Abstract
Zebra Finches (Taeniopygia guttata) are the most commonly used laboratory songbird species, yet their embryological development has been poorly characterized. Most studies to date apply Hamburger and Hamilton stages derived from chicken development; however, significant differences in development between precocial and altricial species suggest that they may not be directly comparable. We provide the first detailed description of embryological development in the Zebra Finch under standard artificial incubation. These descriptions confirm that some of the features used to classify chicken embryos into stages are not applicable in an altricial bird such as the Zebra Finch. This staging protocol will help to standardize future studies of embryological development in the Zebra Finch. J. Morphol. 274:1090–1110, 2013. © 2013 The Authors. Journal of Morphology Published by Wiley Periodicals, Inc.
Keywords: artificial incubation, embryological development, passerine bird, staging, Zebra Finch
INTRODUCTION
In recent years, Zebra Finches (Taeniopygia guttata) have become the predominant laboratory model species for songbird studies and have been used to address questions in a wide array of fields, including behavior (Maney and Goodson, 2011; Rosa et al., 2012), memory and learning (Moorman et al., 2011; Mayer et al., 2012), reproduction (Seguin and Forstmeier, 2012), aging (Austad, 2011; Heidinger et al., 2012), toxicology (Eng et al., 2012), neuroanatomy (Vargha-Khadem et al., 2005), and plasticity and evolution (Chen et al., 2012; Keary and Bischof, 2012; Pytte et al., 2012). Notably, similarities in the mechanisms of song learning in the Zebra Finch and speech learning in humans make this bird a preeminent model system for studying the development of speech (Jarvis, 2004; Bottjer and To, 2012; Moorman et al., 2012; Walton et al., 2012; Winograd and Ceman, 2012). Zebra Finches are easily kept in captivity and breed rapidly and prolifically, thus providing a ready supply of study subjects. Additionally, the genome of the Zebra Finch was recently sequenced, making it only the second bird (after the chicken, Gallus gallus domesticus) and first songbird to have its genome fully sequenced (Warren et al., 2010). This new resource has facilitated the development of valuable genetic and genomic tools for use in this species (Clayton et al., 2009; Srivastava et al., 2012). Given these advantages, there is increasing interest in utilizing the Zebra Finch for questions involving early development (Godsave et al., 2002; Perlman and Arnold, 2003; Perlman et al., 2003; Olson et al., 2006; Olson et al., 2008). However, to address these questions, it is essential to have a detailed and standardized knowledge of the progression of embryological development from fertilization to hatching in the Zebra Finch.
To date, most avian embryological studies have been in the chicken and have used Hamburger and Hamilton’s (1951) article on chicken development. This classic article has been the main source of embryo staging information for nearly all species of birds for over 60 years and has provided the foundation for virtually all subsequent embryo staging work on precocial birds including the Wood Duck (Aix sponsa, Montgomery et al., 1978), Canada Goose (Branta Canadensis, Cooper and Batt, 1972), Northern Bobwhite (Colinus virginianus, Hendrickx and Hanzlik, 1965), Ring-Necked Pheasant (Phasianus colchicus, Fant, 1957), and Domestic Turkey (Meleagris gallopavo, Mun and Kosin,1960), as well as the semi-precocial Adelie Penguin (Pygoscelis adeliae, Herbert, 1967). However, the progress of development can be quite different between commonly studied precocial species, such as chickens and altricial species such as songbirds (Ricklefs and Starck, 1998; Blom and Lilja, 2005). Despite the fact that more than half of all bird species are altricial songbirds, such as the Zebra Finch, relatively few reports have described the progress of embryological development in altricial species. Currently there are studies on the semi-altricial Double-crested Cormorant (Phalacrocorax auritus, Powell et al., 1998), American Kestral (Falco sparverius, Bird et al., 1984), Barn Owl (Tyto alba, Koppl et al., 2005), Rock Dove (Columba livia, Olea and Sandoval, 2012) and the altricial Red-winged Blackbird (Agelaius phoeniceus, Daniel, 1957) and Society Finch (Lonchura striata domestica, Yamasaki and Tonosaki, 1988).
While existing developmental staging studies of altricial and semi-altricial species provide a very useful baseline, many are problematic in that they measure development in 3-day intervals (e.g., Bird et al., 1984), have a low sample size (e.g., Koppl, 2005), and show few or no pictures (e.g., Daniel, 1957), often without adequate labeling (e.g., Yamasaki and Tonosaki, 1988). Moreover, there are currently no staging guides specific for the Zebra Finch. Embryological studies in the Zebra Finch use a combination of incubation length and approximated Hamburger–Hamilton (hereafter “HH”) staging to describe embryos (Chen et al., 2012). While the HH and the Society Finch (Yamasaki and Tonosaki, 1988) study provide an important foundation for staging studies, the detailed description of the progression of development presented here for the Zebra Finch will help to standardize embryological staging among different studies and improve repeatability. This will also allow researchers interested in developmental processes in the Zebra Finch to more easily identify incubation lengths that are required to achieve particular developmental stages.
MATERIALS AND METHODS
Location and Duration of Study
All research for this study was conducted between December 2009 and March 2013 on the campus of The College of William and Mary in Williamsburg, VA.
Animal Care
Unrelated Zebra Finch pairs were housed at 22°C on a 14:10 light:dark cycle in pairs in indoor cages. The pairs were provided with a seed blend (Avian Science Super Finch, Volkman Seed Company, Ceres, CA) and vitamin-enriched water (Vita-Sol Ultravite Multivitamin Supplement, 8 in 1 Pet Products, Islandia, NY) ad libitum. A hanging nesting box and ad libitum hay for nesting material were placed in each cage. All animal care was approved by The College of William and Mary IACUC and adhered to all federal legal requirements.
Egg Collection and Incubation
Captive Zebra Finches usually lay one egg per day in the 3 h after the lights come on in the morning. All eggs were collected approximately 3 h after lights came on. Upon collection, eggs were labeled using a soft graphite pencil with the date of laying and pair number. Eggs were immediately placed within a mechanical incubator designed for chicken eggs (Picture Window Hova-Bator Incubator, Circulated Air Model No.1583 28.3 Watts 115 Volt AC, G.Q.F MFG CO, Savannah GA 31402-1552). Incubators were maintained at 37.5 +/− 1°C and 80–95% humidity. Temperature varied within this range which mimics variation during natural incubation (Gorman et al., 2005). Eggs were placed in a small padded dish on the rotating bars of the incubator. The tilting motion designed to rotate a chicken egg allowed the Zebra Finch eggs to roll within the dish and prevented embryonic adhesion to the shell interior. Incubation in this manner closely approximated normal development with parental care, in which females roll eggs periodically and maintain high temperature and humidity in nest boxes. Eggs in the incubator hatched in 14 days (n = 15), which is the same length of time required for a Zebra Finch egg to hatch under natural incubation.
Dissection
Eggs were dissected at two or three hour increments beginning at 6 h of incubation until the fifth day of incubation. At that point, eggs were dissected at 6 h increments to reflect the reduced rate of development. Eggs were dissected at room temperature on coated weigh paper using a dissecting scope (Olympus SZ61) with fiber optic illuminator lamps (Dolan-Jenner Industries Fiber-Lite MI-150 High Intensity Illuminator). Eggs were candled by lighting from behind using the illuminator prior to dissection. After the second day of incubation, embryological development can be clearly seen through the eggshell and candling allowed us to cut into the egg without damaging the embryo or associated membranes. Shells were cut longitudinally with a sharp scalpel and spread with fine tipped forceps to allow the contents of the egg to flow out onto a clean paper. In eggs that had been incubated between 1 and 4 days, the yolk, embryo, and all membranes can be removed intact using this method. In eggs incubated between 5 and 7 days, the embryo and associated membranes often adhered to the interior of the shell and were carefully removed with forceps. In older eggs, the embryo, yolk, and membranes become less adherent and the shell can be easily peeled away without damage to the structures. In earlier stages, during the first two days of development, a scalpel was used to cut away the yolk membrane from the embryonic disk. In all older dissections, fine-tipped forceps were used to separate the embryo from other egg contents. The amniotic membrane was carefully removed to obtain clearer images of the embryo. For dissections of embryos less than 4-days old, nonsterile 1X PBS (phosphate buffered saline solution) was used to gently wash the embryo free of yolk. These embryos were left attached to the vitelline membrane for ease of imaging. In embryos 4-days old or older, the yolk sack and vitelline membrane were removed. The allantois was also removed in older embryos when it was large enough to obscure other anatomy.
Imaging
Once cleared and freed of extraneous membranes, embryos were submerged in 1X PBS in a glass petri dish. This is in contrast to Hamburger and Hamilton (1951) in which embryos were imaged in formalin which can shrink or distort morphology. Embryos were imaged on a black background with a small amount of water beneath the petri dish to reduce shadowing and provide a darker and more uniform background. Images were taken in original “.tif” format using an Olympus SZH10 Research Stereo Microscope and OPELCO illuminator in conjunction with DPController and DPManager software. All images were taken at the highest possible magnification at which the entire embryo was visible in a single field of view. This magnification ranged from 100 to 16 X. Lengths were manually measured along the dorsal outline of the embryo from full size images within Adobe Photoshop. The length was determined by measuring the embryo from the telencephalon to the tip of the tail bud and scaled appropriately for each magnification setting. Images were annotated in Adobe Photoshop. Anatomical terminology was derived from a variety of developmental atlases (Johnson and Volpe, 1973; Eichler, 1978; Lehman, 1983; Watterson and Schoenwolf, 1984; Schoenwolf, 2001).
Numbers of Embryos Used for the Study
Our study encompasses data from 1063 Zebra Finch embryos. Due to the nature of our study, different data sets had to be gathered from different embryos, with the collection of some data precluding collection of later data from a single individual (i.e., an egg could not be dissected and photographed twice). Thus, 681 embryos were used for the generation of staging protocols for days 0–13; 15 Zebra Finch chicks were hatched in artificial conditions in an incubator; 379 Zebra Finch chicks were hatched in natural conditions from parental incubation; and 300 eggs were weighed (wet weight) at the day of laying. For all stages, 10–44 embryos were evaluated for developmental progress at that stage, with the exception of stages 41–46 for which <10 embryos were evaluated.
RESULTS
Stage 1: Prestreak (0–20 h of development, Fig. 1): The primitive shield is opaque and well defined. A thickened area is apparent at the posterior border of the area pellucida after the first 12–15 h of incubation. The development of the embryonic shield is comparable to the Society Finch and the chicken.
Figure 1.
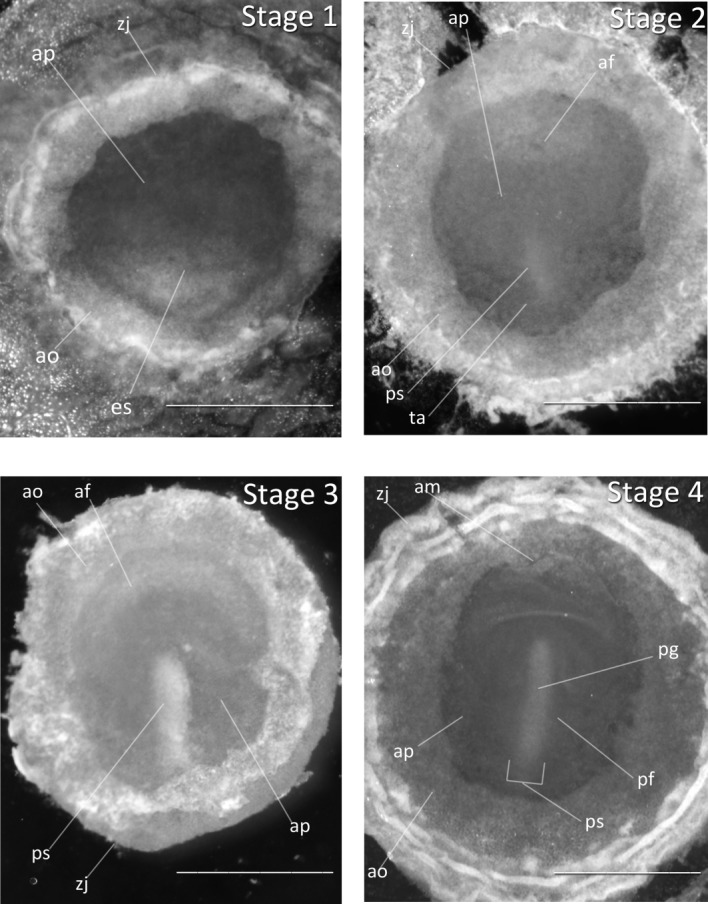
Stages 1–4: All embryos oriented with anterior region at top of image. Scale bar = 1 mm. Stage 1 (0–20 h). Stage 2 (20–22 h). Stage 3 (22 h). Stage 4 (24 h). Abbreviations: af, amniotic fold; am, anterior margin of mesoderm; ao, area opaca; ap, area pellucida; es, embryonic shield; pf, primitive fold, lateral lip; pg, primitive groove, blastopore; ps, primitive streak; ta, thickened area; zj, zone of junction.
Stage 2: Initial Streak (20–22 h, Fig. 1): The amniotic fold is visible. The initial streak is not cone-shaped as in the Society Finch or the chicken, but is short and faint. It appears significantly later than in the chicken, indicating that a greater duration of time is needed for cell migration to occur.
Stage 3: Intermediate Streak (22 h, Fig. 1): The area pellucida is now a teardrop shape. The amniotic fold is more opaque. The primitive streak has thickened and is extended halfway across the length of the area pellucida. The primitive pit and primitive grooves are not distinguishable.
Stage 4: Definitive Streak (24 h, Fig. 1): The primitive streak has reached its maximum length at three-quarters of the total length of area pellucida. The primitive groove and primitive pit are visible. Hensen’s node is not yet visible in Figure 1, but we predict Henson’s node to be present and visible in some embryos of this time point.
Stage 5: Head Process (30 h, Fig. 2): A condensed mesodermal rod has migrated from the anterior of the primitive streak towards the anterior margin to form the head process, or notochord. Hensen’s node is visible between the primitive streak and head process. The posterior region of the embryo is pinched in appearance.
Figure 2.
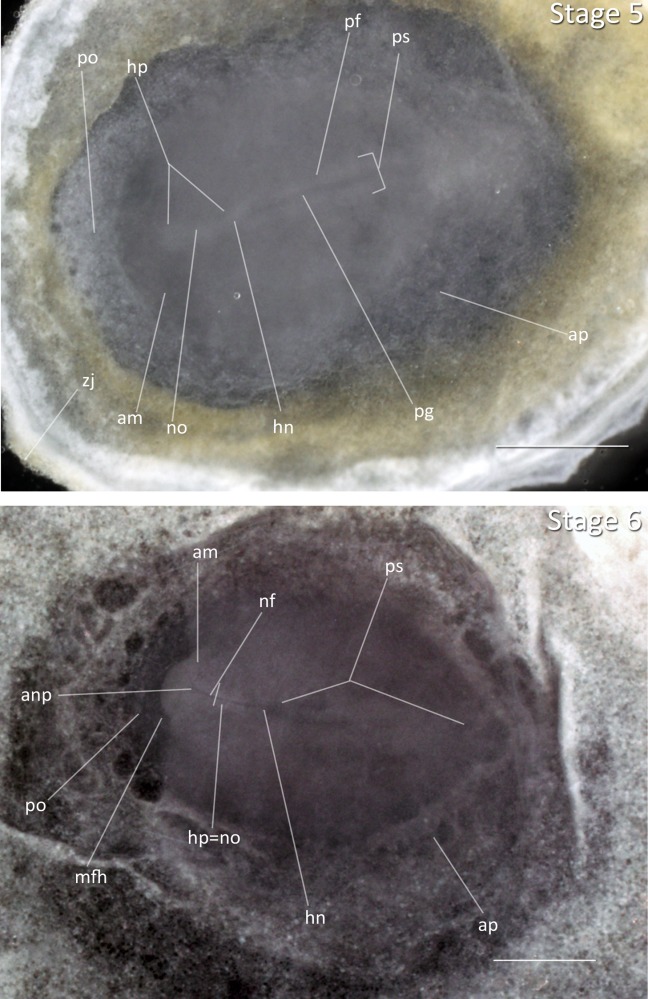
Stages 5 and 6: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 5 (30 h). Stage 6 (36 h). Abbreviations: am, anterior margin of mesoderm; anp, anterior neural pore; ap, area pellucida; hn, Hensen’s node; hp, head process; mfh, margin of free head; nf, neural fold; no, notochord, notochord mesoderm; pf, primitive fold, lateral lip; pg, primitive groove, blastopore; po, proamnion, anterior blastopore; ps, primitive streak; zj, zone of junction. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Stage 6: Neural Fold (36 h, Fig. 2): The neural folds have formed and the notochord has migrated anteriorly from Hensen’s node. No somites are visible. The neural folds appear longer in the Zebra Finch than the chicken, and the head folds are not clearly visible as in chicken development. The presence of neural folds is a better indicator for this stage than the appearance of the head fold.
Stage 7: One to Three Somites (∼38 h, Fig. 3): A single somite is present. Neural folds are thick and easily distinguished. The second and third somites appear within an hour.
Figure 3.
Stages 7 and 8: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 7 (38 h). Two somites. Embryos may have one to three somites visible. Stage 8 (40 h). Four to six somites. Abbreviations: aip, anterior intestinal portal; am, anterior margin of mesoderm; anp, anterior neural pore; ap, area pellucida; bi, blood islands; hf, head fold; hm, head mesoderm; hn, Hensen’s node; lbf, lateral body fold; mfh, margin of free head; mm, mesomere, nephrotome, intermediate mesoderm; nf, neural fold; no, notochord, notochord mesoderm; nt, neural tube; onp, open neural plate; po, proamnion; ps, primitive streak; si, sinus terminalis (circular vein of yolk sac); sm, somite (epimere) mesoderm; usm, unsegmented somite mesoderm; vas, area vasculosa; vit, area vitellina.
Stage 8: Four to Six Somites (40 h, Fig. 3): The neural folds have fused at midbrain. The anterior neuropore is open and visible. The neural folds remain open in the center of the developing foregut. The notochord is visible and blood islands are present in the posterior region of the blastoderm.
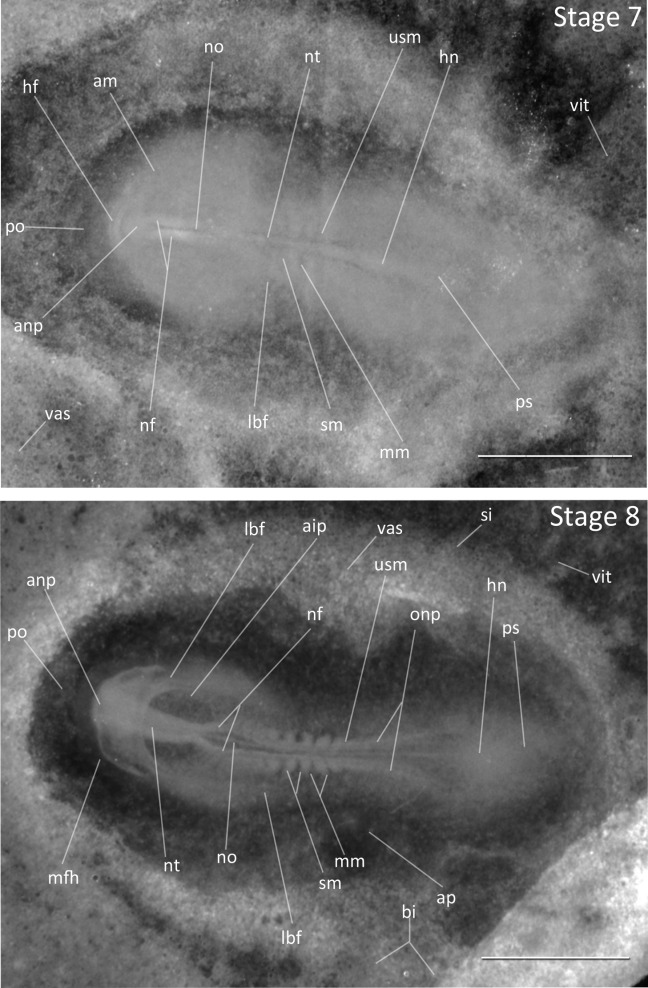
Stage 9: Seven Somites (42 h, Fig. 4): The embryo has narrowed and Hensen’s node is faint but present in the posterior region of the embryo. The forebrain, midbrain, and hindbrain are separated by visible constrictions. This occurs at the 10 somites stage in the chicken. The primary optic vesicles protrude on the side of the prosencephalon and are visible by slight darkening.
Figure 4.
Stages 9 and 10: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 9 (42 h). Seven somites. Stage 10 (2 days). 10 somites. Abbreviations: aip, anterior intestinal portal; amm, anterior margin of invaginated lateral mesoderm; anp, anterior neural pore; ao, area opaca; ap, area pellucida; bi, blood islands; fb, forebrain; fgl, foregut lumen; hb, hindbrain; hm, head mesoderm; hn, Hensen’s node; lbf, lateral body fold mesoderm; mb, midbrain; mfh, margin of free head; mm, mesomere; nf, neural fold; nh, rhombomeres of hindbrain; no, notochord; nt, neural tube; onp, open neural plate; op, optic vesicle; ps, primitive streak; si, sinus terminalis; sm, somite mesoderm; usm, unsegmented somite mesoderm; v, ventricle of heart; vas, area vasculosa; vit, area vitellina; vv, vitelline vein; zj, zone of junction.
Stage 10: 10 Somites (2 days, Fig. 4): The optic vesicles have developed laterally, but are not constricted at their base. The rhombomeres of hindbrain are distinguishable earlier than in the chicken, but all five are not visible at this stage. Cranial flexure has begun and head is slightly tilted to the right. When viewed ventrally, the heart is bent to right of embryo. The heart is not yet visible from dorsal perspective.
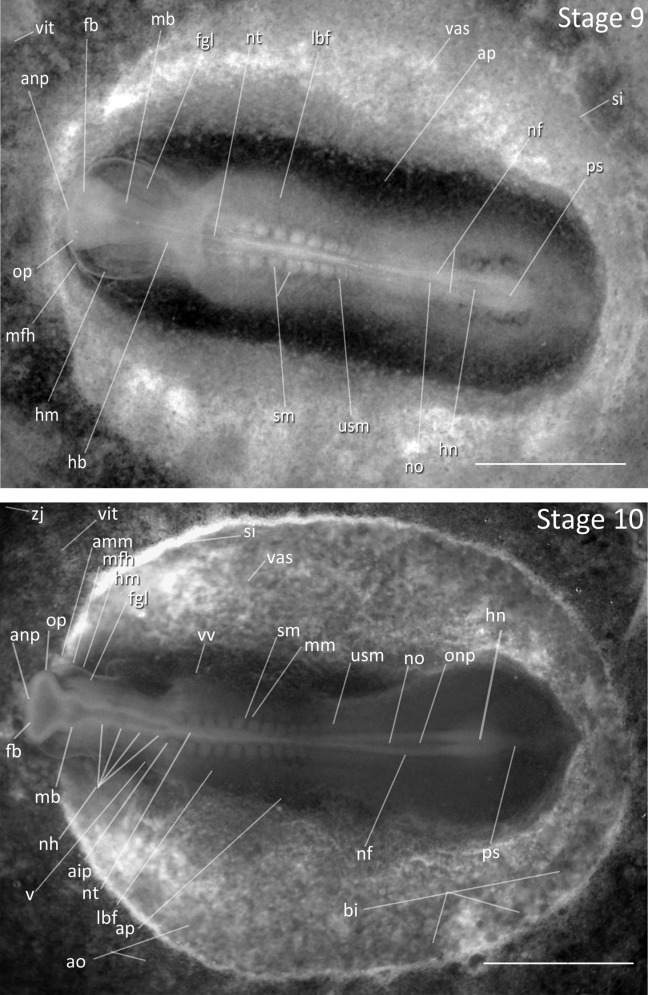
Stage 11: 13 Somites (2 ¼ days, Fig. 5): Five rhombomeres of the hindbrain are visible. The optic vesicles are constricted at their bases. The anterior neuropore is nearly closed.
Figure 5.
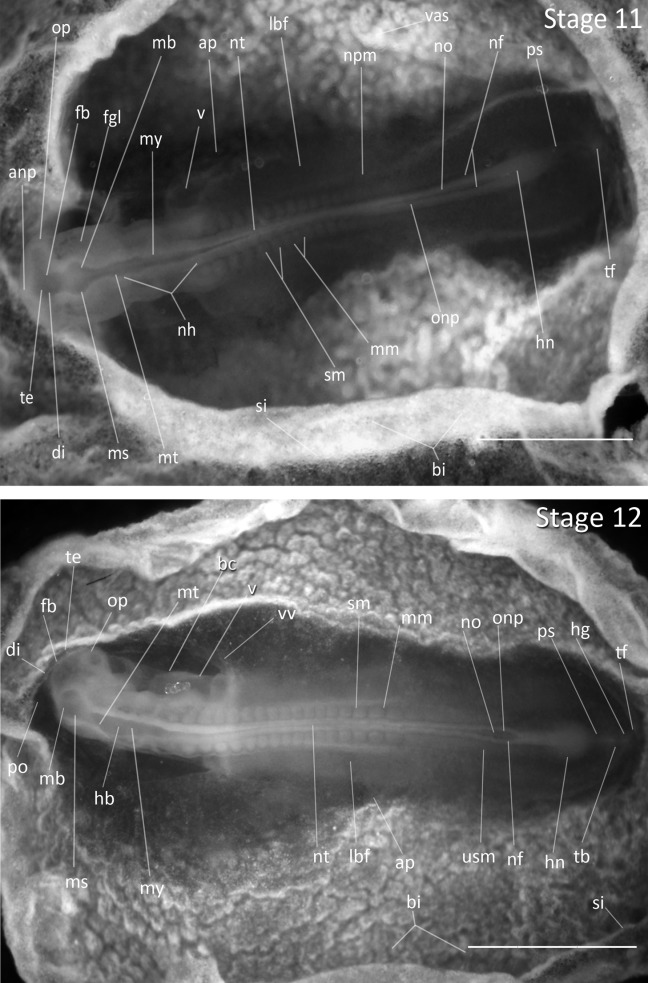
Stages 11 and 12: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 11 (2 ¼ days). Stage 12 (2 ⅓ days). Abbreviations: anp, anterior neural pore; ap, area pellucida; bc, bulbus cordis; bi, blood islands; di, diencephalon; fb, forebrain; fgl, foregut lumen; hb, hindbrain; hg, hindgut lumen; hn, Hensen’s node; lbf, lateral body fold mesoderm; mb, midbrain; mm, mesomere; ms, mesencephalon; mt, metencephalon; my, myelencephalon; nf, neural fold; nh, rhombomeres of hindbrain; no, notochord; npm, nephrogenic mesoderm; nt, neural tube; onp, open neural plate; op, optic vesicle; po, proamnion; ps, primitive streak; si, sinus terminalis; sm, somite mesoderm; tb, tailbud; te, telencephalon; tf, tail fold; usm, unsegmented somite mesoderm; v, ventricle of heart; vas, area vasculosa; vv, vitelline vein.
Stage 12: 16 Somites (2 ⅓ days, Fig. 5): The ventricle and bulbus cordis of heart are bent to the right. The head is turned slightly to the right. The optical stalks are well established.
Stage 13: 19 Somites (2 ½ days, Fig. 6): Cranial flexion and rotation has increased so that the anterior portion of embryo is turned to the right. The optic vesicles are distinct as a darkened bulge framing the telencephalon. The telencephalon has undergone significant expansion from the previous stage. The head fold has progressed posteriorly to fully cover the hindbrain. The atrioventricular canal is constricted. The otic vesicles are first visible bilaterally below the hindbrain, forming the early inner ear openings.
Figure 6.
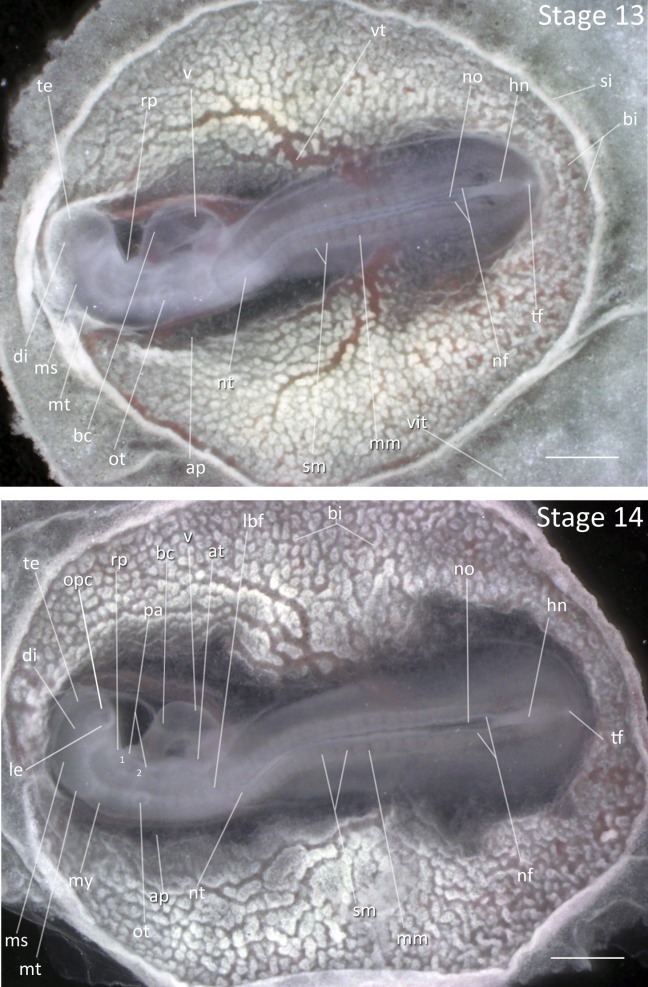
Stages 13 and 14: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 13 (2 ½ days). Stage 14 (2 ⅔ days). Abbreviations: ap, area pellucida; at, atrium of heart; bc, bulbus cordis; bi, blood islands; di, diencephalon; hn, Hensen’s node; lbf, lateral body of foregut; le, lens vesicle; mm, mesomere; ms, mesencephalon; mt, metencephalon; my, myelencephalon; nf, neural fold; no, notochord; nt, neural tube; opc, optic cup; ot, otic vesicle; pa, pharyngeal arch; rp, Rathke’s pouch; si, sinus terminalis; sm, somite mesoderm; te, telencephalon; tf, tail fold; v, ventricle of heart; vit, area vitellina; vt, vitelline artery. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Stage 14: 22 Somites (2 ⅔ days, Fig. 6):
Flexures and Rotation: A 100–110° angle has formed between hindbrain and forebrain at mesencephalon. The anterior portion of the embryo has turned to the right and body rotation extends to somites 7–10, and is usually characterized by a sharp bend in this region.
Eye: The optic vesicles have formed the early optic cup. The lens placode is visible.
Pharyngeal Arches: The first and second pharyngeal arches and the first and second pharyngeal clefts are present.
Lateral Body Fold: A lateral body fold is visible in mid-gut region, extending to the somites 17–18. The amnion is visible posterior to heart, between somites 4–5.
Comparative Embryology: The chicken and the Society Finch at this stage have right angles between forebrain and hindbrain; obtuse angles are typical at this stage in the Zebra Finch. The amnion is further extended in the chicken at this stage than the Zebra Finch.
Stage 15: 25 Somites (2 ¾ days, Fig. 7):
Flexures and Rotation: Rotation in the body has extended to somites 10–13. The forebrain and hindbrain meet at right angles.
Head Region: Telencephalon and mesencephalon are enlarged and distinct.
Pharyngeal Arches: The first three pharyngeal arches are present. The mandibular and hyoid arches have increased in size to extend past the third arch. The first two pharyngeal clefts are oval in shape.
Limbs: The primordial limb areas remain flat. The mesoderm is condensed in the area of the forelimb, but is not clearly defined.
Lateral Body Fold: The lateral body fold has thickened. The amnion has extended below the heart to somites 7–11.
Figure 7.
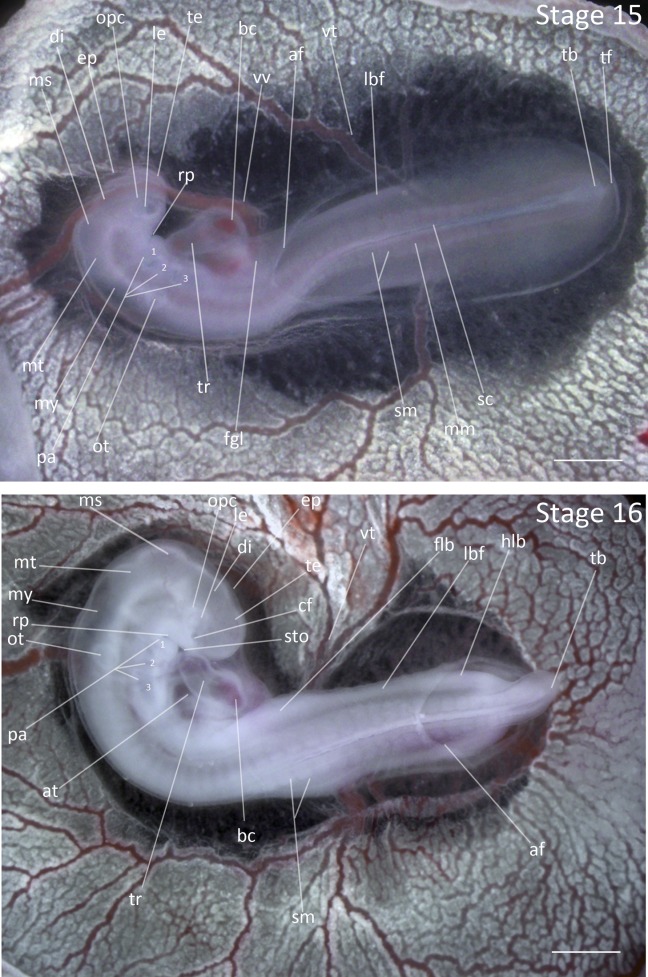
Stages 15 and 16: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 15 (2 ¾ days). Stage 16 (3 days). Abbreviations: af, amniotic fold; at, atrium of heart; bc, bulbus cordis; cf, choroid fissure; di, diencephalon; ep, epiphysis; fgl, foregut lumen; flb, forelimb bud; hlb, hindlimb bud; lbf, lateral body of foregut; le, lens vesicle; mm, mesomere; ms, mesencephalon; mt, metencephalon; my, myelencephalon; opc, optic cup; ot, otic vesicle; pa, pharyngeal arch; rp, Rathke’s pouch; sc, spinal cord; sm, somite mesoderm; sto, stomodeum; tb, tailbud; te, telencephalon; tf, tail fold; tr, truncus arteriosus; vt, vitelline artery; vv, vitelline vein. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Beyond Stage 15, somite number becomes increasingly difficult to identify and represents an average count. The curvature of the tail and thickening mesoderm obscures the somites in these later stages. The limb buds and pharyngeal arches are diagnostic for staging embryos Stage 16–25. The amnion is not a reliable method for determining embryological staging as its progression does not always correspond to a given stage.
Stage 16: Primordial Limb Emergence (3 days, Fig. 7):
Flexures and Rotation: The anterior region of embryo is fully twisted to the region of forelimb buds. The angle of rotation has reached nearly 90° and the anterior region of embryo has gained a hook-like appearance.
Head Region: The telencephalon has doubled in length and the epiphysis is now distinct. The mesencephalon and metencephalon have undergone rapid expansion to be more prominent.
Pharyngeal Arches: The hyoid and mandibular arches have elongated to the anterior edge of the optic cup. The second pharyngeal groove has narrowed but is still distinct.
Limbs: The forelimb buds have thickened and are distinctly visible as small protrusions. The hindlimb buds have appeared as a thickening of mesoderm, but do not protrude from the surface of embryo. The tailbud has formed and has extended beyond the posterior end of the embryo.
Lateral Body Fold: The lateral body fold has expanded throughout the circumference of embryo and is thickened. The amnion is variable, but usually extends to somites 20–24.
Comparative Embryology: In Stage 16, the tailbud of both the Zebra Finch and the Society Finch is longer than the tailbud in the chicken, which is a short cone. The epiphysis emerged one stage earlier in the Zebra Finch than in the chicken.
Stage 17: Four Pharyngeal Arches Present (3 ¼ days, Fig. 8):
Flexures and Rotation: The anterior half of the embryo is rotated further to the right. The angle between forebrain and hindbrain is acute.
Somites: There are 30–32 somites present.
Heart: The bulbus cordis is visible as the heart has assumed the S-shaped form.
Pharyngeal Arches: The fourth pharyngeal arch is clearly outlined by a thickening of mesoderm and three pharyngeal slits are visible as dark, narrow lines. The hyoid arch has grown rapidly so that the mandibular arch and hyoid arch are of equal length.
Limbs: The forelimb bud is broader and longer than the hindlimb bud. The tailbud has curled inward towards the ventral side of the embryo. The amnion has extended to behind the hindlimb buds or over the posterior of the embryo, leaving a small pore at the tailbud.
Comparative Embryology: Both the Society Finch and Zebra Finch are characterized by larger forelimb buds than hindlimb buds at this stage, while the chicken has equal sized forelimb and hindlimb buds.
Figure 8.
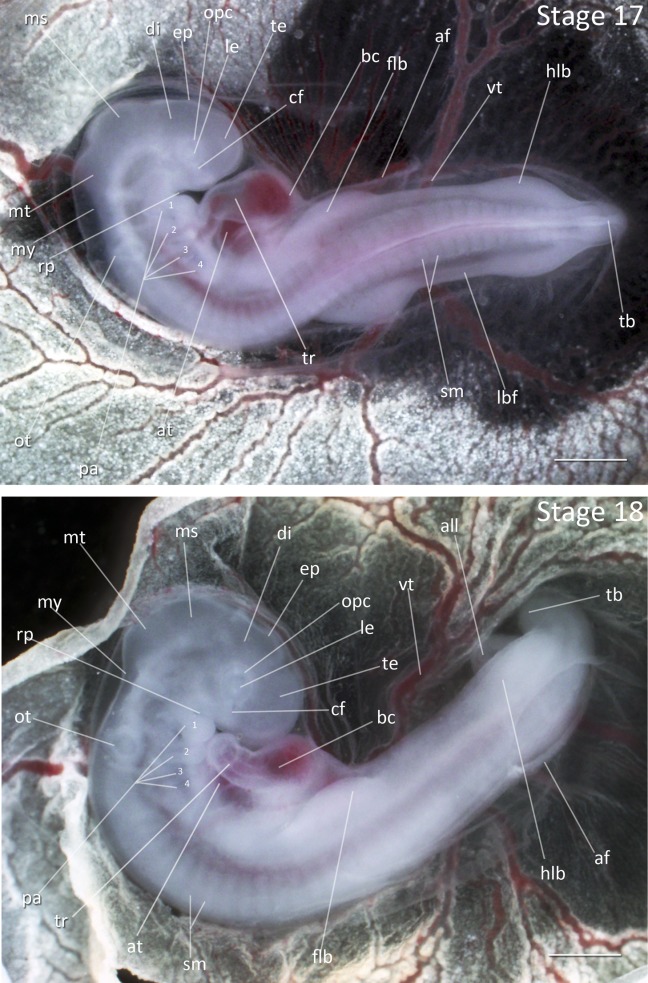
Stages 17 and 18: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 17 (3 ¼ days). Stage 18 (3 ⅓ days). Abbreviations: af, amniotic fold; all, allantois; at, atrium of heart; bc, bulbus cordis; cf, choroid fissure; di, dienchepalon; ep, epiphysis; flb, forelimb bud; hlb, hindlimb bud; lbf, lateral body of foregut; le, lens vesicle; ms, mesencephalon; mt, metencephalon; my, myelencephalon; opc, optic cup; ot, otic vesicle; pa, pharyngeal arch; rp, Rathke’s pouch; sm, somite mesoderm; tb, tailbud; te, telencephalon; tr, truncus arteriosus; vt, vitelline artery. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Stage 18: Emergence of Allantois (3 ⅓ days, Fig. 8):
Flexures and Rotation: The embryo has rotated fully and is often seen in an S-shape during dissection. A 45° angle is present between anterior and posterior regions of body meeting in the thoracic region.
Somites: There are 32–34 somites present.
Head Region: All of the brain structures have enlarged, particularly the diencephalon, such that the head structures are no longer a smooth surface. The nasal pits are faintly visible.
Pharyngeal Arches: The first pharyngeal slit is now oval in shape and the first two arches are partially separated. The fourth pharyngeal slit is faint, but present.
Limbs: The forelimb and hindlimb buds have expanded ventrolaterally. The forelimb buds are wider and longer than the hindlimb buds. The tailbud is curled upward.
Allantois: The allantoic diverticulum is first visible as a thickened pouch.
Comparative Embryology: The chicken hindlimb buds are larger than forelimb buds at this stage. The tailbud is also turned to the right in the chicken but is curled upward both finches. The chicken and the Society Finch do not have a distinct fourth pharyngeal slit that can be seen in the Zebra Finch at this stage. The nasal pit emerges one stage later than in the chicken, but two stages earlier than in the Society Finch.
Stage 19: Lengthened Telencephalon (3 ½ days, Fig. 9):
Flexures and Rotation: The body lies flat on one side. Rotation of the spine reaches the level of hindlimb buds.
Somites: There are 34–38 somites present.
Head Region: The mesencephalon has emerged as distinct feature of head. The choroid fissure is darker and is easily distinguished. The nasal placode is visible.
Pharyngeal Arches: The fourth arch is increased in size, but the first and second arches have undergone the most growth and have extended to the level of the choroid fissure.
Limbs: The limb buds have enlarged ventrocaudally. The forelimb buds are larger than the hindlimb buds. The tail is unsegmented.
Figure 9.
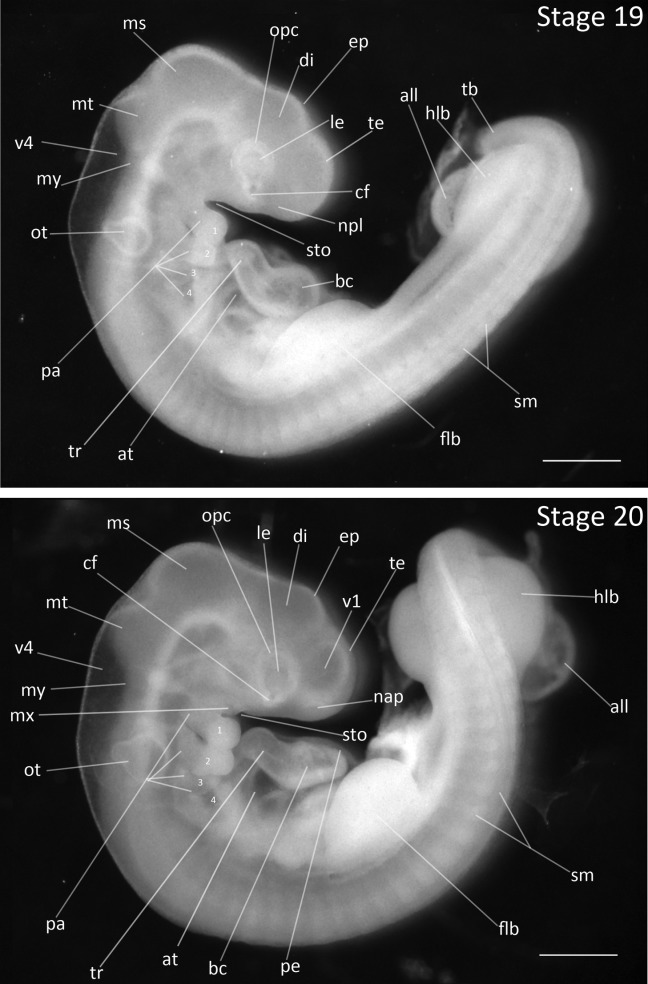
Stages 19 and 20: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 19 (3 ½ days). Stage 20 (3 ⅔ days). Abbreviations: all, allantois; at, atrium; bc, bulbus cordis; cf, choroid fissure; di, diencephalon; ep, epiphysis; flb, forelimb bud; hlb, hindlimb bud; le, lens vesicle; ms, mesencephalon; mt, metencephalon; my, myelencephalon; nap, nasal pit; npl, nasal placode; opc, optic cup; ot, otic vesicle; pa, pharyngeal arch; pe, pericardium; sm, somite mesoderm; sto, stomodeum; tb, tailbud; te, telencephalon; tr, truncus arteriosus; v1, lateral ventricle of telencephalon; v4, fourth vesicle of myelencephalon.
Stage 20: Maxillary Process Establishment (3 ⅔ days, Fig. 9):
Flexures and Rotation: The spinal rotation has reached the tailbud, which now is twisted. During dissections, embryos are characterized by an S-shape.
Somites: The somite number is no longer distinguishable with certainty due to tail rotation.
Head Region: The mesencephalon is the most prominent feature of the head. The metencephalon and diencephalon are relatively equal in size. The lateral ventricle of the telencephalon has emerged. The nasal pits are apparent. The eye region is distinguished by a white optic cup, but no retina pigmentation has yet developed.
Heart: The pericardial epithelium is first observable as a thin membrane overlying the bulbus cordis and truncus arteriosus.
Pharyngeal Arches: The first pharyngeal slit has lengthened and deepened. The mandibular arches are fused at the midline. The maxillary process is visible and is slightly shorter in length than the mandibular process. The mandibular process has enlarged, extending to the anterior edge of the truncus arteriosus. The hyoid arch has enlarged and partially obscures the third arch. All four arches are easy to distinguish at this stage with darkened pharyngeal slits.
Limbs: The forelimb buds have grown ventrally with a caudal slant and continue to be thicker than the hindlimb buds.
Allantois: The allantois has enlarged but is still thick and little vasculature is visible.
Comparative Embryology: The mesencephalon is much larger in the chicken than in the Society Finch and the Zebra Finch from this stage until hatching. Pigmentation of the eye has not yet developed in the Society Finch or the Zebra Finch but begins to develop at this stage in the chicken. The maxillary process develops one stage later in the Society Finch and the Zebra Finch than in the chicken.
Stage 21: Onset of Smooth Curvature of Spine (3 ¾ days, Fig. 10):
Flexures and Rotation: The embryo resembles the letter “c” with a slightly curved flexure in the dorsal spine. Due to the flexure in the spine, the rostral tip of the telencephalon is in contact with the ventral side of the heart.
Head Region: The mesencephalon remains the most prominent head feature. The nasal pits have darkened and are distinct. The eye region is not yet pigmented.
Pharyngeal Arches: The maxillary process is still smaller than the mandibular process. The stomadeum has narrowed as the maxillary process has elongated to the rostral edge of the lens.
Limbs: The forelimb buds and hindlimb buds are still characterized by a caudal tilt; the forelimb buds are larger and thicker than hindlimb buds.
Allantois: The allantois is larger and thin walled.
Comparative Embryology: The maxillary process is longer than mandibular process in the chicken. Faint eye pigmentation develops at this stage in the chicken but not in either of the finches.
Figure 10.
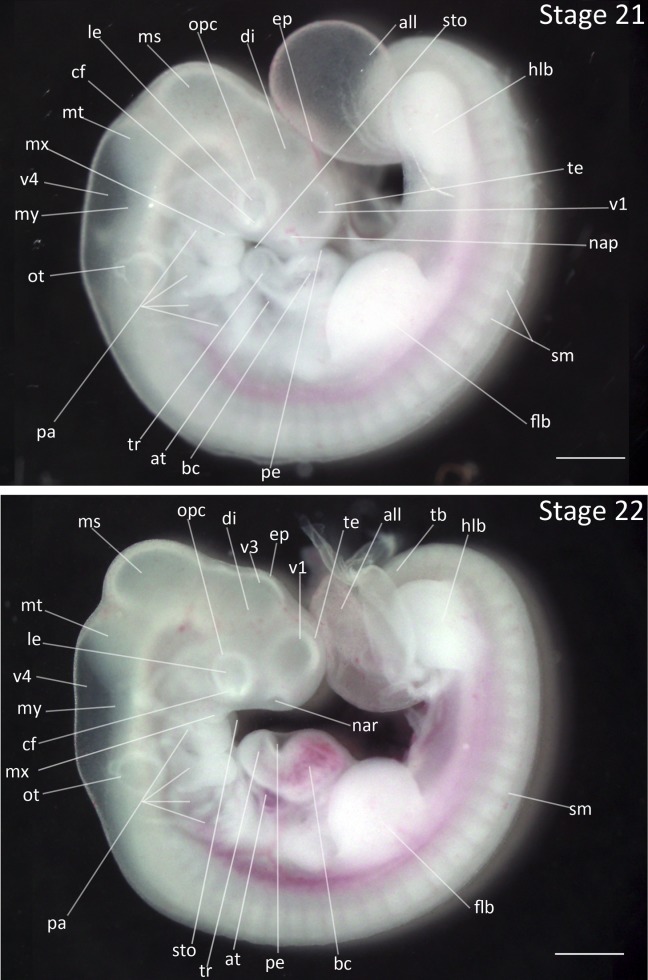
Stages 21 and 22: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 21 (3 ¾ days). Stage 22 (4 days). Abbreviations: all, allantois; at, atrium; bc, bulbus cordis; cf, choroid fissure; di, diencephalon; ep, epiphysis; flb, forelimb bud; hlb, hindlimb bud; le, lens vesicle; ms, mesencephalon; mt, metencephalon; my, myelencephalon; nap, nasal pit; nar, nares; opc, optic cup; ot, otic vesicle; pa, pharyngeal arch; pe, pericardium; sm, somite mesoderm; sto, stomodeum; tb, tailbud; te, telencephalon; tr, truncus arteriosus; v1, lateral ventricle of telencephalon; v3, third ventricle of diencephalon; v4, fourth vesicle of myelencephalon. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Stage 22: Emergence of Equal Limb-buds Lengths (4 days, Fig. 10):
Flexures and Rotation: The dorsal outline of thoracic spine is variable and is either straight or has slight curvature.
Head Region: The third ventricle of the diencephalon has become visible. The epiphysis is pronounced. The nasal pits have deepened to become the nares. The mesencephalon has significantly increased in size relative to the metencephalon.
Pharyngeal Arches: The hyoid arch has enlarged. The maxillary process has grown and is the same length as the mandibular process. The first pharyngeal slit is still visible and the fourth pharyngeal slit has deepened into a narrow slit.
Limbs: The forelimb and hindlimb buds have lengthened ventrally and are no longer angled caudally. This is the first time that the forelimb and hindlimb buds are equal in length. The tail is segmented.
Allantois: The allantois is vesicular and thin walled.
Comparative Embryology: The chicken and Zebra Finch have segmented tails at this stage while the Society Finch does not.
Stage 23: Eye Pigment Initiation (4 ⅓ days, Fig. 11):
Head Region: The pigmentation of the eye has appeared and is faint.
Pharyngeal Arches: The first pharyngeal slit is closed. The mandibular and maxillary processes are equal in length. The third and fourth arches remain exposed.
Limbs: Limbs are lengthened and have thickened significantly to appear knob-like in shape. The forelimb bud is thicker than the hindlimb bud, although both are still equal in length.
Allantois: The allantois is very thin and may obscure head features.
Comparative Embryology: The pigmentation of the eye develops three stages later in the Zebra Finch than in the chicken, and emerges one stage earlier than in the Society Finch.
Figure 11.
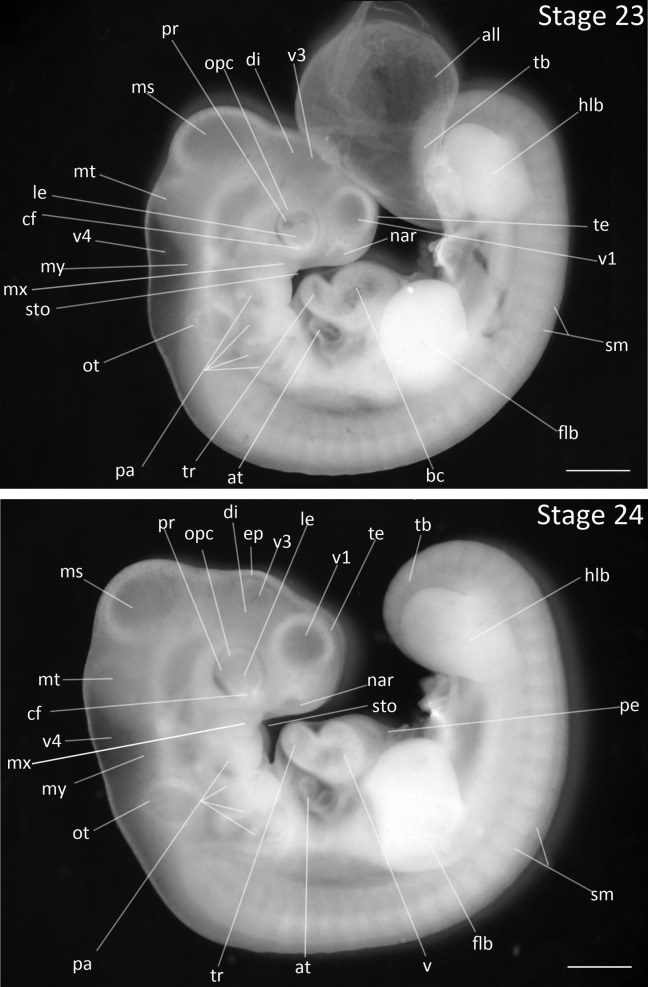
Stages 23 and 24: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 23 (4 ⅓ days). Stage 24 (4 ½ days). Abbreviations: all, allantois; at, atrium; bc, bulbus cordis; cf, choroid fissure; di, diencephalon; ep, epiphysis; flb, forelimb bud; hlb, hindlimb bud; le, lens vesicle; ms, mesencephalon; mt, metencephalon; my, myelencephalon; nar, nares; opc, optic cup; ot, otic vesicle; pa, pharyngeal arch; pe, pericardium; sm, somite mesoderm; sto, stomodeum; tb, tailbud; te, telencephalon; tr, truncus arteriosus; v, ventricle of heart; v1, lateral ventricle of telencephalon; v3, third ventricle of diencephalon; v4, fourth vesicle of myelencephalon.
Stage 24: Lengthened Limb-buds (4 ½ days, Fig. 11):
Head Region: The first ventricle of telencephalon is roughly equal in diameter to eye.
Flexures and Rotation: The outline of the dorsal flexure is no longer a smooth curve but is angled at the intersection of the thoracic and lumbar regions of spine.
Heart: The right ventricle develops from the bulbus cordis, indicated by a deep groove that separates the right and left ventricles (de la Cruz et al., 1972).
Pharyngeal Arches: The hyoid arch is enlarged and is greater in length than mandibular and maxillary processes. The hyoid arch has also obscured the posterior arches.
Limbs: The forelimb and hindlimb buds are relatively equal in length and width. Both are narrowed but have no indication of a wrist/ankle or elbow/knee.
Comparative Embryology: Zebra Finch limb development closely follows the progression of Society Finch development and at no point does the hindlimb bud exceed the forelimb bud in size at these earlier stages as seen in chicken development.
Stage 25: Wrist and Elbow Development (4 ¾ days, Fig. 12):
Head Region: The first ventricle of telencephalon has increased in size to exceed diameter of eye.
Limbs: The limb buds are elongated so that the forelimb buds have obscured the dorsal region of ventricle and the hindlimb buds have obscured part of the tailbud. The distal end of each limb bud is a digital plate. The contour of the digital plate is rounded. Faint digit demarcation may be visible as a shadowing on the hind limb buds, though no grooves are visible on either the fore or hind limb buds. The first indications of constrictions demarcating wrist and ankle are visible. The elbow and knee joints are visible.
Pharyngeal Arches: The posterior arches are visible, but their ends are not distinguishable. The hyoid arch is the largest arch. The maxillary process is longer than the mandibular process.
Comparative Embryology: In the chicken, the maxillary process has migrated to meet the wall of the nasal groove; this has not occurred in the Zebra Finch at this stage.
Figure 12.
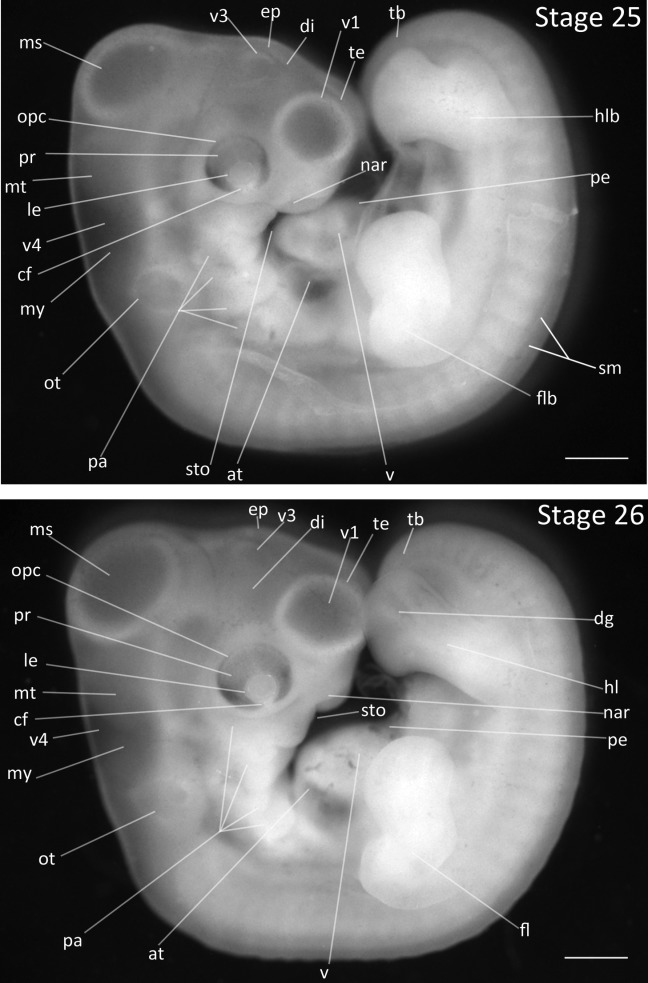
Stages 25 and 26: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 25 (4 ¾ days). Stage 26 (5 days). Abbreviations: all, allantois; at, atrium; cf, choroid fissure; dg, digit of limb; di, diencephalon; ep, epiphysis; fl, forelimb; flb, forelimb bud; hl, hindlimb; hlb, hindlimb bud; le, lens vesicle; ms, mesencephalon; mt, metencephalon; my, myelencephalon; nar, nares; opc, optic cup; ot, otic vesicle; pa, pharyngeal arch; pe, pericardium; sm, somite mesoderm; sto, stomodeum; tb, tailbud; te, telencephalon; v, ventricle of heart; v1, lateral ventricle of telencephalon; v3, third ventricle of diencephalon; v4, fourth vesicle of myelencephalon.
Stage 26: Digit Appearance (5 days, Fig. 12):
Limbs: The limb buds are further elongated so that the hindlimbs have extended past the tailbud. The elbow joint has become more acute than in the previous stage. Digits are now visible in both the forelimb and hindlimb digital plates with darkened regions separating the opaque digits. The elbow flexure is distinct. Constriction of the wrist and ankle are accentuated from previous stage.
Pharyngeal Arches: The maxillary process has fused with wall of the nasal groove, creating the upper beak anlage. The frontal process is prominent.
Eyes: Pigmentation of retina is darker relative to previous stage.
Comparative Embryology: The maxillary process has fused with the nasal groove one stage later than in the chicken.
Stage 27: Pericardium Thickens (5 ¼ days, Fig. 13):
Heart: The interior chambers have become increasingly obscured due to thickening of the pericardial epithelium.
Limbs: The limbs have elongated so that the forelimb has obscured the dorsal half of the heart. The digital plate is wider relative to the previous stage. The demarcation of four digits in hindlimb and three digits in forelimb is distinct with darkened regions between digits.
Pharyngeal Arches: The collar has begun to form over the posterior pharyngeal arches. The frontal process is increasingly distinct.
Eyes: Retina pigmentation has darkened from medium grey to black at the ventral-most regions of eye.
Tail: The tailbud has shortened and is thicker at its base.
Figure 13.
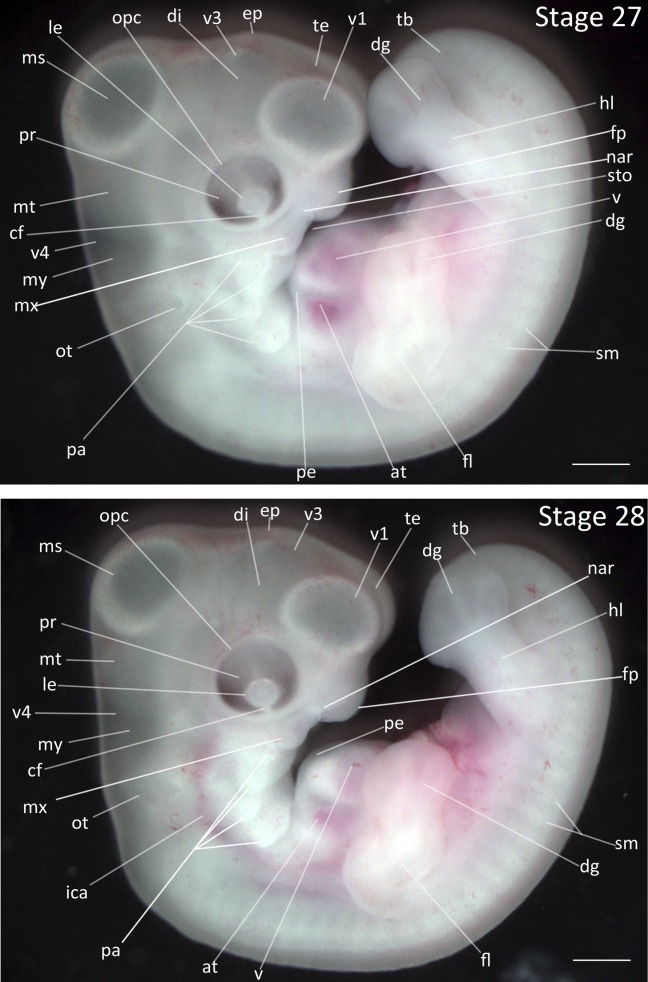
Stages 27 and 28: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 27 (5 ¼ days). Stage 28 (5 ½ days). Abbreviations: all, allantois; at, atrium; cf, choroid fissure; dg, digit of limb; di, diencephalon; ep, epiphysis; fl, forelimb; fp, frontal process; hl, hindlimb; ica, internal carotid artery; le, lens vesicle; ms, mesencephalon; mt, metencephalon; mx, maxillary process; my, myelencephalon; nar, nares; opc, optic cup; ot, otic vesicle; pa, pharyngeal arch; pe, pericardium; sm, somite mesoderm; sto, stomodeum; tb, tailbud; te, telencephalon; v, ventricle of heart; v1, lateral ventricle of telencephalon; v3, third ventricle of diencephalon; v4, fourth vesicle of myelencephalon. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Stage 28: Maxillary Migration (5 ½ days, Fig. 13):
Limbs: The digital plate is slightly wider in both forelimb and hind limbs. The four digits in the hindlimb are distinct.
Eyes: Increased retinal pigmentation is present in the upper regions of the eye.
Pharyngeal Arches: All four pharyngeal arches are still distinguishable, though the development of the collar has partially obscured the third and fourth arch. The maxillary process is now located immediately caudal to frontal process and has developed an uneven ventral surface.
Stage 29: Collar Formation (5 ¾ days, Fig. 14):
Heart: The interior chambers are no longer distinguishable due to the extent of the pericardial epithelium development.
Pharyngeal Arches: The collar has developed further and creates a smooth outline in the anterior region of the neck. The mandibular process protrudes forward. The maxillary process is located beyond the rostral edge of eye and is at the level of the midpoint of the telencephalon.
Limbs: Shallow grooves between the digits of both the forelimb and hindlimb are visible, and the outline of the digital plate has slight indentations corresponding to the appearance of interphalangeal furrows and formation of webbing. The second digit of the forelimb is slightly shorter than third digit.
Tail: The tailbud is dramatically shorter relative to the previous stage.
Comparative Embryology: The chicken forelimb at this stage has a longer third digit relative to the other digits in the forelimb; the difference in digit length is less pronounced in the Zebra Finch at this stage. Note that the three digits present in the forelimb in avian development are usually identified by embryologists as the second, third, and fourth digit although controversy over their identity exists (reviewed in Young et al., 2011).
Figure 14.
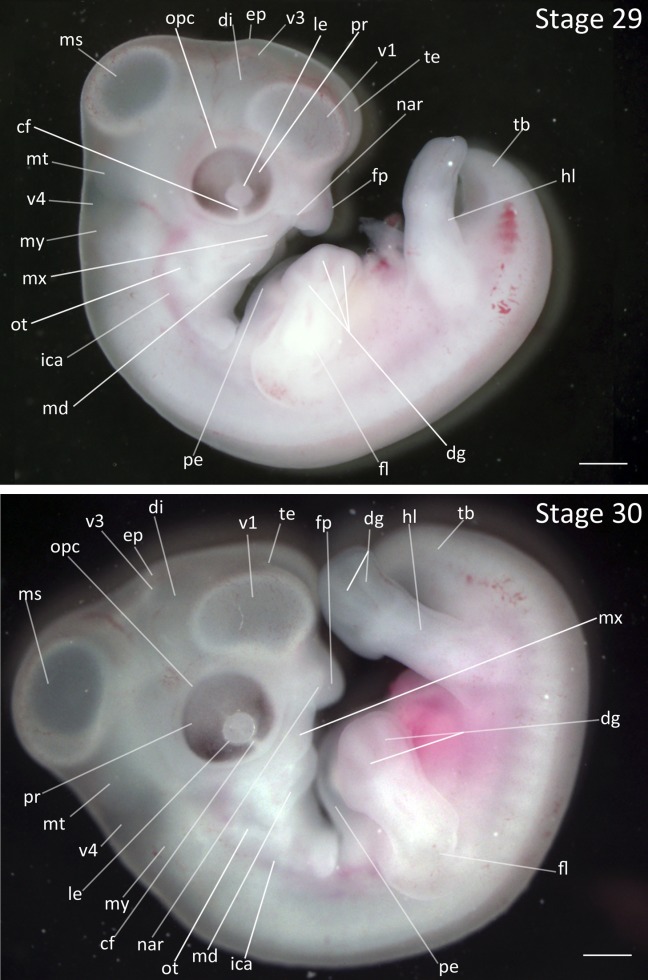
Stages 29 and 30: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 29 (5 ¾ days). Stage 30. (6 days). Abbreviations: all, allantois; cf, choroid fissure; dg, digit of limb; di, diencephalon; ep, epiphysis; fl, forelimb; fp, frontal process; hl, hindlimb; ica, internal carotid artery; le, lens vesicle; md, mandibular process; ms, mesencephalon; mt, metencephalon; mx, maxillary process; my, myelencephalon; nar, nares; opc, optic cup; ot, otic vesicle; pe, pericardium; sm, somite mesoderm; sto, stomodeum; tb, tailbud; te, telencephalon; v1, lateral ventricle of telencephalon; v3, third ventricle of diencephalon; v4, fourth vesicle of myelencephalon. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Stage 30: Pharyngeal Arch Migration (6 days, Fig. 14):
Limbs: The interphalangeal furrows have deepened.
Pharyngeal Arches: The mandibular and maxillary processes have continued to migrate in a rostral direction to meet the frontal process. The maxillary and mandibular processes are of equal length. The collar has obscured the posterior pharyngeal arches completely.
Comparative Embryology: The first scleral papillae are present in the chicken at this stage; the Zebra Finch lacks scleral papillae.
Stage 31: Mandible Formation (6 ¼ days, Fig. 15):
Beak: The maxillary process has fused with the frontal process, creating the maxilla. The maxilla is longer than the mandible. The collar remains visible posterior to the mandible, creating the neck.
Eyes: The eyelid is first visible beyond the perimeter of the pigmented retina.
Limbs: The third digit on the forelimb is 50% longer than the second digit. The interphalangeal furrows have deepened, but digits have not yet separated.
Comparative Embryology: In the Society Finch, there is a deepening and splitting of the first interphalangeal furrow concurrent with rapid growth of the third digit; this does not occur in the Zebra Finch at this stage.
Figure 15.
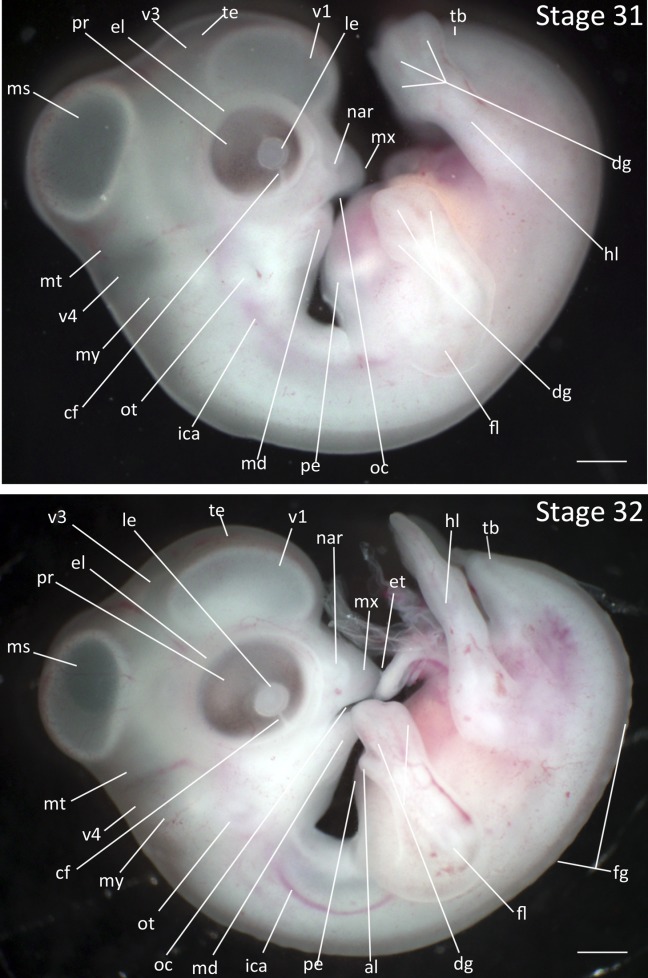
Stages 31 and 32: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 31 (6 ¼ days). Stage 32 (6 ¾ days). Abbreviations: all, allantois; cf, choroid fissure; dg, digit of limb; di, diencephalon; el, eyelid; ep, epiphysis; fg, feather germ; fl, forelimb; hl, hindlimb; ica, internal carotid artery; le, lens vesicle; md, mandibular process; ms, mesencephalon; mt, metencephalon; mx, maxillary process; my, myelencephalon; nar, nares; opc, optic cup; ot, otic vesicle; pe, pericardium; sm, somite mesoderm; sto, stomodeum; tb, tailbud; te, telencephalon; v1, lateral ventricle of telencephalon; v3, third ventricle of diencephalon; v4, fourth vesicle of myelencephalon. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Stage 32: Eggtooth Appearance (6 ¾ days, Fig. 15):
Beak: The eggtooth has appeared. The mandible has not yet reached the base of the maxilla.
Limbs: The third digit is twice as long as second digit and the furrow between the second and third digits is partially split. The elbow joint has become distinct and accentuated, with forelimb now forming an approximate right angle held perpendicular to rostrocaudal axis.
Tail: The tail has regressed in length to below the level of the foot.
Feathers: The first feather germs have appeared, emerging as a single row on the spinal pteryla.
Brain: The lateral ventricle of the telencephalon is distinctly more opaque.
Ear: The external ear is clearly defined as double circular outline of the shallow external auditory pore and external auditory meatus.
Ventral Exterior: The ventral outline is smooth; the outline of heart is no longer perceptible.
Comparative Embryology: The emergence of feather germs occurs at the same stage in the Zebra Finch as in the Society Finch; feather germ emergence occurs two stages earlier in the chicken. Additionally, the egg tooth develops in the Zebra Finch one stage earlier than in the Society Finch, and two stages later than in the chicken.
Stage 33: Second and Third Digit Separation (7 days, Fig. 16):
Beak: The mandible has reached the base of the maxilla.
Eyes: The eyelid has advanced to cover the retina but only the outermost margins of the pigmented retina are obscured.
Limbs: The second and third digits of the fore limb are completely separated with the second digit (alula) having gained its distinctive shape. The third digit is approximately three times the length of the second digit. Webbing at the inner elbow between the radial margin and the humerus is first observable.
Feathers: Additional rows of feather germs have appeared in the spinal pteryla. Feather germs have also appeared on the lateral surface of the tail.
Figure 16.
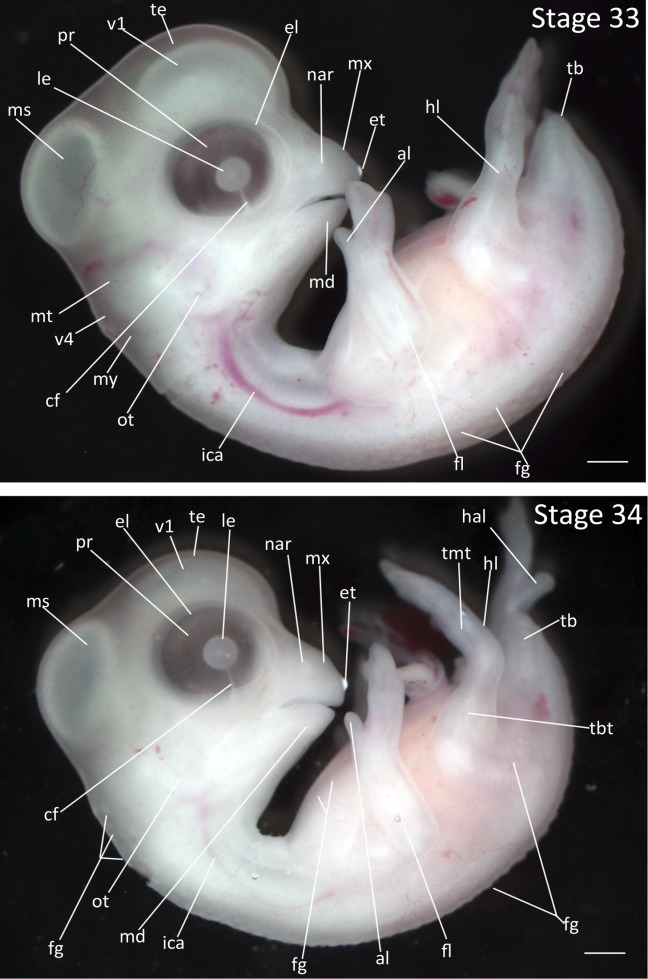
Stages 33 and 34: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 33 (7 days). Stage 34 (7 ¼ days). Abbreviations: al, alula; cf, choroid fissure; el, eyelid; et, egg tooth; fg, feather germ; fl, forelimb; hal, hallux; hl, hindlimb; ica, internal carotid artery; le, lens vesicle; md, mandibular process; ms, mesencephalon; mt, metencephalon; mx, maxillary process; my, myelencephalon; nar, nares; ot, otic vesicle; pr, pigmented retina of eye; tb, tailbud; te, telencephalon; tmt, tarsometatarsus; v1, lateral ventricle of telencephalon; v4, fourth vesicle of myelencephalon. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Stage 34: Feather Germs on Ventral Surface (7 ¼ days, Fig. 16):
Brain: The third ventricle of the diencephalon is no longer visible.
Eyes: The eyelid has advanced to cover the outer edges of the retina and the circumference of the eyelid opening is slightly constricted.
Feathers: Feather germs have appeared in the capital pteryla on the dorsal side of the neck and in the ventral pterylae on the chest wall. A single row of feather germs is present on the femoral pteryla.
Limbs: The third digit of the forelimb is approximately four times as long as the alula. The forelimb now has wing-like appearance. The first digit of the hindlimb (hallux) is separated and is rotated relative to other hindlimb digits.
Tail: The tail has now regressed in length to be approximately level with the ankle joint.
Comparative Embryology: In the chicken, the nictating membrane has extended to a point halfway between outer rim of eye (eyelid) and scleral papillae; the nictating membrane is not visible at this stage in the Zebra Finch.
Stage 35: Feather Germs on Eyebrow (7 ½ days, Fig. 17):
Eyes: The eyelid has advanced to cover the outer portion of the retina with a corresponding reduction of the circumference of the eyelid opening.
Feathers: Feather germs are apparent in the capital pteryla above the eye. Additional rows of feather germs appear in the femoral pteryla.
Limbs: The second, third, and forth digits on the hindlimb split to form distinct toes.
Figure 17.
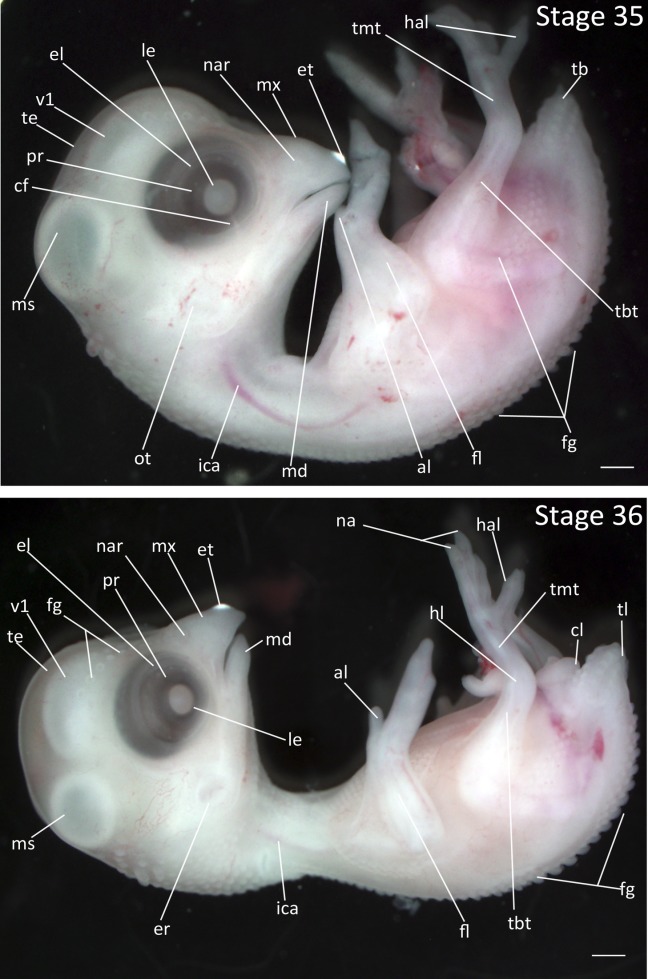
Stages 35 and 36: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 35 (7 ½ days). Stage 36 (7 ¾ days). Abbreviations: al, alula; cf, choroid fissure; el, eyelid; er, opening to ear; et, egg tooth; fg, feather germ; fl, forelimb; hal, hallux; hl, hindlimb; ica, internal carotid artery; le, lens vesicle; md, mandibular process; ms, mesencephalon; mx, maxillary process; na, nail; nar, nares; ot, otic vesicle; pr, pigmented retina of eye; tb, tailbud; tbt, tibiotarsus; te, telencephalon; tl, tail; tmt, tarsometatarsus; v1, lateral ventricle of telencephalon. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Stage 36: Prominent Spinal Feather Germs (7 ¾ days, Fig. 17):
Eyes: The choroid fissure is no longer visible as it is obscured by the developing eyelids.
Feathers: The feather germs all along spinal pteryla have reached greatest prominence. Feather germs have appeared on the capital pteryla on the ventral side of the chin and mandible.
Limbs: Nails have formed at the tips of the toes.
Excretory System: The cloaca is prominent.
Tail: The tail is smaller and less curved, reaching its final approximate size and shape relative to the body.
Comparative Embryology: Nails appear one stage later in the Society Finch than in the Zebra Finch.
Stage 37: First Row of Alar Feather Germs (8 days, Fig. 18):
Eyes: The eyelids have become more prominent, covering about half of the eye.
Feathers: The feather germs on the spinal pteryla are reduced in prominence. A single row of feather germs have appeared on the alar pteryla distal to the wrist joint. Feather germs are present on the ventral pteryla posterior to the forelimbs.
Limbs: The toes have increased in size by about 50%. The nails on the toes are longer and pointed. The third and fourth digits on the forelimb are nearly indistinguishable.
Figure 18.
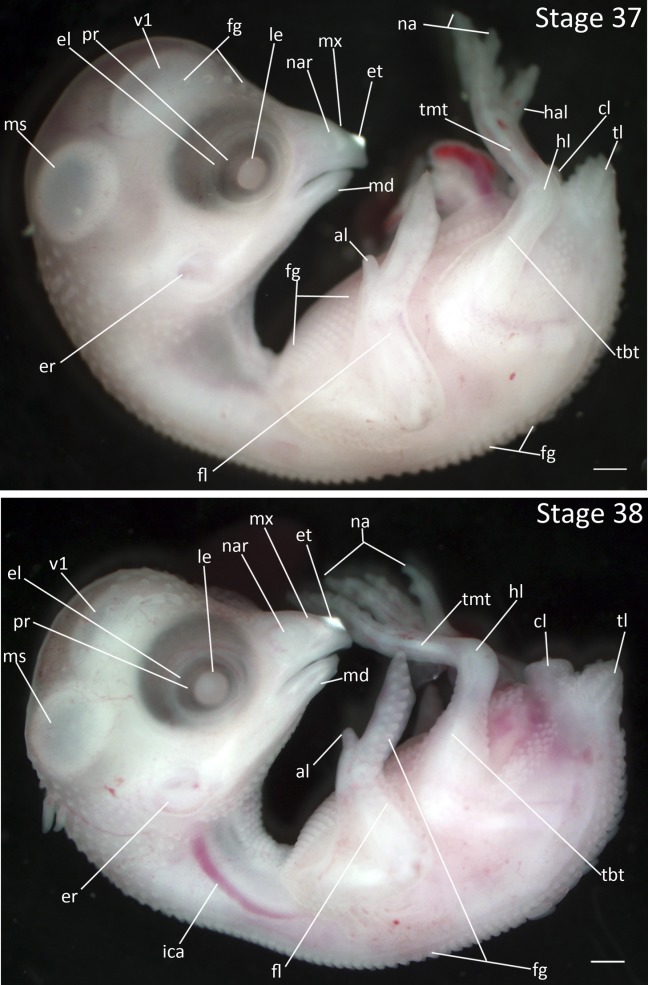
Stages 37 and 38: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 37 (8 days). Stage 38 (8 ½ days). Abbreviations: al, alula; cl, cloaca; el, eyelid; er, opening to ear; et, egg tooth; fg, feather germ; fl, forelimb; hal, hallux; hl, hindlimb; ica, internal carotid artery; le, lens vesicle; md, mandibular process; ms, mesencephalon; mx, maxillary process; na, nail; nar, nares; pr, pigmented retina of eye; tbt, tibiotarsus; tl, tail; tmt, tarsometatarsus; v1, lateral ventricle of telencephalon. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Stage 38: Second Row of Alar Feather Germs (8 ½ days, Fig. 18):
Eyes: The eyelids cover about 75% of the eye. The opening between the eyelids remains roughly circular.
Feathers: Some feather germs on the capital and spinal pterylae have become more prominent. A second row of feather germs have appeared in the alar pteryla on the forelimb distal to the wrist joint. Feather germs have appeared in the capital pteryla on the dorsal side of the head and the ventral side of the neck. Feather germs are present in the crural pteryla.
Limbs: The toes reach final proportion relative to the rest of the leg. The joints form in the toes. The nails are long and hooked.
Comparative Embryology: Nails develop earlier in the Zebra Finch than in the Society Finch but do not become hooked until Stage 41.
Stage 39: Feather Germ Elongation (9 days, Fig. 19):
Eyes: The eyelids have grown such that the opening between them is ellipsoid. The lens is not yet covered by the eyelids.
Brain: The mesencephalon and telencephalon are reduced in size by approximately half.
Feathers: Some feather germs on the capital, spinal, humeral, and femoral pterylae have elongated. Note that the number of elongated feather germs is variable between individuals and not diagnostic for staging.
Limbs: The third and fourth digits of the forelimb are indistinguishable. The tarsometatarsus has reached its final proportions relative to the rest of the leg.
Figure 19.
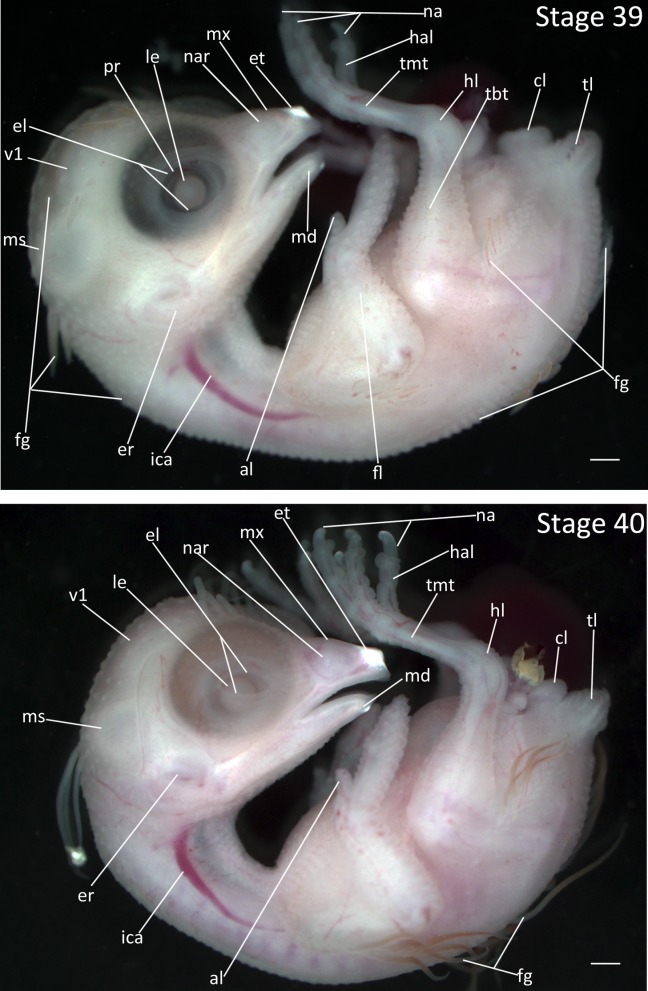
Stages 39 and 40: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 39 (9 days). Stage 40 (9 ½ days). Anterior–posterior axis reads left to right. Abbreviations: al, alula; cl, cloaca; el, eyelid; er, opening to ear; et, egg tooth; fg, feather germ; fl, forelimb; hal, hallux; hl, hindlimb; ica, internal carotid artery; le, lens vesicle; md, mandibular process; ms, mesencephalon; mx, maxillary process; na, nail; nar, nares; pr, pigmented retina of eye; tbt, tibiotarsus; tl, tail; tmt, tarsometatarsus; v1, lateral ventricle of telencephalon. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Stage 40: Onset of Cornification (9 ½ days, Fig. 19):
Eyes: The gap between eyelids is a narrow ellipsoid with the lower lid covering about half of the lens.
Brain: The mesencephalon and telencephalon are reduced in size and are barely discernible through the developing skull. The outline of the head no longer bulges up above and behind the eye making the head’s shape typical of a hatchling Zebra Finch.
Beak: Cornification is present at the tip of the maxilla near the egg tooth and at the tip of the mandible and appears whiter than the surrounding tissue.
Limbs: Cornification is visible at the tips of the nails.
Feathers: The feather germs on the capital, spinal, humeral, and femoral pterylae are now flagelliform. Feather germs in other tracks throughout the rest of the embryo appear less pronounced as they are surrounded by developing epidermis.
Comparative Embryology: Stages 40–45 in the chicken are distinguished largely by the increasing length of the beak and the third toe. These measurements are not useful in the Zebra Finch. Additionally, many of the diagnostic traits for later stages of the Zebra Finch (e.g., cornification, eyelid development, growth of feathers) occur at earlier stages in the chicken. In the Society Finch, the lower eyelid has only reached the bottom of the lens at this stage.
Stage 41: Formation of Gape Flange (10 days, Fig. 20):
Eyes: The gap between the eyelids is narrowed to a thin slit with the lower eyelid covering more than half of the lens.
Brain: The vesicles of the brain are no longer visible through the developing skull.
Beak: Cornification has spread posteriorly from the tip of the beak. Gape flange has developed at the corner of the mouth.
Feathers: The growing feathers have a stiffer, pin feather-like appearance and are no longer pink.
Comparative Embryology: In the Society Finch, the lower eyelid covers no more than half of the lens at this stage.
Figure 20.
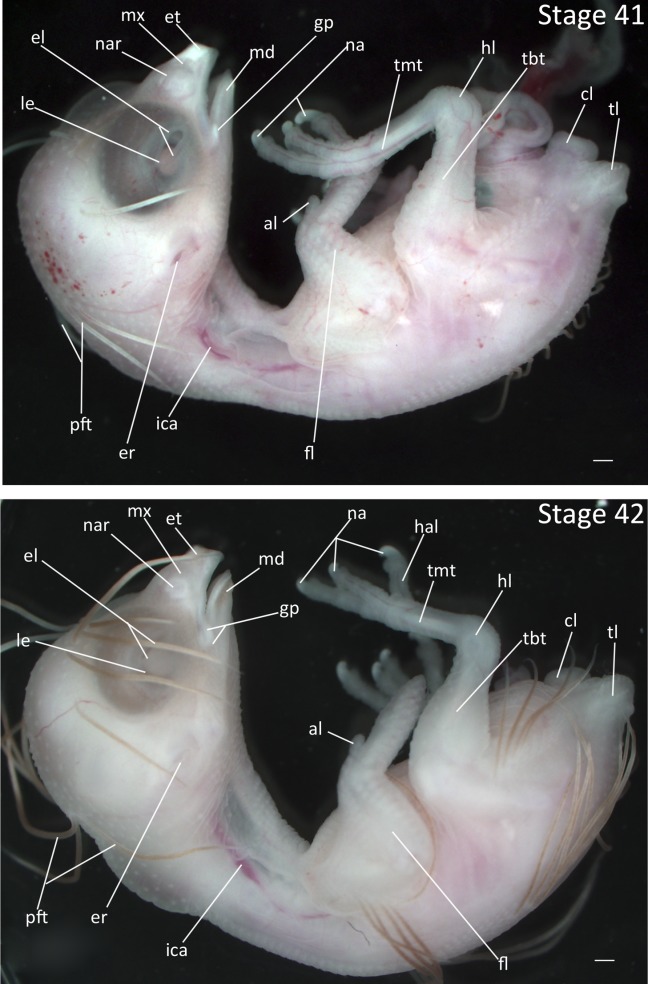
Stages 41 and 42: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 41 (10 days). Stage 42 (10 ½ days). Abbreviations: al, alula; cl, cloaca; el, eyelid; er, opening to ear; et, egg tooth; fl, forelimb; gp, gape flange; hal, hallux; hl, hindlimb; ica, internal carotid artery; le, lens vesicle; md, mandibular process; mx, maxillary process; na, nail; nar, nares; pft, pin feathers; tbt, tibiotarsus; tl, tail; tmt, tarsometatarsus. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Stage 43: Opaque Outline of Nares (10 ½ days, Fig. 20):
Beak: Cornification is present throughout tip of the beak and edges of the mouth. Gape flange is somewhat enlarged. Area around the nasal pit is raised and cornified, forming the circular outline of nares.
Feathers: The pin feathers are nearly fully elongated.
Epidermis: The epidermis is thickened and becomes somewhat opaque.
Limbs: The nails are more than 50% cornified.
Stage 43: Onset of pigmentation (11 days, Fig. 21):
Ears: The epidermis around the ear has formed a flap that gives the opening to the ear its final crescent shape.
Beak: Dark pigmentation is present on the maxilla and mandible near the tips and around the nares. Cornification has spread from the tip of the beak, edges of the mouth, and nares, covering over than half of the beak. Gape flange is larger and more prominent.
Feathers: The pin feathers have reached their final length and take on straw-like appearance. Note: absolute feather length varies greatly between individuals and is not diagnostic of stage.
Limbs: Scaling appears on the tarsometatarsus.
Comparative Embryology: Pigmentation of the beak does not appear in the Society Finch until Stage 44.
Figure 21.
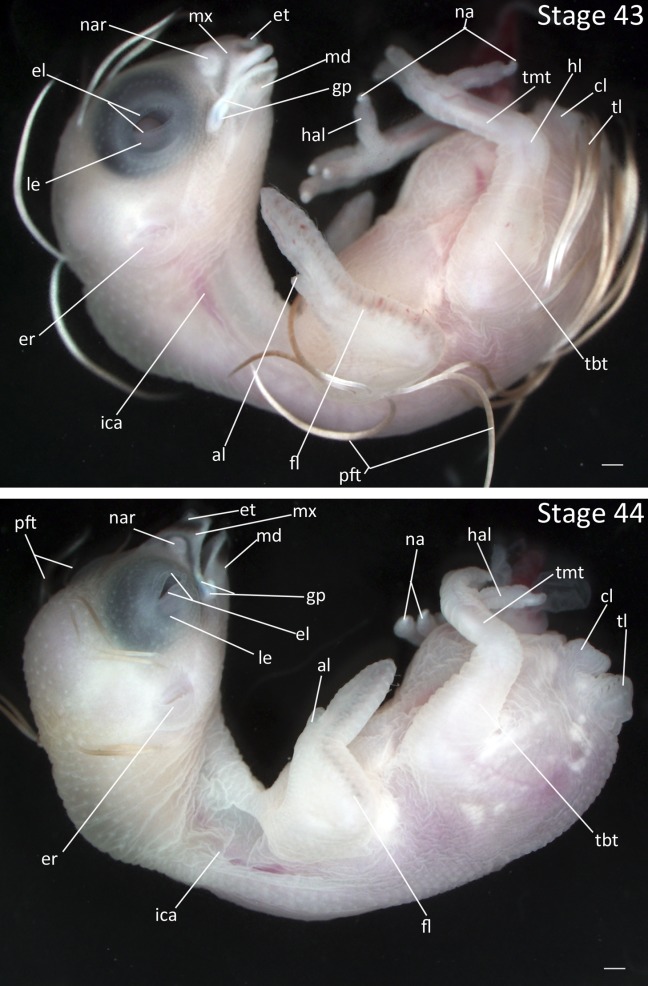
Stages 43 and 44: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 43 (11 days). Stage 44 (12 days). Abbreviations: al, alula; cl, cloaca; el, eyelid; er, opening to ear; et, egg tooth; fl, forelimb; gp, gape flange; hal, hallux; hl, hindlimb; ica, internal carotid artery; le, lens vesicle; md, mandibular process; mx, maxillary process; na, nail; nar, nares; pft, pin feathers; tbt, tibiotarsus; tl, tail; tmt, tarsometatarsus. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Stage 44: Beak Cornification. (12 days, Fig. 21):
Eyes: The opening between eyelids is a thin crescent.
Beak: The beak is dark at the tip and around the nares. Cornification of the beak is nearly complete.
Epidermis: The epidermis around the neck appears loose and wrinkled.
Limbs: Scaling has spread to the toes.
Stage 45: Epidermal Maturation (13 days, Fig. 22):
Eyes: The eyelids are closed.
Ears: The crescent shaped opening to ear canal is pigmented.
Beak: The mandible and maxilla are fully cornified. Surfaces of the beak are darkly pigmented. Gape flange is fully enlarged and white. Begging pigmentations are present on the interior of the mouth.
Limbs: The prominence of the alula has receded somewhat as it becomes incorporated into the outline of the wing. The nails are fully cornified.
Epidermis: The epidermis is pigmented, particularly along the trailing edge of the wing, dorsal surface, and the area around the beak. The epidermis appears loose and wrinkled over the entire body. The skin also becomes water repellent such that water beads up on the surface of the embryo. Note that in white plumage morphs the pigmentation of the skin may be absent while pigmentation in the beak remains.
Figure 22.
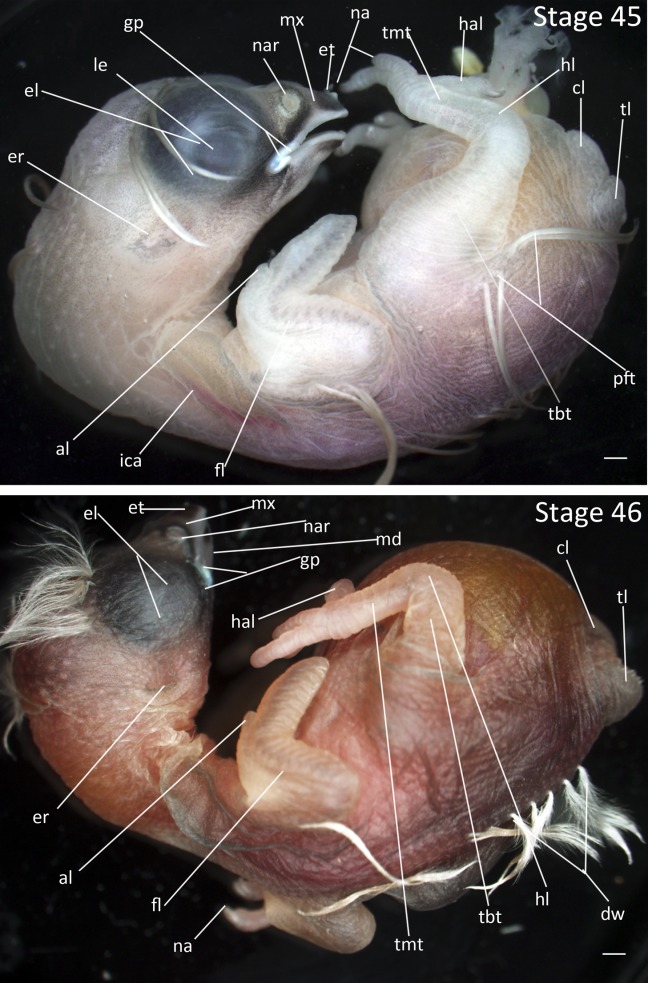
Stages 45 and 46: Anterior–posterior axis reads left to right in both images. Scale bar = 1 mm. Stage 45 (13 days). Stage 46 (14 days). Abbreviations: al, alula; cl, cloaca; dw, down feathers; el, eyelid; er, opening to ear; et, egg tooth; fl, forelimb; gp, gape flange; hal, hallux; hl, hindlimb; ica, internal carotid artery; le, lens vesicle; md, mandibular process; mx, maxillary process; na, nail; nar, nares; pft, pin feathers; tbt, tibiotarsus; tl, tail; tmt, tarsometatarsus. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Stage 46: Newly Hatched Chick (14 days, Fig. 22): The newly hatched Zebra Finch chick has sparse patches of natal down along the spinal, humeral, femoral, and capital pterylae. The eyes are tightly closed. It is incapable of moving any distance as limbs are small relative to the body. All these features are typical of an altricial songbird.
DISCUSSION
This description of embryological development confirms substantial developmental differences between the chicken, a precocial species, and the Zebra Finch, an altricial songbird, thus illustrating the need for a songbird-specific staging guide. Starting as early in development as the primitive streak, there are structural differences and timing differences in the embryos of the Zebra Finch and the chicken, including the shape of the primitive streak, the appearance of the neural and head folds, the relative size of limb buds, and presence of scleral papillae. Additionally, while the total incubation time for the chicken is substantially longer than the incubation time for Zebra Finch (∼21 days vs. 14 days), the chicken reaches the early stages in development (through stage 28) in less time than the Zebra Finch, indicating faster development in the early stages (Fig. 23). Variation in developmental rate is apparent even when comparing the Zebra Finch to a closely related altricial species, the Society Finch. Because of the Society Finch’s longer incubation period (17 days vs. the Zebra Finch’s 14 days), the Zebra Finch reached every developmental stage in less time than it took for the Society Finch (Fig. 23).
Figure 23.
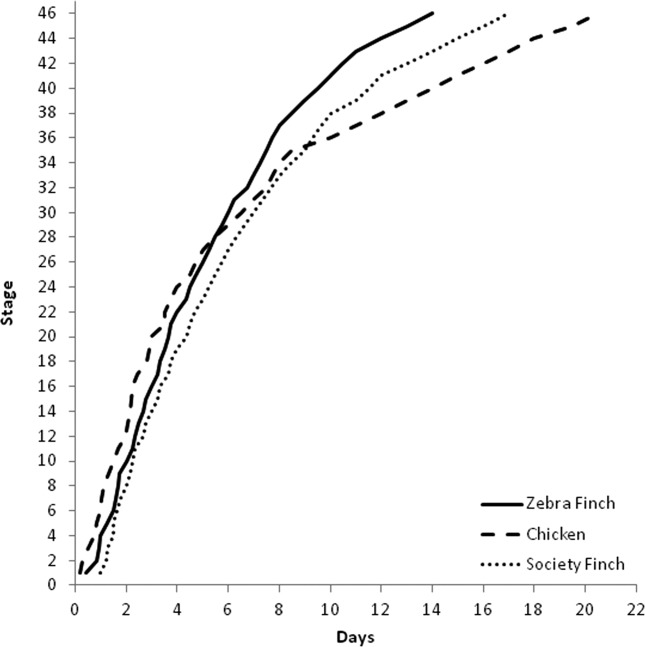
Timing of the stages in the Zebra Finch, Society Finch, and chicken. Solid line is the development of the Zebra Finch, dashed line is the development of the chicken, and dotted line is the development of Society Finch. The timing of the stages is determined from the average time reported in staging papers (Hamburger and Hamilton, 1951; Yamasaki and Tonosaki, 1988).
In the later stages, the differences between the chicken and the Zebra Finch become more striking as the last six stages of chicken development are characterized primarily by growth (Hamburger and Hamilton, 1951), whereas in the Zebra Finch, these stages are characterized by integumentary maturation, most of which is complete by Stage 40 in the chicken. At hatch, precocial species must be able to move about, thermoregulate, feed themselves, and perform other complex behaviors necessary for survival, whereas altricial species are unable to do any of these things at hatching and are entirely dependent on the parent for survival. These last days of growth prior to hatch may explain the increased tissue maturation observed in precocial species at hatching when compared to altricial species (Starck and Ricklefs, 1998).
In the Zebra Finch under artificial incubation, there is typically a short, single-day hatching window in comparison to the longer, 2-day hatching window for the chicken. Zebra Finch eggs left to incubate a full 14 days in artificial incubation conditions generally hatched early on the 14th day, whereas in the chicken, eggs hatch within the span of the 20th to 21st day. Additionally, even under the less standardized condition of parental incubation where parental attentiveness and other such factors can prolong incubation length, with a sample size of 379, the average length of incubation was 14.38 with a 95% confidence interval of 14.23–14.52 days. While eggs do occasionally hatch in more or less time, they are the exception rather than the rule.
Although most Zebra Finch embryos develop at approximately the same rate, they are often significantly different in size. The variation in size contrasts with chicken embryos, which are relatively more uniform in size for a given stage, but take different amounts of time to reach a given stage (Hamburger and Hamilton, 1951). Notably and in contrast to some other species (e.g., Mun and Kosin, 1960; Hendrickx and Hanzlik, 1965), there is variation in both egg size and embryo size in Zebra Finch embryos of the same age. Freshly laid Zebra Finch eggs weigh 1.1 g on average, but range from 0.8–1.5 g (N = 300). This variation in egg size may correspond to variation in embryo size at specific ages. At day 6 of development, embryo wet weights ranged between 0.047 and 0.192 g (n = 35) and embryo lengths (as measured from the tip of telencephalon to the tip of the tail bud) ranged from 21.8 to 23.4 mm (n = 3). At day 8 of development (n = 17), embryo wet weights ranged between 0.121 and 0.303 g while embryo lengths ranged between 20.7 to 23.1 mm (n = 3), which is slightly shorter than day 6 development. This can be attributed to shortening of the tail bud and greater length in the head region beyond the telencephalon as the beak develops. The variability in size and weight for a given stage suggests that both interspecific and intraspecific comparisons using stages based upon discrete measurements is not an optimal methodology. The problems inherent in using discrete measurements in species with embryonic size variability have been noted by Mun et al. (1960), Daniel et al. (1957), and Ricklefs and Starck (1998).
In summary, the number of physical and temporal developmental differences present between altricial and precocial birds supports the creation of a Zebra Finch-specific staging guide. High-resolution, labeled images and detailed corresponding text organized by tissue system presented in this staging guide will significantly aid in both consistency of terminology usage and the ability of the researcher to recognize developmental trends in the individual embryo. Building upon and incorporating HH and Yamasaki and Tonosaki (1998), our guide standardizes Zebra Finch development, thus preventing the guesswork and potential inaccuracies that result from approximation of altricial development using HH alone. This enables researchers to correlate organismal with cellular-molecular aspects of development with greater precision in the Zebra Finch, an important model organism that is being increasingly utilized for addressing questions across a diverse array of biological disciplines.
Acknowledgments
The authors thank Dan Cristol not only for his suggestions on the manuscript but for his ongoing support of this work. They also thank the reviewers for their very helpful suggestions. They also acknowledge support from the College of William and Mary, Department of Biology and College of Arts and Sciences for assistance with animal care.
LITERATURE CITED
- Austad SN. Candidate bird species for use in aging research. ILAR J. 2011;52:89–96. doi: 10.1093/ilar.52.1.89. [DOI] [PubMed] [Google Scholar]
- Bird DM, Gautier J, Montpetit V. Embryonic growth of American Kestrels. Auk. 1984;101:392–396. [Google Scholar]
- Blom J, Lilja C. A comparative study of embryonic development of some bird species with different patterns of postnatal growth. Zoology. 2005;108:81–95. doi: 10.1016/j.zool.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, To M. Afferents from vocal motor and respiratory effectors are recruited during vocal production in juvenile songbirds. J Neurosci. 2012;32:10895–10906. doi: 10.1523/JNEUROSCI.0990-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-C, Balaban E, Jarvis ED. 2012. Interspecies avian brain chimeras reveal that large brain size differences are influenced by cell-interdependent processes. PLoS One 7:e42477. [DOI] [PMC free article] [PubMed]
- Clayton DF, Balakrishnan CN, London SE. Integrating genomes, brain and behavior in the study of songbirds. Curr Biol. 2009;19:R865–R873. doi: 10.1016/j.cub.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA, Batt BDJ. Criteria for aging giant Canada Goose embryos. J Wildlife Manage. 1972;34:1267–1270. [Google Scholar]
- Daniel JC., Jr An embryological comparison of the domestic fowl and the red-winged Blackbird. Auk. 1957;74:340–358. [Google Scholar]
- de la Cruz V, Munoz-Armas S, Munoz-Castellanos L. Development of the Chick Heart. Baltimore: The Johns Hopkins University Press; 1972. pp. 7–41. [Google Scholar]
- Eichler VB. Atlas of Comparative Embryology. St. Louis, MO: The C.V. Mosby Company; 1978. [Google Scholar]
- Eng ML, Elliott JE, MacDougall-Shackleton SA, Letcher RJ, Williams TD. Early exposure to 2,2′,4,4′,5-pentabromodiphenyl ether (BDE-99) affects mating behavior of Zebra Finches. Toxicol Sci. 2012;127:269–276. doi: 10.1093/toxsci/kfs076. [DOI] [PubMed] [Google Scholar]
- Fant RJ. Criteria for aging pheasant embryos. J Wildlife Manage. 1957;21:324–328. [Google Scholar]
- Godsave SF, Lohmann R, Vloet RPM, Gahr M. Androgen receptors in the embryonic Zebra Finch hindbrain suggest a function for maternal androgens in perihatching survival. J Comp Neurol. 2002;453:57–70. doi: 10.1002/cne.10391. [DOI] [PubMed] [Google Scholar]
- Gorman HE, Orr KJ, Adam A, Nager RG. Effect of incubation conditions and offspring sex on embryonic development and survival in the Zebra Finch (Taeniopygia guttata) Auk. 2005;122:1239–1248. [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. Telomere length in early life predicts lifespan. Proc Natl Acad Sci USA. 2012;109:1743–1748. doi: 10.1073/pnas.1113306109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickx AG, Hanzlik R. Developmental stages of the Bob-White Quail embryo (Colinus virginianus) Biol Bull. 1965;129:523–531. doi: 10.2307/1539730. [DOI] [PubMed] [Google Scholar]
- Herbert C. A timed series of embryonic developmental stages of the Adelie Penguin (Pygoscelis adeliae) from Signy Island, South Orkney Islands. Br Antarct Surv Bull. 1967;14:45–67. [Google Scholar]
- Jarvis ED. Learned birdsong and the neurobiology of human language. Ann N Y Acad Sci. 2004;1016:749–777. doi: 10.1196/annals.1298.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LG, Volpe EP. Patterns and Experiments in Developmental Biology. Dubuque, IA: WM. C. Brown Company Publishers; 1973. [Google Scholar]
- Keary N, Bischof HJ. Activation changes in Zebra Finch (Taeniopygia guttata) brain areas evoked by alterations of the earth magnetic field. PLoS One. 2012;7:e38697. doi: 10.1371/journal.pone.0038697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppl C, Futterer E, Nieder B, Sisterman R, Wagner H. Embryonic and posthatching development of the Barn Owl (Tyto alba): Reference data for age determination. Dev Dyn. 2005;233:1248–1260. doi: 10.1002/dvdy.20394. [DOI] [PubMed] [Google Scholar]
- Lehman HE. Chordate Development: A Practical Textbook with Atlases and Techniques for Experimental and Descriptive Embryology. Winston-Salem, NC: 1983. p. Hunter Textbooks Inc. [Google Scholar]
- Maney DL, Goodson JL. Neurogenomic mechanisms of aggression in songbirds. Adv Genet. 2011;75:83–119. doi: 10.1016/B978-0-12-380858-5.00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U, Watanabe S, Bischof HJ. Spatial memory and the avian hippocampus: Research in Zebra Finches. J Physiol Paris, in press. 2012 doi: 10.1016/j.jphysparis.2012.05.002. DOI: 10.1016/j.jphysparis.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Montgomery RA, Burke CJ, Byers SM. A field guide to the aging of Wood Duck embryos. J Wildlife Manage. 1978;42:432–437. [Google Scholar]
- Moorman S, Mello CV, Bolhuis JJ. From songs to synapses: Molecular mechanisms of birdsong memory. Molecular mechanisms of auditory learning in songbirds involve immediate early genes, including zenk and arc, the ERK/MAPK pathway and synapsins. Bioessays. 2011;33:377–385. doi: 10.1002/bies.201000150. [DOI] [PubMed] [Google Scholar]
- Moorman S, Gobes SM, Kuijpers M, Kerkhofs A, Zandbergen MA, Bolhuis JJ. Human-like brain hemispheric dominance in birdsong learning. Proc Natl Acad Sci USA. 2012;109:12782–12787. doi: 10.1073/pnas.1207207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun AM, Kosin IL. Developmental stages of the Broad Breasted Bronze Turkey embryo. Biol Bull. 1960;119:90–97. [Google Scholar]
- Olea GB, Sandoval MT. Embryonic development of Columba livia (Aves: Columbiformes) from an altricial-precocial perspective. Rev Colomb Cienc Pec. 2012;25:3–13. [Google Scholar]
- Olson CR, Vleck CM, Vleck D. Periodic cooling of bird eggs reduces embryonic growth efficiency. Physiol Biochem Zool. 2006;79:927–936. doi: 10.1086/506003. [DOI] [PubMed] [Google Scholar]
- Olson CR, Vleck CM, Adams DC. Decoupled morphological development from growth in periodically cooled Zebra Finch embryos. J Morphol. 2008;269:875–883. doi: 10.1002/jmor.10635. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Arnold AP. Expression of estrogen receptor and aromatase mRNAs in embryonic and posthatch Zebra Finch brain. J Neurobiol. 2003;55:204–219. doi: 10.1002/neu.10190. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Ramachandran B, Arnold AP. Expression of androgen receptor mRNA in the late embryonic and early posthatch Zebra Finch brain. J Comp Neurol. 2003;455:513–530. doi: 10.1002/cne.10510. [DOI] [PubMed] [Google Scholar]
- Powell DC, Aulerich RJ, Balander RJ, Stromborg KL, Bursian SJ. A photographic guide to the development of double-crested Cormorant embryos. Colon Waterbird. 1998;21:348–355. [Google Scholar]
- Pytte CL, George S, Korman S, David E, Bogdan D, Kirn JR. Adult neurogenesis is associated with the maintenance of a stereotyped, learned motor behavior. J Neurosci. 2012;32:7052–7057. doi: 10.1523/JNEUROSCI.5385-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs RE, Starck JM. Embryonic growth and development. In: Ricklefs RE, Starck JM, editors. Avian Growth and Development: Evolution within the Altricial-Precocial Spectrum. New York: Oxford University Press; 1998. pp. 31–58. [Google Scholar]
- Rosa P, Nguyen V, Dubois F. Individual differences in sampling behavior predict social information use in Zebra Finches. Behav Ecol Sociobiol. 2012;66:1259–1265. [Google Scholar]
- Schoenwolf GC. Laboratory Studies of Vertebrate and Invertebrate Embryos: Guide and Atlas of Descriptive and Experimental Development. 8th ed. Upper Saddle River, NJ: 2001. p. Prentice Hall. [Google Scholar]
- Seguin A, Forstmeier W. No band color effects on male courtship rate or body mass in the Zebra Finch: Four experiments and a meta-analysis. PLoS One. 2012;7:e37785. doi: 10.1371/journal.pone.0037785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Winker K, Shaw TI, Jones KL, Glenn TC. Transcriptome analysis of a North American songbird, Melospiza melodia. DNA Res. 2012;19:325–333. doi: 10.1093/dnares/dss015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starck JM, Ricklefs RE. Patterns of development: The altricial-precocial spectrum. In: Ricklefs RE, Starck JM, editors. Avian Growth and Development: Evolution within the Altricial-Precocial Spectrum. New York: Oxford University Press; 1998. pp. 3–30. [Google Scholar]
- Vargha-Khadem F, Gadian DG, Copp A, Mishkin M. FOXP2 and the neuroanatomy of speech and language. Nat Rev Neurosci. 2005;6:131–138. doi: 10.1038/nrn1605. [DOI] [PubMed] [Google Scholar]
- Walton C, Pariser E, Nottebohm F. The Zebra Finch paradox: Song is little changed, but number of neurons doubles. J Neurosci. 2012;32:761–774. doi: 10.1523/JNEUROSCI.3434-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren WC, Clayton DF, Ellegren H, Arnold AP, Hillier LW, Kunstner A, Searle S, White S, Vilella AJ, Fairley S, Heger A, Kong LS, Ponting CP, Jarvis ED, Mello CV, Minx P, Lovell P, Velho TAF, Ferris M, Balakrishnan CN, Sinha S, Blatti C, London SE, Li Y, Lin YC, George J, Sweedler J, Southey B, Gunaratne P, Watson M, Nam K, Backstrom N, Smeds L, Nabholz B, Itoh Y, Whitney O, Pfenning AR, Howard J, Voelker M, Skinner BM, Griffin DK, Ye L, McLaren WM, Flicek P, Quesada V, Velasco G, Lopez-Otin C, Puente XS, Olender T, Lancet D, Smit AFA, Hubley R, Konkel MK, Walker JA, Batzer MA, Gu WJ, Pollock DD, Chen L, Cheng Z, Eichler EE, Stapley J, Slate J, Ekblom R, Birkhead T, Burke T, Burt D, Scharff C, Adam I, Richard H, Sultan M, Soldatov A, Lehrach H, Edwards SV, Yang SP, Li XC, Graves T, Fulton L, Nelson J, Chinwalla A, Hou SF, Mardis ER, Wilson RK. The genome of a songbird. Nature. 2010;464:757–762. doi: 10.1038/nature08819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson RL, Schoenwolf GC. Laboratory Studies of Chick, Pig, and Frog Embryos: Guide and Atlas of Vertebrate Embryology. 5th ed. Minneapolis, MN: Burgess Publishing Company; 1984. [Google Scholar]
- Winograd C, Ceman S. Exploring the Zebra Finch Taeniopygia guttata as a novel animal model for the speech-language deficit of fragile X syndrome. Results Probl Cell Differ. 2012;54:181–197. doi: 10.1007/978-3-642-21649-7_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki M, Tonosaki A. Developmental stages of the Society Finch Lonchura striata var dornestica. Dev Growth Differ. 1988;30:515–542. doi: 10.1111/j.1440-169X.1988.00515.x. [DOI] [PubMed] [Google Scholar]
- Young RL, Bever GS, Wang Z, Wagner GP. Identity of the avian wing digits: Problems resolved and unsolved. Dev Dyn. 2011;240:1042–1053. doi: 10.1002/dvdy.22595. [DOI] [PubMed] [Google Scholar]


