evidence of variability in the activation patterns of these neural networks, with a recent meta-analysis reporting hippocampal over-engagement rather than hypoactivation in PTSD (Patel et al., 2012). Examining heterogeneity in neural network coupling as a function of the PTSD symptom clusters can be a stepping stone toward testing whether affective and cognitive processing vary with symptom prominence.
Interest in dysfunctional neural networks in PTSD is growing, and preliminary research suggests that PTSD moderates network connectivity. PTSD modulates resting-state connectivity between posterior cingulate/precuneus and regions consistently linked to PTSD (Bluhm et al., 2009). Studies have also identified modified connectivity in PTSD in response to unpleasantly valenced stimuli, with one study suggesting that insula positively influences cortical regions typically involved in cognitive control (Mazza et al., 2013). Given that research in this area is currently limited, continued investigation of functional coupling can help further characterize neurocircuitry abnormalities associated with PTSD.
The present study tested the hypothesis that PTSD severity moderates functional connectivity in trauma-exposed individuals during emotional interference using an emotion-word Stroop task. The vast majority of pathophysiology studies have examined the presence or absence of PTSD. However, taxometric analyses indicate that the latent structure of the disorder is dimensional (Ruscio, Ruscio, & Keane, 2002), and preliminary data suggest there is heterogeneity in the neural correlates of the symptom clusters (Hopper, Frewen, van der Kolk, & Lanius, 2007; Lanius et al., 2005). Thus, identifying relationships between individual symptom clusters and patterns of neural connectivity is a potential means for parsing etiological heterogeneity in PTSD. Emotion Stroop tasks have been widely utilized in research on PTSD, because they assess the interplay between sensitivity to emotional distraction and cognitive control. Previous work using emotion Stroop tasks has found diminished anterior cingulate activation in PTSD vs. non-PTSD comparison groups to emotionally distracting stimuli (Bremner et al., 2004; Shin et al., 2001). We predicted that overall PTSD severity would moderate coupling between amygdala, mPFC, and hippocampus when participants encountered unpleasantly valenced stimuli, based on evidence that PTSD is associated with inefficient cognitive control when negative valence systems are activated (Bremner et al., 2004; Cisler et al., 2011; Shin et al., 2001). Given that few studies have examined functional coupling relationships with individual symptom clusters, specific hypotheses were not made about these relationships. However, because reexperiencing is associated with dysfunctional inhibition of intrusive thoughts (Vasterling, Brailey, Constans, & Sutker, 1998) and greater insula activation during emotional processing (Hopper et al., 2007), these symptoms may be particularly likely to moderate functional coupling with insula.
Methods
Participants
Participants were 35 adults (74% women) ages 18–50 (M = 33.8; SD = 11.2) who experienced a traumatic event meeting Diagnostic and Statistical Manual-IV-TR PTSD Criterion A (DSM-IV-TR; American Psychiatric Association, 2000). Participants identified as White (79%), Asian (9%), Black (6%), Hispanic (3%), and mixed/other (3%). Information about income/socioeconomic status was not collected. The categories of traumatic events endorsed were: witnessing death/injury (e.g., assault, combat, n = 12), sexual crime (e.g., rape, stalking, n = 10), accident (e.g., car accident, n = 5), physical assault (e.g., domestic violence, n = 5), natural disaster (e.g., typhoon, n = 2), and other crime (burglary, n = 1).
Measures
PTSD symptoms
Symptoms were assessed using the Structured Clinical Interview for DSM-IV-TR (SCID-IV-TR; First, Spitzer, Gibbon, & Williams, 2002). An advanced doctoral student rated the presence/absence of PTSD symptoms based on behavioral observations and participant self-report. Decisions were determined in consultation with GAM, a clinical psychologist very experienced in SCID diagnosis. Symptoms were summed to create a total PTSD severity score (M = 4.3, SD = 3.7) and three symptom-cluster scores: reexperiencing (M = 1.9, SD = 1.6), avoidance/emotional numbing (M = 1.1, SD = 1.3), and hyperarousal (M = 1.4, SD = 1.5). Although all participants were exposed to a Criterion A traumatic event, only a subset (n = 5) met full criteria for PTSD, with 25 participants meeting Criterion B, six participants meeting Criterion C, and 13 participants meeting Criterion D.
Emotion-word Stroop
On each trial, participants responded via button press to the ink color (red, yellow, green, blue) of a pleasant, unpleasant, or neutral word, presented in counterbalanced blocks (four pleasant, four unpleasant, eight neutral) of 16 words each. Word stimuli were selected from the Affective Norms for English Words (Bradley & Lang, 1998). Sixty-four were pleasant (e.g. ecstasy, laughter), 64 were unpleasant (e.g. suicide, war, victim) and 128 were neutral (e.g. hydrant, carpet). Additional details are available in Sadeh et al. (2013).
Data Analysis
Reaction time (RT) and error frequency were analyzed using repeated-measures ANCOVAs with Emotion (pleasant, neutral, unpleasant) as the within-subject factor (contrasts were Valence [pleasant vs. unpleasant] & Arousal [emotion vs. neutral]), and continuous PTSD symptom counts (total severity or reexperiencing, avoidance, & hyperarousal entered simultaneously using the covariate function) as between-subjects predictors. Counterbalancing order was entered as a covariate.
fMRI collection/preprocessing is described in Sadeh et al. (2013), with the addition here of slice-timing correction. fMRI pre/processing was conducted with FSL tools. Left/right amygdalae/hippocampi were used as seed clusters and segmented in each anatomical via FMRIB’s Integrated Registration and Segmentation Tool (FIRST). Functional data were registered to the anatomical using Boundary Based Registration, and the inverse of this transform was applied to segmentations. Mean signal across voxels in each ROI was computed for each timepoint to create amygdalae/hippocampi timeseries predictors. To model potentially confounding brain-wide fluctuations (Fox, Zhang, Snyder, & Raichle, 2009), the mean across all intra-cerebral voxels was calculated for each timepoint.
Predictors in timeseries regressions included an amygdala/hippocampus timeseries (one per analysis), task contrast (valence: pleasant = 1, unpleasant = −1; arousal: pleasant/unpleasant = 1, neutral = −1), and the timeseries X contrast predictor interaction. Three covariates were included, two modeling task-related variance and one brain-wide signal fluctuations. Task predictors were convolved with a double-gamma function.
Timeseries X task contrast interaction β-maps were warped into MNI152 space using FMRIB’s Nonlinear Image Registration Tool (FNIRT) and used as group-level dependent variables. Four analyses (one each for right/left amygdala/hippocampus) were conducted with total PTSD symptom score as a predictor, covarying counterbalancing order. Similar analyses were conducted with the three symptom-cluster scores entered simultaneously. Two-tailed t-tests were conducted on β’s and converted to z-scores. Gaussian-random-field theory was used for multiple comparisons correction (z-threshold = 2.0537, overall p ≤ .05). A gray-matter mask was used to constrain the number of voxels under consideration.
To ascertain the impact of mPFC-amygdala coupling on task-related activation in amygdala and mPFC, first-level analyses were re-computed as in Sadeh et al. (2013). Mean amygdala/mPFC activation to unpleasant was extracted and regressed on a variable representing the degree of amygdala-mPFC coupling, covarying counterbalancing order. Total PTSD symptom score was also covaried to ensure that variance shared with PTSD did not bias the analyses. Amygdala activation data from one participant were not used given data > 3 SD from the mean across participants. A similar analysis investigated the relevance of dysfunctional amygdala-mPFC coupling to real-world impairment by regressing the Global Assessment of Functioning (GAF) SCID-IV-TR rating on the degree of amygdala-mPFC coupling during the unpleasant condition. Only significant findings are reported.
Results
Behavioral Results
Analysis of RT produced a valence effect (F(1,31) = 12.25, p < .01). Participants responded more slowly to color name unpleasant (M = 699.6, SD = 112.2) than pleasant words (M = 685.6, SD = 107.1).
PTSD severity moderated the impact of valence on error frequency (F(1,30) = 4.54, p = .04), whereby more symptoms were related to greater color naming errors to unpleasant than pleasant words (r = −.36). PTSD severity also modulated the impact of arousal on error frequency (F(1,30) = 4.13, p = .05), with more symptoms related to more color naming errors to emotion than neutral words (r = .35). Across conditions, greater PTSD severity was negatively associated with RT (F(1,30) = 4.39, p = .04) and error frequency (F(1,30) = 4.18, p = .05).
For symptom clusters, reexperiencing moderated the impact of valence on RT (F(1,30) = 4.27, p = .05), with longer RT to color name pleasant than unpleasant words. Further examination revealed that individuals high on reexperiencing had similar RT for both conditions, whereas individuals low on reexperiencing had longer RT to color name unpleasant words.
Moderation of Coupling by PTSD Severity
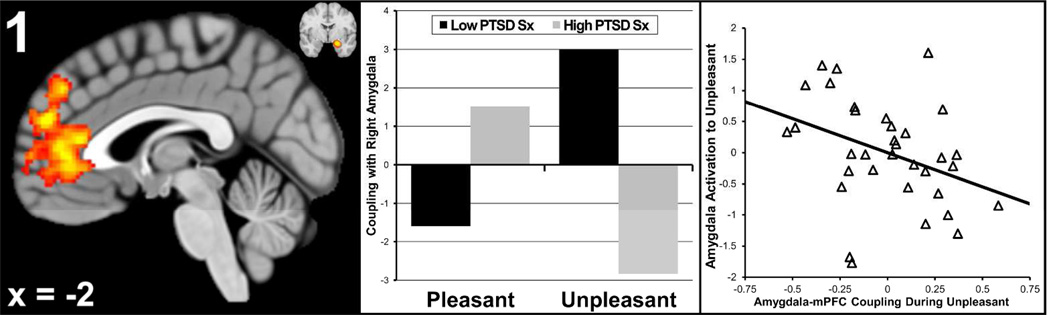
PTSD severity moderated valence-related coupling between right amygdala and a cluster in mPFC that included pregenual anterior cingulate and paracingulate (21,296 mm3, p < .01, max z = 3.93 at xyz = [0,34,12]). Individuals with more severe PTSD had a small degree of positive coupling during pleasant and strong negative coupling during unpleasant stimuli (Figure 1).
Figure 1. Total Posttraumatic Stress Disorder (PTSD) Symptoms Moderates Amygdala-mPFC Coupling.
mPFC = medial prefrontal cortex. Total PTSD symptoms moderated valence-related coupling between right amygdala and the cluster in mPFC pictured in the left panel. Also pictured in the upper right corner of the left panel is the average location of the right amygdala seed clusters at y = −3. The middle panel represents the degree of coupling for low and high levels of PTSD (created via median-split) for the pleasant and unpleasant conditions. The right panel is a scatterplot of the relationship between task-related activation in right amygdala (y-axis) and the degree of coupling amygdala-mPFC coupling, during the unpleasant condition (x-axis).
To aid in interpreting this finding, an analysis was conducted that examined whether greater negative coupling during the unpleasant condition was associated with greater amygdala reactivity (possibly due to impaired top-down mPFC regulation of amygdala). Amygdala activation to unpleasant stimuli was regressed on the degree of amygdala-mPFC coupling during unpleasant words. As illustrated in the rightmost panel in Figure 1, coupling was negatively related to amygdala activation (β = −.54, p = .05, ΔR2 = .12), consistent with a model of impaired down-regulation of amygdala by mPFC. In contrast, coupling was unrelated to mPFC activation (β = −.01, p = .98, ΔR2 = .00). Additionally, coupling correlated positively with GAF (β = .54, p < .01, ΔR2 = .26), suggesting that greater (negative) amygdala-mPFC coupling relates to worse functional impairment in trauma-exposed individuals.
Total PTSD symptom score also moderated valence-related coupling between a highly overlapping cluster in mPFC and left amygdala (8,496 mm3, p < .01, max z = 3.75 at xyz = [0,32,14]), right hippocampus (9,416 mm3, p < .01, max z = 3.61 at xyz = [−4,46,8]), and left hippocampus (2,904 mm3, p = .05, max z = 3.16 at xyz = [0,30,16]). Given the similarity of these findings, moderation of connectivity with each subcortical structure was re-examined while partialling out variance related to connectivity with the other subcortical structures. Only coupling with right amygdala survived this analysis (t = 2.42, p = .02), suggesting that the findings for left amygdala and hippocampus are due to shared variance.
Moderation of Coupling by PTSD Symptom Clusters
Hyperarousal moderated valence-related coupling between right amygdala and a mPFC cluster (3,656 mm3, p = .02, max z = 3.77 at xyz = [4,44,16]) that overlapped considerably with that found in the analyses above (that examined total PTSD symptoms), and reflected stronger negative coupling during unpleasant stimuli. To determine whether particular hyperarousal symptoms drove this moderation, the degree of amygdala-mPFC coupling was extracted for each participant and the analysis repeated with individual symptoms entered simultaneously as predictors. Exaggerated startle (t = 3.32, p < .01), difficulty concentrating (t = 2.12, p = .05), and hypervigilance (t = 2.01, p = .05) each contributed unique variance. Hyperarousal also moderated valence-related coupling between right hippocampus and a similar mPFC cluster (3,488 mm3, p = .03, max z = 3.06 at xyz = [4,52,32]), although this finding is likely due to shared variance with right amygdala, as observed for total symptom score.
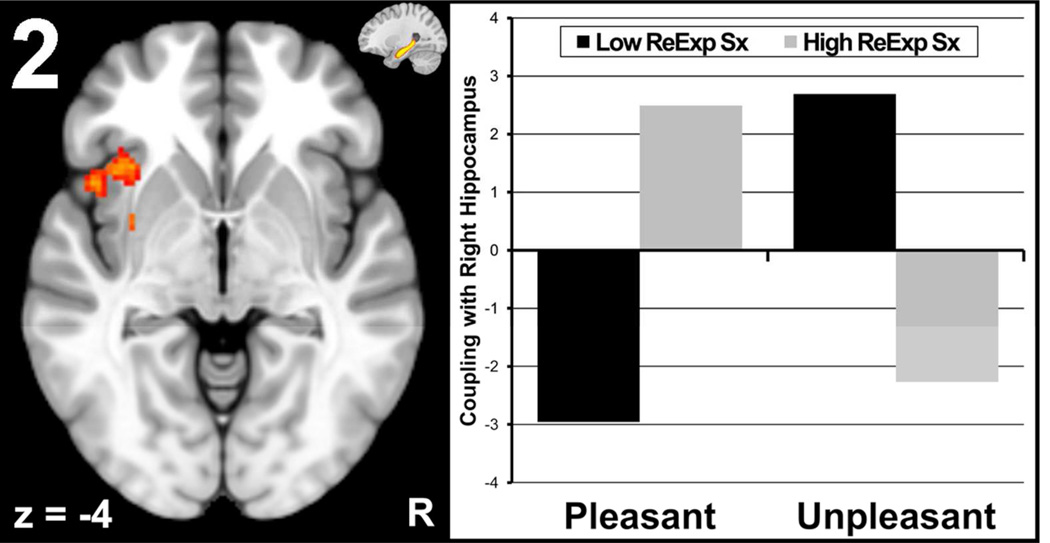
Reexperiencing moderated valence-related coupling between right hippocampus and a cluster in left anterior insula/putamen (2,416mm3, p = .03, max z = 3.23 at xyz = [−32,10,−10]). Individuals with more severe reexperiencing symptoms had a larger degree of positive coupling during pleasant words, whereas coupling was strongly negative during unpleasant words (Figure 2). When examining specific symptoms, both acting/feeling as if the event were recurring (t = 2.13, p = .04) and psychological distress in response to reminders of the event (t = 2.31, p = .03) moderated hippocampus-insula/putamen coupling. Avoidance symptoms did not moderate coupling between brain regions of interest.
Figure 2. Reexperiencing Symptom Score Moderates Hippocampus-Insula Coupling.
Severity of reexperiencing symptoms (ReExp Sx) moderated valence-related right hippocampus-insula/putamen coupling pictured in the left panel. Also pictured in the upper right corner of the left panel is the average location of the right hippocampus seed clusters at x = 26. The right panel represents the degree of coupling for low and high levels of reexperiencing (created via median-split) for the pleasant and unpleasant conditions.
Discussion
Clarifying network connectivity in trauma-exposed individuals has the potential to advance etiological models of posttraumatic stress, improve diagnostic reliability/validity, and identify innovative intervention targets. Accordingly, the present study tested the hypothesis that PTSD severity would moderate functional connectivity in trauma-exposed individuals during an emotional Stroop task. First, findings provide evidence of PTSD-related moderation of mPFC-amygdala connectivity, characterized by strong negative coupling during unpleasant distractors. This inverse coupling was strongest for the hyperarousal symptom cluster and is consistent with widely studied models of PTSD that emphasize the centrality of deficits in cognitive control when negative valence systems are activated (Rauch et al., 2006). Moderation of mPFC-amygdala connectivity by PTSD severity corresponded with real-world impairment, illustrating the potential clinical significance of this neural circuit for adaptive function. Second, reexperiencing severity was related to stronger negative coupling between hippocampus and anterior insula/putamen during unpleasant than pleasant words. Findings provide evidence that PTSD symptoms differentially moderate functional coupling during emotional interference and underscore the importance of examining network connectivity in future research on PTSD.
PTSD Severity
Analysis of overall PTSD severity showed it moderated coupling between right amygdala and mPFC. Individuals with more severe PTSD evidenced strongly negative amygdala-mPFC coupling during the unpleasant condition (Figure 1), and this negative coupling was positively associated with impairment in real-world function (indexed by GAF). Notably, greater negative coupling predicted increased amygdala reactivity to unpleasant stimuli (Figure 1) but was unrelated to mPFC reactivity, suggesting that PTSD-related changes in coupling may be more relevant for amygdala than mPFC function. This negative coupling is consistent with the behavioral finding that PTSD severity was associated with greater color naming errors to unpleasant than pleasant words, suggesting more interference during unpleasant distractors. Interpreted in the context of theoretical models of neural dysfunction in PTSD, these findings suggest that individuals with more severe PTSD experience deficits in top-down mPFC regulation of amygdala. However, present analyses provide only a suggestion regarding the direction of influence, not a definitive test.
These results add novel information about potentially dysfunctional amygdala-mPFC coupling in trauma-exposed individuals during emotional interference. The identified mPFC region is central to several key affective processes, including negative affect reduction (Myers-Schulz & Koenigs, 2012), attention to emotion (Grimm et al., 2006), and recalling/recognizing affective aspects of memory (Grimm et al., 2006). Of note, research indicates that this region is crucial to regulating affect via top-down control of amygdala. For example, stimulation of mPFC in rodents decreases responsiveness of output neurons in amygdala (Quirk, Likhtik, Pelletier, & Pare, 2003). Mounting evidence also implicates ventral mPFC in successful fear extinction (Phelps et al., 2004). The essential role of the mPFC-amygdala circuit in emotion regulation and fear extinction is consistent with the finding that coupling strength in this circuit predicted greater functional impairment in everyday life.
Hyperarousal and Reexperiencing Symptoms
Modulation of mPFC-amygdala neurocircuitry was particularly salient for hyperarousal symptoms. Insufficient regulation of amygdala in response to unpleasant/threatening stimuli may manifest phenotypically as symptoms of hyperarousal, with exaggerated startle, hypervigilance, and difficulty concentrating emerging as the strongest indicators of this relationship. Individuals with hyperarousal are particularly reactive to threatening stimuli, and results suggest that this reactivity reflects negative coupling between top-down resources and amygdala, especially when attentional resources are taxed by competing emotional distraction. Disrupted top-down mPFC control may contribute to these symptoms and be self-maintaining, given that repeated activation of these neural pathways will increase efficiency over time (Collingridge, Peineau, Howland, & Wang, 2010), potentially producing chronic hyperarousal.
Right hippocampus-left insula coupling was strongly positive during the pleasant condition and strongly negative during the unpleasant condition in individuals with more severe reexperiencing symptoms (see Figure 2). Strong negative hippocampus-insula coupling during unpleasant words may reflect a tendency to disengage attention when confronted with unpleasant stimuli, given that RT interference from unpleasant words decreased as reexperiencing symptoms increased. Findings converge with evidence that individuals with prominent dissociative symptoms show greater insula activation to emotional stimuli (Aupperle, Melrose, Stein, & Paulus, 2012), greater connectivity between regions associated with the representation of bodily states (insula, thalamus) during trauma recall, and reduced activation in parahippocampal gyrus (Lanius et al., 2005; Lanius et al., 2002). Similarly, reexperiencing severity has shown positive associations with right anterior insula activity and negative associations with rostral ACC during exposure to script-driven imagery (Hopper et al., 2007).
Reexperiencing symptoms are theorized to reflect greater encoding and organization of traumatic experience in somatic than declarative memory (van der Kolk, 1994). Strong negative coupling between insula and hippocampus may reflect greater activation of somatosensory representations and reduced recruitment of hippocampally mediated episodic memory when processing unpleasant stimuli (Schonfeld, Ehlers, Bollinghaus, & Rief, 2007), although the direction of the influence cannot be directly inferred, and other interpretations are possible. Research indicates that stress leads to worse recall, less effective hippocampal processing, and greater insula reactivity (Qin, Hermans, van Marle, & Fernandez, 2012). Thus, traumatic stress may lead to “gist-based” memory consolidation that incorporates more general somatosensory representations rather than context-specific details (Oyarzun & Packard, 2012), potentially leading to overgeneralization of memory (Schonfeld et al., 2007).
Implications and Conclusions
Of clinical relevance, research on neural networks can help identify potential etiological heterogeneity within trauma-exposed individuals. For instance, strong negative mPFC-amygdala coupling may be a transdiagnostic indicator cutting across disorders with prominent hyperarousal symptoms. Similarly, dysfunctional coupling between hippocampus and anterior insula may represent a vulnerability to reexperiencing and dissociative symptoms extending beyond PTSD to other disorders commonly associated with trauma exposure (e.g., Borderline Personality Disorder). Linking PTSD symptoms to neural networks can be used to develop cognitive training interventions aimed at altering network dysfunction. Treatments aimed at strengthening top-down amygdala coupling or reducing disengagement when confronted with unpleasant stimuli may be particularly relevant for improving cognitive control in individuals with hyperarousal and reexperiencing symptoms, respectively.
Present findings cannot speak to causal relationships between network connectivity and symptom manifestation, and prospective research is needed to ascertain whether network abnormalities represent vulnerabilities for PTSD and/or consequences of trauma exposure. Further, null results should be interpreted with caution due to the modest sample size and potential restricted range in symptoms by not including more participants who met full DSM-IV criteria. Strengths of the study include a well validated emotional interference task and a theory-based analysis of key brain regions implicated in the pathophysiology of PTSD. The findings shed new light on neural mechanisms of PTSD, and provide preliminary evidence that features of the disorder show separable patterns of connectivity during emotional interference.
Acknowledgments
The study was supported in part by the National Institute of Mental Health (R01 MH61358, P50 MH079485) and the Beckman Institute of the University of Illinois Urbana-Champaign. Views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of Veterans Affairs.
Ten participants overlapped with Sadeh et al. (2013), nine with Spielberg et al. (2012), seven with Warren et al. (2010) and 22 with Crocker et al. (2012). These studies examined neither PTSD nor connectivity.
Footnotes
N.S. and J.M.S. formulated study questions, performed data analysis, and drafted the manuscript. G.A.M. and W.H. designed the study and provided critical revisions. J.M.S. and S.L.W. performed data collection/testing. All authors approved the final manuscript.
References
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411(6835):305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR®. American Psychiatric Pub; 2000. [Google Scholar]
- Aupperle RL, Melrose AJ, Stein MB, Paulus MP. Executive function and PTSD: disengaging from trauma. Neuropharmacology. 2012;62(2):686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, Lanius RA. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci. 2009;34(3):187–194. [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective norms for English words. Gainesville, FL: University of Florida, NIMH Center for the Study of Emotion and Attention; 1998. [Google Scholar]
- Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga B, Charney DS. Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biol Psychiatry. 2004;55(6):612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Wolitzky-Taylor KB, Adams TG, Jr, Babson KA, Badour CL, Willems JL. The emotional Stroop task and posttraumatic stress disorder: a meta-analysis. Clin Psychol Rev. 2011;31(5):817–828. doi: 10.1016/j.cpr.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci. 2010;11(7):459–473. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- Crocker LD, Heller W, Spielberg JM, Warren SL, Bredemeier K, Sutton BP, Miller GA. Neural mechanisms of attentional control differentiate trait and state negative affect. Front Psychol. 2012;3:298. doi: 10.3389/fpsyg.2012.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structural Clinical Interview for DSM-IV-TR, Research Version, Non-patient Edition. Biometrics Research, New York State Psychiatric Institute: New York. 2002 [Google Scholar]
- 11.Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101(6):3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia R, Vouimba RM, Baudry M, Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402(6759):294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- 13.Grimm S, Schmidt CF, Bermpohl F, Heinzel A, Dahlem Y, Wyss M, Northoff G. Segregated neural representation of distinct emotion dimensions in the prefrontal cortex-an fMRI study. Neuroimage. 2006;30(1):325–340. doi: 10.1016/j.neuroimage.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Hopper JW, Frewen PA, van der Kolk BA, Lanius RA. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Trauma Stress. 2007;20(5):713–725. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- 15.Lanius RA, Williamson PC, Bluhm RL, Densmore M, Boksman K, Neufeld RW, Menon RS. Functional connectivity of dissociative responses in posttraumatic stress disorder: a functional magnetic resonance imaging investigation. Biol Psychiatry. 2005;57(8):873–884. doi: 10.1016/j.biopsych.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RW, Menon RS. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol Psychiatry. 2002;52(4):305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- 17.Mazza M, Tempesta D, Pino MC, Catalucci A, Gallucci M, Ferrara M. Regional cerebral changes and functional connectivity during the observation of negative emotional stimuli in subjects with post-traumatic stress disorder. Eur Arch Psychiatry Clin Neurosci. 2013;263(7):575–583. doi: 10.1007/s00406-013-0394-3. [DOI] [PubMed] [Google Scholar]
- 18.McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44(1):1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- 19.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163(1):109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- 21.Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. 2012;14(1):29–37. doi: 10.31887/DCNS.2012.14.1/smorris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Mol Psychiatry. 2012;17(2):132–141. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oyarzun JP, Packard PA. Stress-induced gist-based memory processing: a possible explanation for overgeneralization of fear in posttraumatic stress disorder. J Neurosci. 2012;32(29):9771–9772. doi: 10.1523/JNEUROSCI.2318-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2012;36(9):2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 26.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106(2):274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 27.Qin S, Hermans EJ, van Marle HJ, Fernandez G. Understanding low reliability of memories for neutral information encoded under stress: alterations in memory-related activation in the hippocampus and midbrain. J Neurosci. 2012;32(12):4032–4041. doi: 10.1523/JNEUROSCI.3101-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23(25):8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Rauch SL, Shin LM, Whalen PJ, Pitman RK. Neuroimaging and the neuroanatomy of PTSD. CNS Spectr. 1998;3(Suppl 2):30–41. [Google Scholar]
- 31.Ruscio AM, Ruscio J, Keane TM. The latent structure of posttraumatic stress disorder: a taxometric investigation of reactions to extreme stress. J Abnorm Psychol. 2002;111(2):290–301. [PubMed] [Google Scholar]
- 32.Sadeh N, Spielberg JM, Heller W, Herrington JD, Engels AS, Warren SL, Miller GA. Emotion disrupts neural activity during selective attention in psychopathy. Soc Cogn Affect Neurosci. 2013;8(3):235–246. doi: 10.1093/scan/nsr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schonfeld S, Ehlers A, Bollinghaus I, Rief W. Overgeneral memory and suppression of trauma memories in post-traumatic stress disorder. Memory. 2007;15(3):339–352. doi: 10.1080/09658210701256571. [DOI] [PubMed] [Google Scholar]
- 34.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 35.Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Rauch SL. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 36.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 37.Spielberg JM, Miller GA, Warren SL, Engels AS, Crocker LD, Sutton BP, Heller W. Trait motivation moderates neural activation associated with goal pursuit. Cogn Affect Behav Neurosci. 2012;12(2):308–322. doi: 10.3758/s13415-012-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Kolk BA. The body keeps the score: memory and the evolving psychobiology of posttraumatic stress. Harv Rev Psychiatry. 1994;1(5):253–265. doi: 10.3109/10673229409017088. [DOI] [PubMed] [Google Scholar]
- 39.Vasterling JJ, Brailey K, Constans JI, Sutker PB. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12(1):125–133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- 40.Warren SL, Bost KK, Roisman GI, Silton RL, Spielberg JM, Engels AS, Heller W. Effects of adult attachment and emotional distractors on brain mechanisms of cognitive control. Psychol Sci. 2010;21(12):1818–1826. doi: 10.1177/0956797610388809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilker S, Kolassa IT. The formation of a neural fear network in Posttraumatic Stress Disorder: insights from molecular genetics. Clin Psychol Sci. 2013;1(4):452–469. [Google Scholar]