Abstract
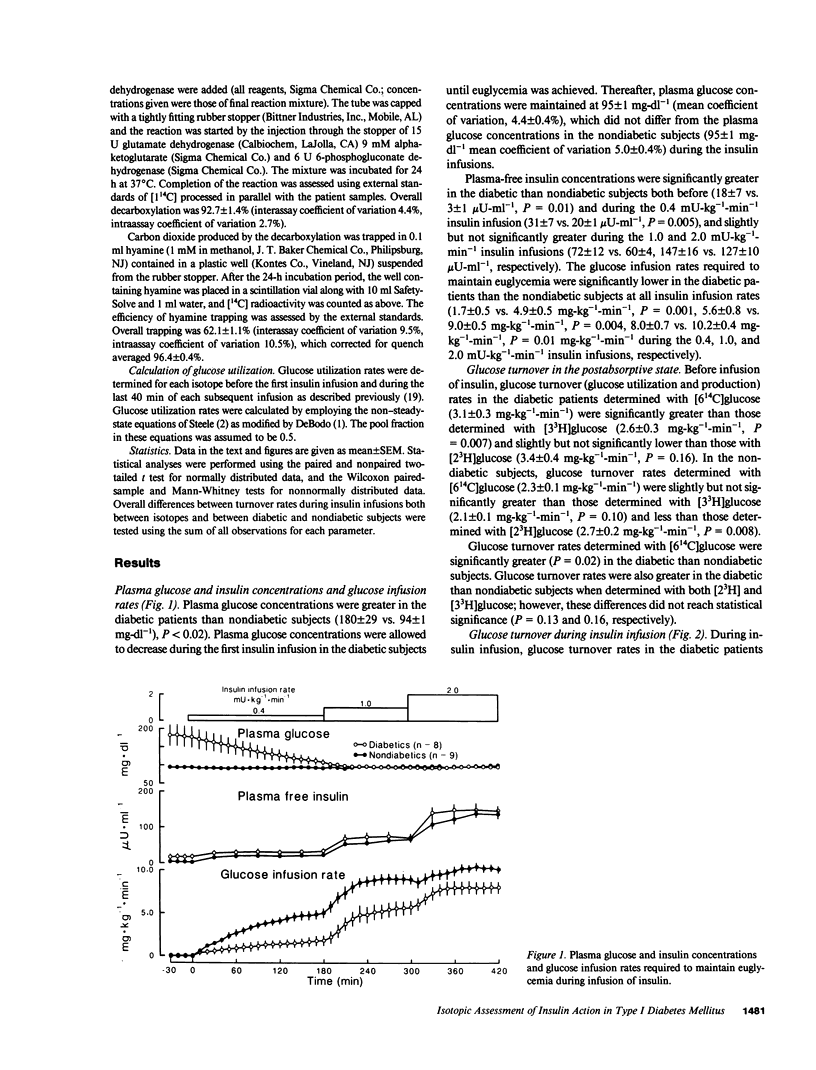
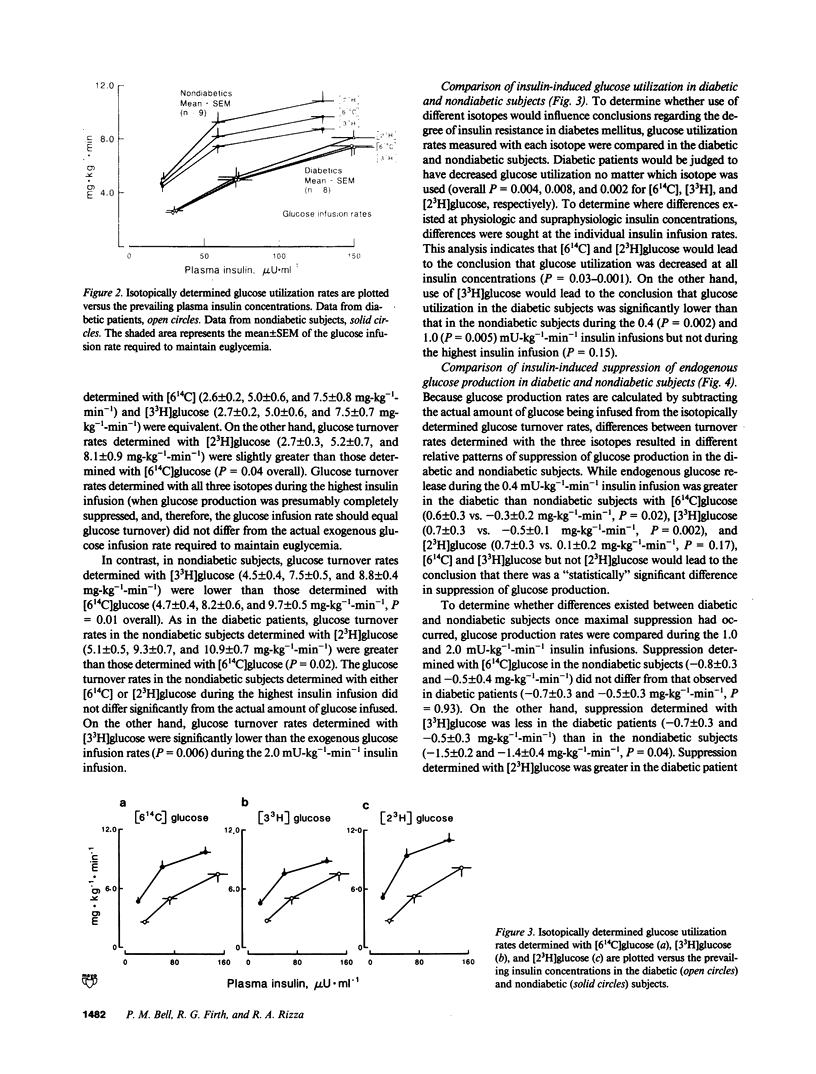
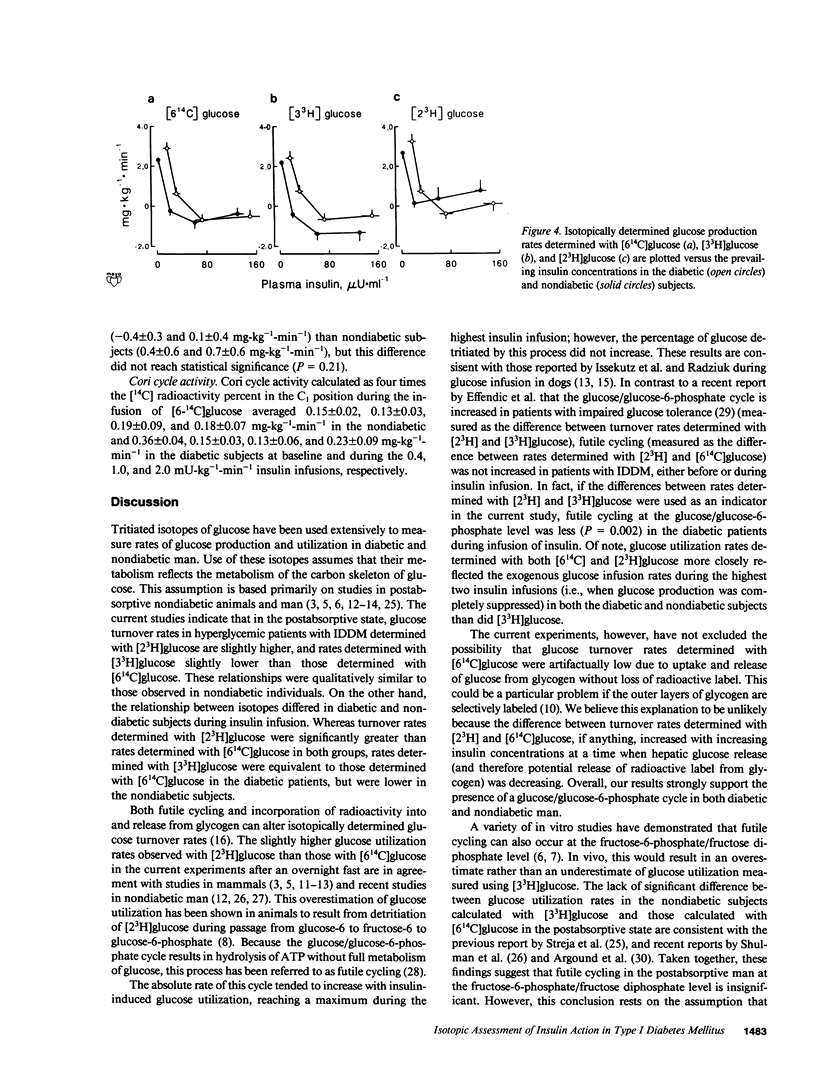
To determine whether [2(3)H], [3(3)H], and [6(14)C]glucose provide an equivalent assessment of glucose turnover in insulin-dependent diabetes mellitus (IDDM) and nondiabetic man, glucose utilization rates were measured using a simultaneous infusion of these isotopes before and during hyperinsulinemic euglycemic clamps. In the nondiabetic subjects, glucose turnover rates determined with [6(14)C]glucose during insulin infusion were lower (P less than 0.02) than those determined with [2(3)H]glucose and higher (P less than 0.01) than those determined with [3(3)H]glucose. In IDDM, glucose turnover rates measured with [6(14)C]glucose during insulin infusion were lower (P less than 0.05) than those determined with [2(3)H]glucose, but were not different from those determined with [3(3)H]glucose. All three isotopes indicated the presence of insulin resistance. However, using [3(3)H]glucose led to the erroneous conclusion that glucose utilization was not significantly decreased at high insulin concentrations in the diabetic patients. [6(14)C] and [3(3)H]glucose but not [2(3)H]glucose indicated impairment in insulin-induced suppression of glucose production. These results indicate that tritiated isotopes do not necessarily equally reflect the pattern of glucose metabolism in diabetic and nondiabetic man.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altszuler N., Barkai A., Bjerknes C., Gottlieb B., Steele R. Glucose turnover values in the dog obtained with various species of labeled glucose. Am J Physiol. 1975 Dec;229(6):1662–1667. doi: 10.1152/ajplegacy.1975.229.6.1662. [DOI] [PubMed] [Google Scholar]
- Bell P. M., Firth R. G., Rizza R. A. Effects of hyperglycemia on glucose production and utilization in humans. Measurement with [23H]-, [33H]-, and [614C]glucose. Diabetes. 1986 Jun;35(6):642–648. doi: 10.2337/diab.35.6.642. [DOI] [PubMed] [Google Scholar]
- Bergman R. N., Finegood D. T., Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev. 1985 Winter;6(1):45–86. doi: 10.1210/edrv-6-1-45. [DOI] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Mott D., Reaven G. R., Kashiwagi A., Foley J. E. Relationship between obesity and maximal insulin-stimulated glucose uptake in vivo and in vitro in Pima Indians. J Clin Invest. 1984 Mar;73(3):800–805. doi: 10.1172/JCI111274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D., Lee D., Rognstad R., Katz J. Futile cycles in isolated perfused rat liver and in isolated rat liver parenchymal cells. Biochem Biophys Res Commun. 1975 Nov 3;67(1):212–219. doi: 10.1016/0006-291x(75)90304-6. [DOI] [PubMed] [Google Scholar]
- DEBODO R. C., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S. ON THE HORMONAL REGULATION OF CARBOHYDRATE METABOLISM; STUDIES WITH C14 GLUCOSE. Recent Prog Horm Res. 1963;19:445–488. [PubMed] [Google Scholar]
- DeFronzo R. A., Simonson D., Ferrannini E. Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1982 Oct;23(4):313–319. doi: 10.1007/BF00253736. [DOI] [PubMed] [Google Scholar]
- Del Prato S., Nosadini R., Tiengo A., Tessari P., Avogaro A., Trevisan R., Valerio A., Muggeo M., Cobelli C., Toffolo G. Insulin-mediated glucose disposal in type I diabetes: evidence for insulin resistance. J Clin Endocrinol Metab. 1983 Nov;57(5):904–910. doi: 10.1210/jcem-57-5-904. [DOI] [PubMed] [Google Scholar]
- Devos P., Hers H. G. A molecular order in the synthesis and degradation of glycogen in the liver. Eur J Biochem. 1979 Aug 15;99(1):161–167. doi: 10.1111/j.1432-1033.1979.tb13242.x. [DOI] [PubMed] [Google Scholar]
- Dunn A., Katz J., Golden S., Chenoweth M. Estimation of glucose turnover and recycling in rabbits using various [3H, 14C]glucose labels. Am J Physiol. 1976 Apr;230(4):1159–1162. doi: 10.1152/ajplegacy.1976.230.4.1159. [DOI] [PubMed] [Google Scholar]
- Efendić S., Wajngot A., Vranić M. Increased activity of the glucose cycle in the liver: early characteristic of type 2 diabetes. Proc Natl Acad Sci U S A. 1985 May;82(9):2965–2969. doi: 10.1073/pnas.82.9.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth R. G., Bell P. M., Marsh H. M., Hansen I., Rizza R. A. Postprandial hyperglycemia in patients with noninsulin-dependent diabetes mellitus. Role of hepatic and extrahepatic tissues. J Clin Invest. 1986 May;77(5):1525–1532. doi: 10.1172/JCI112467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L. The role of futile cycles in the regulation of carbohydrate metabolism in the liver. Adv Enzymol Relat Areas Mol Biol. 1981;52:247–331. doi: 10.1002/9780470122976.ch4. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr Studies on hepatic glucose cycles in normal and methylprednisolone-treated dogs. Metabolism. 1977 Feb;26(2):157–170. doi: 10.1016/0026-0495(77)90051-8. [DOI] [PubMed] [Google Scholar]
- Judson G. J., Leng R. A. Estimation of the total entry rate and resynthesis of glucose in sheep using glucoses uniformly labelled with 14 C and variously labelled with 3 H. Aust J Biol Sci. 1972 Dec;25(6):1313–1332. doi: 10.1071/bi9721313. [DOI] [PubMed] [Google Scholar]
- Kahn C. R. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism. 1978 Dec;27(12 Suppl 2):1893–1902. doi: 10.1016/s0026-0495(78)80007-9. [DOI] [PubMed] [Google Scholar]
- Kalhan S. C., Savin S. M., Adam P. A. Estimation of glucose turnover with stable tracer glucose-1-13C. J Lab Clin Med. 1977 Feb;89(2):285–294. [PubMed] [Google Scholar]
- Katz J., Dunn A. Glucose-2-t as a tracer for glucose metabolism. Biochemistry. 1967 Jan;6(1):1–5. doi: 10.1021/bi00853a001. [DOI] [PubMed] [Google Scholar]
- Katz J., Rognstad R. Futile cycles in the metabolism of glucose. Curr Top Cell Regul. 1976;10:237–289. doi: 10.1016/b978-0-12-152810-2.50013-9. [DOI] [PubMed] [Google Scholar]
- Lager I., Lönnroth P., von Schenck H., Smith U. Reversal of insulin resistance in type I diabetes after treatment with continuous subcutaneous insulin infusion. Br Med J (Clin Res Ed) 1983 Dec 3;287(6406):1661–1664. doi: 10.1136/bmj.287.6406.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laville M., Riou J. P., Bougneres P. F., Canivet B., Beylot M., Cohen R., Serusclat P., Dumontet C., Berthezene F., Mornex R. Glucose metabolism in experimental hyperthyroidism: intact in vivo sensitivity to insulin with abnormal binding and increased glucose turnover. J Clin Endocrinol Metab. 1984 Jun;58(6):960–965. doi: 10.1210/jcem-58-6-960. [DOI] [PubMed] [Google Scholar]
- Miller T. B., Jr Effects of diabetes on glucose regulation of enzymes involved in hepatic glycogen metabolism. Am J Physiol. 1978 Jan;234(1):E13–E19. doi: 10.1152/ajpendo.1978.234.1.E13. [DOI] [PubMed] [Google Scholar]
- Nakagawa S., Nakayama H., Sasaki T., Yoshino K., Yu Y. Y. A simple method for the determination of serum free insulin levels in insulin-treated patients. Diabetes. 1973 Aug;22(8):590–600. doi: 10.2337/diab.22.8.590. [DOI] [PubMed] [Google Scholar]
- Newgard C. B., Foster D. W., McGarry J. D. Evidence for suppression of hepatic glucose-6-phosphatase with carbohydrate feeding. Diabetes. 1984 Feb;33(2):192–195. doi: 10.2337/diab.33.2.192. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A. Substrate cycles: their metabolic, energetic and thermic consequences in man. Biochem Soc Symp. 1978;(43):183–205. [PubMed] [Google Scholar]
- RIEDER S. V., ROSE I. A. The mechanism of the triosephosphate isomerase reaction. J Biol Chem. 1959 May;234(5):1007–1010. [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Experimental validation of measurements of glucose turnover in nonsteady state. Am J Physiol. 1978 Jan;234(1):E84–E93. doi: 10.1152/ajpendo.1978.234.1.E84. [DOI] [PubMed] [Google Scholar]
- Revers R. R., Kolterman O. G., Scarlett J. A., Gray R. S., Olefsky J. M. Lack of in vivo insulin resistance in controlled insulin-dependent, type I, diabetic patients. J Clin Endocrinol Metab. 1984 Feb;58(2):353–358. doi: 10.1210/jcem-58-2-353. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Haymond M. W., Gerich J. E. Adrenergic mechanisms for the effects of epinephrine on glucose production and clearance in man. J Clin Invest. 1980 Mar;65(3):682–689. doi: 10.1172/JCI109714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol. 1981 Jun;240(6):E630–E639. doi: 10.1152/ajpendo.1981.240.6.E630. [DOI] [PubMed] [Google Scholar]
- STEELE R., WALL J. S., DE BODO R. C., ALTSZULER N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956 Sep;187(1):15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- Seitz H. J., Müller M. J., Krone W., Tarnowski W. Rapid conversion by insulin of hepatic intermediary metabolism from glucose production to glucose utilization in the liver of alloxan-diabetic rats. Diabetes. 1977 Dec;26(12):1159–1174. doi: 10.2337/diab.26.12.1159. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Ladenson P. W., Wolfe M. H., Ridgway E. C., Wolfe R. R. Substrate cycling between gluconeogenesis and glycolysis in euthyroid, hypothyroid, and hyperthyroid man. J Clin Invest. 1985 Aug;76(2):757–764. doi: 10.1172/JCI112032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streja D. A., Steiner G., Marliss E. B., Vranic M. Turnover and recycling of glucose in man during prolonged fasting. Metabolism. 1977 Oct;26(10):1089–1098. doi: 10.1016/0026-0495(77)90035-x. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Hue L., Hers H. G. Control of the fructose-6-phosphate/fructose 1,6-bisphosphate cycle in isolated hepatocytes by glucose and glucagon. Role of a low-molecular-weight stimulator of phosphofructokinase. Biochem J. 1980 Dec 15;192(3):887–895. doi: 10.1042/bj1920887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin E., Castillo M., Luyckx A. S., Lefebvre P. J. Similar metabolic effects of pulsatile versus continuous human insulin delivery during euglycemic, hyperinsulinemic glucose clamp in normal man. Diabetes. 1984 Dec;33(12):1169–1174. doi: 10.2337/diab.33.12.1169. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Koivisto V. A. Insulin sensitivity in newly diagnosed type 1 diabetics after ketoacidosis and after three months of insulin therapy. J Clin Endocrinol Metab. 1984 Sep;59(3):371–378. doi: 10.1210/jcem-59-3-371. [DOI] [PubMed] [Google Scholar]




