Abstract
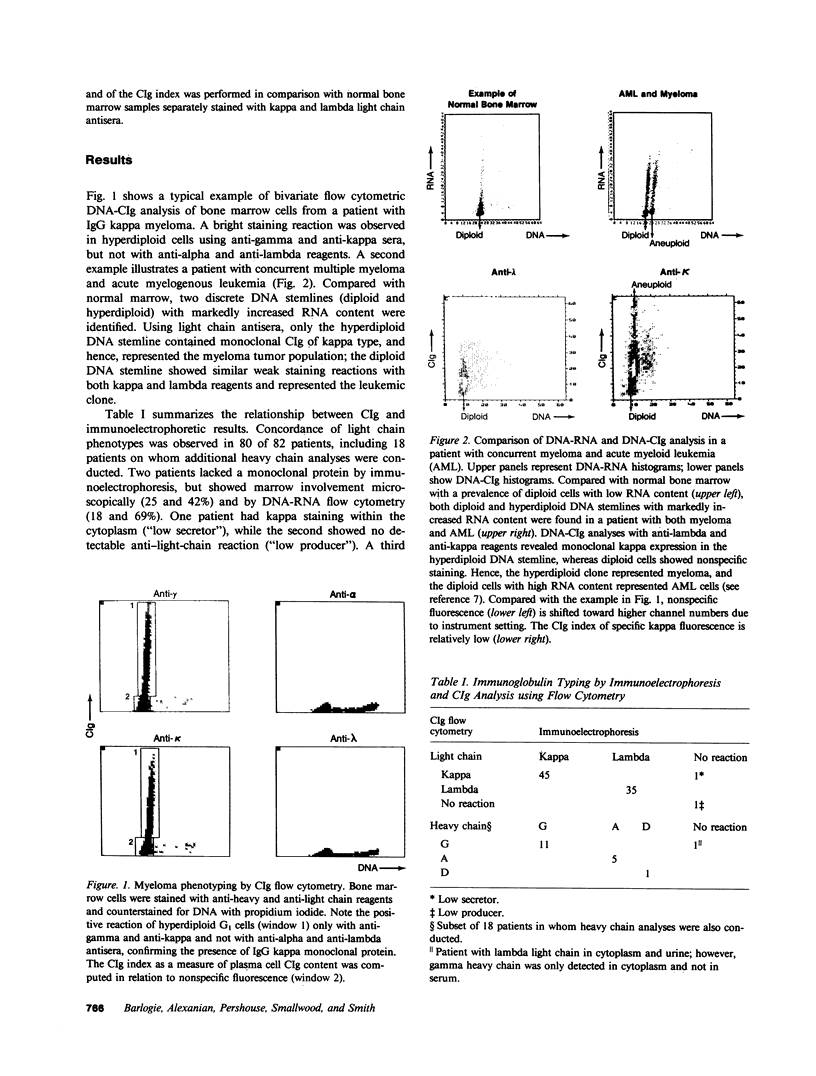
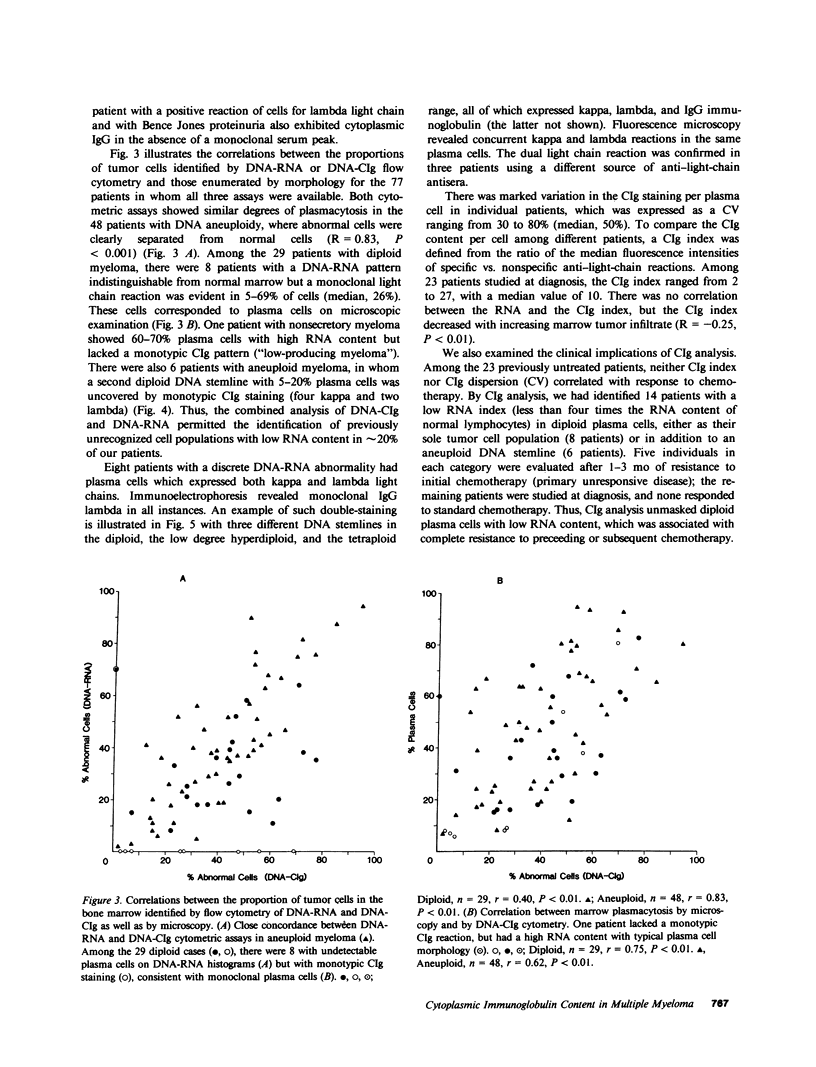
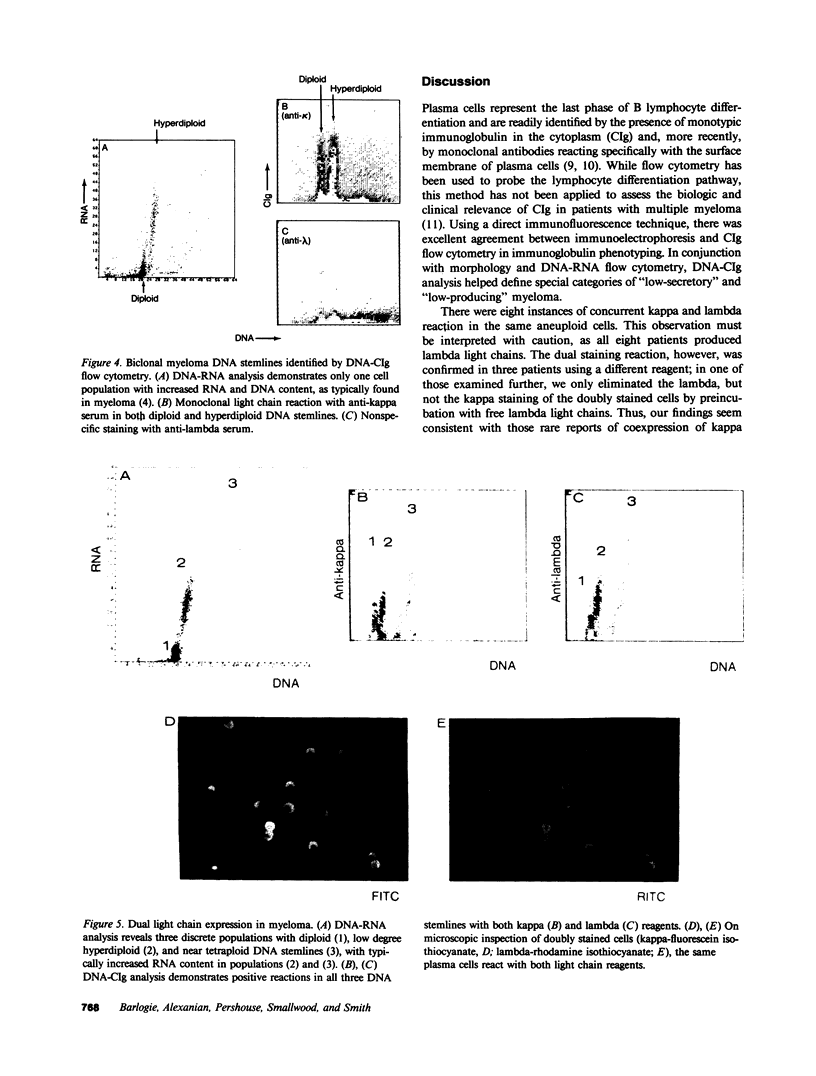
Bone marrow cells of 82 patients with multiple myeloma were subjected to flow cytometric analysis of DNA and cytoplasmic immunoglobulin (CIg) content using propidium iodide and direct immunofluorescence assays. Except for two patients with nonsecretory myeloma, there was conformity in the immunoglobulin type derived from immunoelectrophoresis and plasma cell CIg staining. One patient with nonsecretory myeloma exhibited monotypic CIg staining, while the second showed no reaction. In eight patients with IgG lambda myeloma, the same tumor cells contained both lambda and kappa light chains, suggesting the productive rearrangement of both light chain genes. 14 patients with previously unrecognized plasma cells of low RNA content, all of whom were resistant to chemotherapy, were identified by CIg staining. By revealing previously unrecognized plasma cells with low RNA content, CIg analysis identified more patients with treatment-refractory myeloma.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. C., Park E. K., Bates M. P., Leonard R. C., Hardy R., Schlossman S. F., Nadler L. M. Antigens on human plasma cells identified by monoclonal antibodies. J Immunol. 1983 Mar;130(3):1132–1138. [PubMed] [Google Scholar]
- Barlogie B., Alexanian R., Gehan E. A., Smallwood L., Smith T., Drewinko B. Marrow cytometry and prognosis in myeloma. J Clin Invest. 1983 Sep;72(3):853–861. doi: 10.1172/JCI111056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlogie B., Latreille J., Freireich E. J., Fu C. T., Mellard D., Meistrich M., Andreeff M. Characterization of hematologic malignancies by flow cytometry. Blood Cells. 1980;6(4):719–744. [PubMed] [Google Scholar]
- Barlogie B., Raber M. N., Schumann J., Johnson T. S., Drewinko B., Swartzendruber D. E., Göhde W., Andreeff M., Freireich E. J. Flow cytometry in clinical cancer research. Cancer Res. 1983 Sep;43(9):3982–3997. [PubMed] [Google Scholar]
- Barlogie B., Smith L., Alexanian R. Effective treatment of advanced multiple myeloma refractory to alkylating agents. N Engl J Med. 1984 May 24;310(21):1353–1356. doi: 10.1056/NEJM198405243102104. [DOI] [PubMed] [Google Scholar]
- Bouvet J. P., Buffe D., Oriol R., Liacopoulos P. Two myeloma globulins IgG1-kappa and IgG1-lambda, from a single patient (Im). II. Their common cellular origin as revealed by immunofluorescence studies. Immunology. 1974 Dec;27(6):1095–1101. [PMC free article] [PubMed] [Google Scholar]
- Bunn P. A., Jr, Krasnow S., Makuch R. W., Schlam M. L., Schechter G. P. Flow cytometric analysis of DNA content of bone marrow cells in patients with plasma cell myeloma: clinical implications. Blood. 1982 Mar;59(3):528–535. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Choi Y. J., Wong M. S. Double light-chain production by leukemic cells of common clonal origin: a case report with review of pertinent literature. Am J Hematol. 1981;11(1):93–98. doi: 10.1002/ajh.2830110111. [DOI] [PubMed] [Google Scholar]
- Crissman H. A., Mullaney P. F., Steinkamp J. A. Methods and applications of flow systems for analysis and sorting of mammalian cells. Methods Cell Biol. 1975;9(0):179–246. doi: 10.1016/s0091-679x(08)60076-x. [DOI] [PubMed] [Google Scholar]
- Goldie J. H., Coldman A. J. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat Rep. 1979 Nov-Dec;63(11-12):1727–1733. [PubMed] [Google Scholar]
- Hieter P. A., Korsmeyer S. J., Waldmann T. A., Leder P. Human immunoglobulin kappa light-chain genes are deleted or rearranged in lambda-producing B cells. Nature. 1981 Apr 2;290(5805):368–372. doi: 10.1038/290368a0. [DOI] [PubMed] [Google Scholar]
- Hopper J. E. Comparative studies on monotypic IgM lambda and IgG kappa from an individual patient. IV. Immunofluorescent evidence for a common clonal synthesis. Blood. 1977 Aug;50(2):203–211. [PubMed] [Google Scholar]
- Korsmeyer S. J., Hieter P. A., Ravetch J. V., Poplack D. G., Waldmann T. A., Leder P. Developmental hierarchy of immunoglobulin gene rearrangements in human leukemic pre-B-cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7096–7100. doi: 10.1073/pnas.78.11.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Argüelles G. J., Katzmann J. A., Greipp P. R., Gonchoroff N. J., Garton J. P., Kyle R. A. Multiple myeloma: circulating lymphocytes that express plasma cell antigens. Blood. 1984 Aug;64(2):352–356. [PubMed] [Google Scholar]
- Zeile G. Intracytoplasmic immunofluorescence in multiple myeloma. Cytometry. 1980 Jul;1(1):37–41. doi: 10.1002/cyto.990010109. [DOI] [PubMed] [Google Scholar]