Abstract
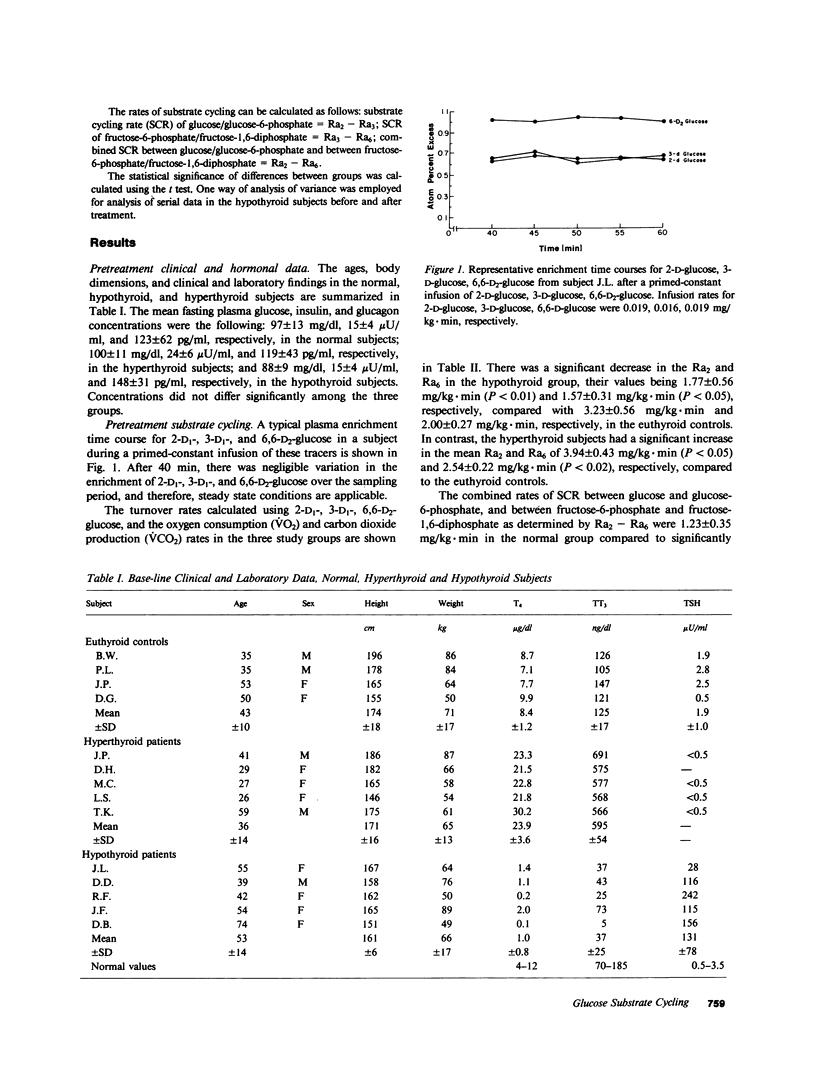
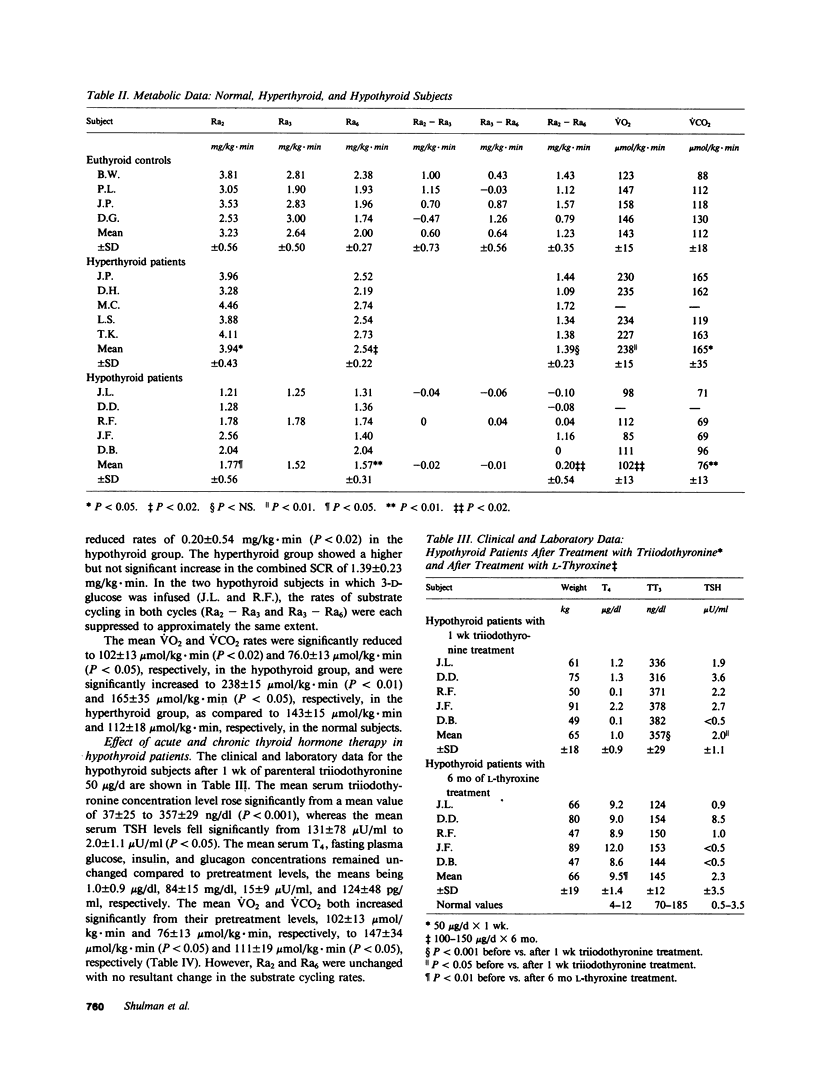
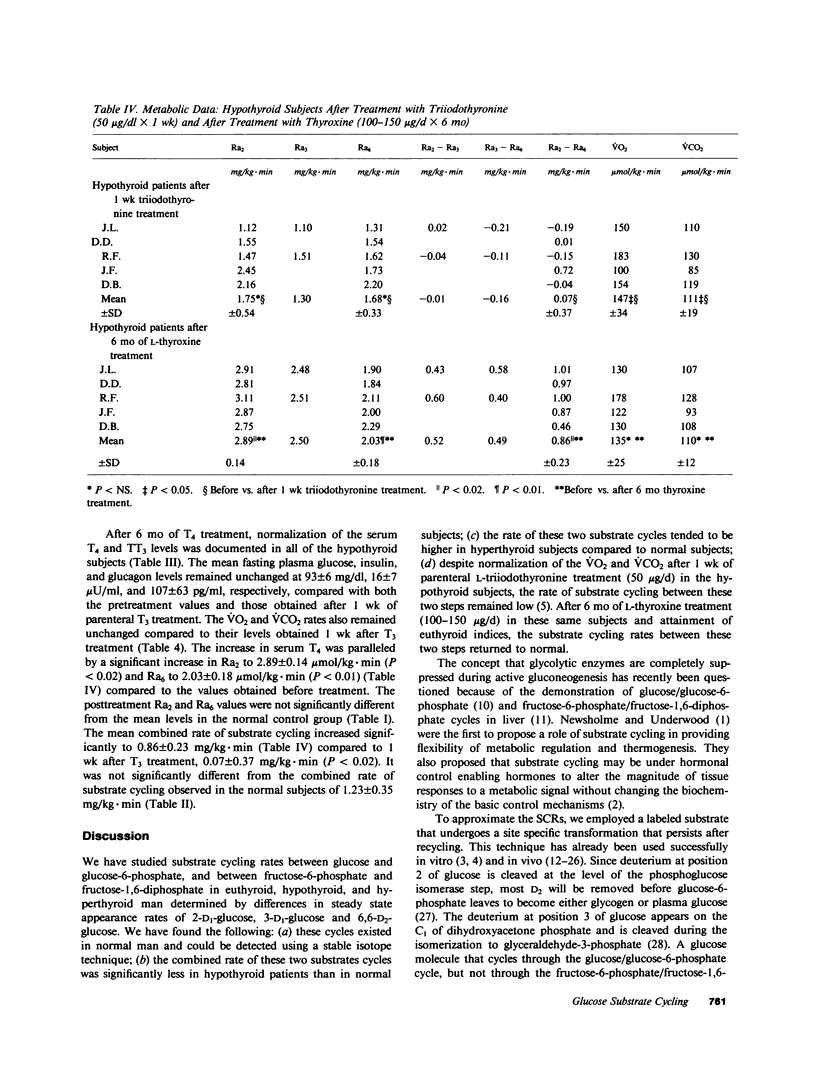
Substrate, or futile cycles, have been hypothesized to be under hormonal control, and important in metabolic regulation and thermogenesis. To define the role of thyroid hormones in the regulation of substrate cycling in glycolysis and gluconeogenesis, we measured rates of cycling in normal (n = 4), hypothyroid (n = 5), and hyperthyroid (n = 5) subjects employing a stable isotope turnover technique. Glucose labeled with deuterium at different positions (2-D1-, 3-D1-, and 6,6-D2-glucose) was given as a primed-constant infusion in tracer doses, and arterialized plasma samples were obtained and analyzed by gas-chromatography mass-spectrometry for the steady state enrichment of glucose that was labeled at the various positions. The rate of appearance (Ra) was then calculated for each isotopic tracer. The difference between the Ra determined by 2-D1-glucose (Ra2) and the Ra determined by 3-D1-glucose (Ra3) represents the substrate cycling rate (SCR) between glucose and glucose-6-phosphate. The difference between the Ra determined by 3-D1-glucose (Ra3) and the Ra determined by 6,6-D2-glucose (Ra6) represents the SCR between fructose-6-phosphate and fructose-1,6-diphosphate. The difference between Ra2 and Ra6 represents the combined SCR of both cycles. In normal subjects (serum thyroxine [T4] = 8.4 +/- 1.2 microgram/dl (all expressions, mean +/- SD), n = 4), the rates of appearance for Ra2, Ra3, and Ra6 were 3.23 +/- 0.56, 2.64 +/- 0.50, and 2.00 +/- 0.27 mg/kg X min, respectively, whereas those in the hypothyroid subjects (T4 = 1.0 +/- 0.8 microgram/dl; n = 5) were 1.77 +/- 0.56 (P less than 0.01), 1.52, 1.57 +/- 0.31 (P less than 0.05) mg/kg X min, respectively. Conversely, the rates of appearance for Ra2 and Ra6 in the hyperthyroid subjects (T4 = 23.9 +/- 3.6 micrograms/dl) were 3.94 +/- 0.43 (P less than 0.05) and 2.54 +/- 0.22 (P less than 0.02), respectively, compared with the normal subjects. On the basis of these data, we noted that the normal subjects had a combined SCR of 1.23 +/- 0.35 mg/kg X min. In contrast, the hypothyroid patients had a significantly decreased combined SCR, 0.20 +/- 0.54 mg/kg X min (P less than 0.02). The hyperthyroid patients had a combined SCR of 1.39 +/- 0.23 mg/kg X min (P less than NS). To determine whether these cycles responded to thyroid hormone treatment, these same hypothyroid subjects were acutely treated for 1 wk with parenteral 50 micrograms/d sodium L-triiodothyronine and chronically with 100-150 micrograms/d L-thyroxine. After 7 d, their mean oxygen consumption rate and carbon dioxide production rate increased significantly from 102+/-13 micromol/kg.min, to 147+/-34 micromol/kg.min (P<0.05), and from 76+/-13 micromol/kg.min to 111+/-19 micromol/kg.min (P<0.05), respectively. The combined SCR (Ra(2)--Ra(6) remained unchanged at 0.07+/-0.37 mg/kg.min. However, after 6 mo of oral L-thyroxine therapy (T(4)=9.5+/-1.4 microgram/kl) the treated hypothyroid patients had increased their combined SCR (Ra(2)--Ra(6)) to 0.86 +/-0.23 mg/kg.min (P<0.02), a value not significantly different from the combined SCR of normal subjects. We conclude that substrate cycling between glucose and glucose-6-phosphate and between fructose-6-phosphate and fructose-1,6-diphosphate occurs in man and is affected by thyroid hormone. Substrate cycles may represent a mechanism by which thyroid hormone alters the sensitivity of certain reactions to metabolic signals.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. N., Rabin D., Diamond M. P., Lacy W. W. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism. 1981 Sep;30(9):936–940. doi: 10.1016/0026-0495(81)90074-3. [DOI] [PubMed] [Google Scholar]
- Albano J. D., Ekins R. P., Maritz G., Turner R. C. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol (Copenh) 1972 Jul;70(3):487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- Altszuler N., Barkai A., Bjerknes C., Gottlieb B., Steele R. Glucose turnover values in the dog obtained with various species of labeled glucose. Am J Physiol. 1975 Dec;229(6):1662–1667. doi: 10.1152/ajplegacy.1975.229.6.1662. [DOI] [PubMed] [Google Scholar]
- Anwer M. S., Chapman T. E., Gronwall R. Glucose utilization and recycling in ponies. Am J Physiol. 1976 Jan;230(1):138–142. doi: 10.1152/ajplegacy.1976.230.1.138. [DOI] [PubMed] [Google Scholar]
- Battarbee H. D. The effects of thyroid state on rat liver glucose-6-phosphatase activity and glycogen content. Proc Soc Exp Biol Med. 1974 Nov;147(2):337–343. doi: 10.3181/00379727-147-38337. [DOI] [PubMed] [Google Scholar]
- Brady L. J., Romsos D. R., Leveille G. A. Glucose turnover in the young chicken (Gallus domesticus) using variously labeled (3H, U-14C) glucose tracers. Comp Biochem Physiol B. 1977;56(4):421–425. doi: 10.1016/0305-0491(77)90242-5. [DOI] [PubMed] [Google Scholar]
- Chenoweth M., Dunn A. Fructose-6-phosphate substrate cycling and hormonal regulation of gluconeogenesis in vivo. Am J Physiol. 1978 Sep;235(3):E295–E303. doi: 10.1152/ajpendo.1978.235.3.E295. [DOI] [PubMed] [Google Scholar]
- Clark D. G., Rognstad R., Katz J. Isotopic evidence for futile cycles in liver cells. Biochem Biophys Res Commun. 1973 Oct 1;54(3):1141–1148. doi: 10.1016/0006-291x(73)90811-5. [DOI] [PubMed] [Google Scholar]
- Clark D., Lee D., Rognstad R., Katz J. Futile cycles in isolated perfused rat liver and in isolated rat liver parenchymal cells. Biochem Biophys Res Commun. 1975 Nov 3;67(1):212–219. doi: 10.1016/0006-291x(75)90304-6. [DOI] [PubMed] [Google Scholar]
- Clark M. G., Bloxham D. P., Holland P. C., Lardy H. A. Estimation of the fructose 1,6-diphosphatase-phosphofructokinase substrate cycle and its relationship to gluconeogenesis in rat liver in vivo. J Biol Chem. 1974 Jan 10;249(1):279–290. [PubMed] [Google Scholar]
- Clark M. G., Bloxham D. P., Holland P. C., Lardy H. A. Estimation of the fructose diphosphatase-phosphofructokinase substrate cycle in the flight muscle of Bombus affinis. Biochem J. 1973 Jun;134(2):589–597. doi: 10.1042/bj1340589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. M., Glynn P., Shulman R. G. 13C NMR study of gluconeogenesis from labeled alanine in hepatocytes from euthyroid and hyperthyroid rats. Proc Natl Acad Sci U S A. 1981 Jan;78(1):60–64. doi: 10.1073/pnas.78.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. M., Ogawa S., Shulman R. G. 13C NMR studies of gluconeogenesis in rat liver cells: utilization of labeled glycerol by cells from euthyroid and hyperthyroid rats. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1603–1609. doi: 10.1073/pnas.76.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A., Chenoweth M. Fructose-6-phosphate substrate cycling and glucose and insulin regulation of gluconeogenesis in vivo. Am J Physiol. 1979 Apr;236(4):E410–E415. doi: 10.1152/ajpendo.1979.236.4.E410. [DOI] [PubMed] [Google Scholar]
- Dunn A., Katz J., Golden S., Chenoweth M. Estimation of glucose turnover and recycling in rabbits using various [3H, 14C]glucose labels. Am J Physiol. 1976 Apr;230(4):1159–1162. doi: 10.1152/ajplegacy.1976.230.4.1159. [DOI] [PubMed] [Google Scholar]
- Hetenyi G., Jr, Mak D. 3H-2-glucose in turnover studies. Can J Physiol Pharmacol. 1970 Oct;48(10):732–734. doi: 10.1139/y70-105. [DOI] [PubMed] [Google Scholar]
- Hue L., Hers H. G. On the use of (3H, 14C)labelled glucose in the study of the so-called "futile cycles" in liver and muscle. Biochem Biophys Res Commun. 1974 Jun 4;58(3):532–539. doi: 10.1016/s0006-291x(74)80453-5. [DOI] [PubMed] [Google Scholar]
- Hue L. The role of futile cycles in the regulation of carbohydrate metabolism in the liver. Adv Enzymol Relat Areas Mol Biol. 1981;52:247–331. doi: 10.1002/9780470122976.ch4. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Allen M., Borkow I. Estimation of glucose turnover in the dog with glucose-2-T and glucose-U- 14 C. Am J Physiol. 1972 Mar;222(3):710–712. doi: 10.1152/ajplegacy.1972.222.3.710. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr Studies on hepatic glucose cycles in normal and methylprednisolone-treated dogs. Metabolism. 1977 Feb;26(2):157–170. doi: 10.1016/0026-0495(77)90051-8. [DOI] [PubMed] [Google Scholar]
- Katz J., Dunn A., Chenoweth M., Golden S. Determination of synthesis, recycling and body mass of glucose in rats and rabbits in vivo 3H-and 14C-labelled glucose. Biochem J. 1974 Jul;142(1):171–183. doi: 10.1042/bj1420171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Dunn A. Glucose-2-t as a tracer for glucose metabolism. Biochemistry. 1967 Jan;6(1):1–5. doi: 10.1021/bi00853a001. [DOI] [PubMed] [Google Scholar]
- Katz J., Golden S., Dunn A., Chenoweth M. Estimation of glucose turnover in rats in vivo with tritium labeled glucoses. Hoppe Seylers Z Physiol Chem. 1976 Dec;357(10):1387–1394. doi: 10.1515/bchm2.1976.357.2.1387. [DOI] [PubMed] [Google Scholar]
- Katz J., Rognstad R. Futile cycles in the metabolism of glucose. Curr Top Cell Regul. 1976;10:237–289. doi: 10.1016/b978-0-12-152810-2.50013-9. [DOI] [PubMed] [Google Scholar]
- McCulloch A. J., Nosadini R., Pernet A., Piniewska M., Cook D. B., Clark F., Johnston D. G., Alberti K. G. Glucose turnover and indices of recycling in thyrotoxicosis and primary thyroid failure. Clin Sci (Lond) 1983 Jan;64(1):41–47. doi: 10.1042/cs0640041. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B. Substrate cycles in metabolic regulation and in heat generation. Biochem Soc Symp. 1976;(41):61–109. [PubMed] [Google Scholar]
- Newsholme E. A., Gevers W. Control of glycolysis and gluconeogenesis in liver and kidney cortex. Vitam Horm. 1967;25:1–87. doi: 10.1016/s0083-6729(08)60033-3. [DOI] [PubMed] [Google Scholar]
- Okajima F., Ui M. Metabolism of glucose in hyper- and hypo-thyroid rats in vivo. Glucose-turnover values and futile-cycle activities obtained with 14C- and 3H-labelled glucose. Biochem J. 1979 Aug 15;182(2):565–575. doi: 10.1042/bj1820565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARLE G. L., STRISOWER E. H., CHAIKOFF I. L. Glucose pool and glucose space in the normal and diabetic dog. Am J Physiol. 1954 Feb;176(2):190–194. doi: 10.1152/ajplegacy.1954.176.2.190. [DOI] [PubMed] [Google Scholar]
- Saunders J., Hall S. E., Sönksen P. H. Glucose and free fatty acid turnover in thyrotoxicosis and hypothyroidism, before and after treatment. Clin Endocrinol (Oxf) 1980 Jul;13(1):33–44. doi: 10.1111/j.1365-2265.1980.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Stevenson R. W., Parsons J. A., Alberti K. G. Effect of intraportal and peripheral insulin on glucose turnover and recycling in diabetic dogs. Am J Physiol. 1983 Feb;244(2):E190–E195. doi: 10.1152/ajpendo.1983.244.2.E190. [DOI] [PubMed] [Google Scholar]
- Surholt B., Newsholme E. A. The rate of substrate cycling between glucose and glucose 6-phosphate in muscle and fat-body of the hawk moth (Acherontia atropos) at rest and during flight. Biochem J. 1983 Jan 15;210(1):49–54. doi: 10.1042/bj2100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tserng K. Y., Kalhan S. C. Estimation of glucose carbon recycling and glucose turnover with [U-13C] glucose. Am J Physiol. 1983 Nov;245(5 Pt 1):E476–E482. doi: 10.1152/ajpendo.1983.245.5.E476. [DOI] [PubMed] [Google Scholar]
- Winnick S. Response of hepatic glucokinase and glucose-6-phosphatase activities in juvenile and adult hyperthyroid mice. Endocrinology. 1970 Jul;87(1):124–128. doi: 10.1210/endo-87-1-124. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., O'Donnell T. F., Jr, Stone M. D., Richmand D. A., Burke J. F. Investigation of factors determining the optimal glucose infusion rate in total parenteral nutrition. Metabolism. 1980 Sep;29(9):892–900. doi: 10.1016/0026-0495(80)90130-4. [DOI] [PubMed] [Google Scholar]


